Highlights
► rB/HPIV3 & rHPIV3-NB contain ≥1 internal gene from BPIV3 & HN & F genes from HPIV3. ► In phase I trials both vaccines induced antibody in HPIV3-naïve children. ► rB/HPIV3 was significantly more restricted in replication than rHPIV3-NB. ► rB/HPIV3 induced significantly higher HPIV3 antibody titers than rHPIV3-NB. ► rB/HPIV3 is the preferred candidate for further clinical development.
Keywords: Parainfluenza, Live-attenuated vaccine, Clinical trial
Abstract
Human parainfluenza virus type 3 (HPIV3) is an important cause of lower respiratory tract illness in children, yet a licensed vaccine or antiviral drug is not available. We evaluated the safety, tolerability, infectivity, and immunogenicity of two intranasal, live-attenuated HPIV3 vaccines, designated rHPIV3-NB and rB/HPIV3, that were cDNA-derived chimeras of HPIV3 and bovine PIV3 (BPIV3). These were evaluated in adults, HPIV3 seropositive children, and HPIV3 seronegative children. A total of 112 subjects participated in these studies. Both rB/HPIV3 and rHPIV3-NB were highly restricted in replication in adults and seropositive children but readily infected seronegative children, who shed mean peak virus titers of 102.8 vs. 103.7 pfu/mL, respectively. Although rB/HPIV3 was more restricted in replication in seronegative children than rHPIV3-NB, it induced significantly higher titers of hemagglutination inhibition (HAI) antibodies against HPIV3. Taken together, these data suggest that the rB/HPIV3 vaccine is the preferred candidate for further clinical development.
1. Introduction
Human parainfluenza virus type 3 (HPIV3) accounts for at least 11,000 pediatric hospitalizations annually in the United States and is an important cause of acute lower respiratory illness (ALRI) in infants and young children worldwide [1], [2], [3], [4], [5], [6]. Like respiratory syncytial virus (RSV), HPIV3 can cause bronchiolitis, viral pneumonia and apnea in infants and has been associated with wheezing episodes in older children with asthma [4], [5], [7], [8], [9]. Although precise estimates do not exist, the outpatient burden of HPIV3 is likely substantial, since the number of emergency room and outpatient visits for HPIV3-associated illness may exceed hospitalizations by 10–80-fold [4], [6]. Since approximately two-thirds of HPIV3 hospitalizations occur in the first year of life [4], [6], development of a vaccine capable of inducing protective immunity in infancy is urgently needed.
Live-attenuated, intranasally administered HPIV3 offers several potential advantages as a vaccine for infants, including ease of administration, reduction of the likelihood of interference with the many childhood vaccines that are given parenterally during the first year of life, lack of restriction of vaccine virus replication by the presence of HPIV3-specific maternal serum antibodies that are present in early infancy [10], [11], and induction of local immunity [12], [13]. Since the immunogenicity of vaccines administered during the first few months of life is typically less than that observed in older children, more than one dose of an HPIV3 vaccine would likely be needed to induce lasting protective immunity in infants and children [10], [11], [14], [15]. Serum antibody responses to the surface antigens of HPIV3, namely the hemagglutinin-neuraminidase (HN) and fusion (F) glycoproteins, are recognized correlates of protective immunity [7], [16], [17], [18], [19], [20]. The roles of other aspects of host immunity in protective responses against HPIV3 are not well understood, particularly in the pediatric population.
Two approaches have been used to generate HPIV3 vaccines for clinical development. First, wild-type (wt) HPIV3 was attenuated by serial passage at low temperatures to yield cp45 HPIV3, which was shown to be well-tolerated, infectious, immunogenic, and phenotypically stable in HPIV3-seronegative children as young as one month of age [10], [11]. More recently, the tolerability, level of attenuation, and immunogenicity of a cDNA-derived recombinant version of cp45, designated rcp45, was shown to be comparable to what was previously observed with the biologically derived cp45 vaccine [11a]. The use of cDNA-derived virus has the advantage of a short, well-characterized passage history as well as the ability to readily regenerate and modify the virus as needed.
Another approach to HPIV3 vaccine development has been based on a related animal virus, bovine parainfluenza type 3 (BPIV3). BPIV3 has evolved in bovines and is restricted for replication in humans, i.e., it exhibits a host-range phenotype. BPIV3 has been shown to be well-tolerated and attenuated in adults, children, and infants as young as one month of age [10], [21]. However, cross-neutralization studies have demonstrated that the BPIV3 HN and F proteins are only 25% antigenically related to the HPIV3 HN and F, and antibody responses to HPIV3 in children administered BPIV3 have been suboptimal [10], [21], [22]. For this reason, cDNA-derived chimeric viruses have been developed that contain the HN and F genes of HPIV3 in a backbone containing one or more BPIV3 genes. The purpose of this strategy is to combine the antigenic specificity of the HPIV3 HN and F glycoproteins with the host range restriction of BPIV3.
Previous studies in non-human primates demonstrated that each of the genes of BPIV3 independently contributes to the host-range attenuation phenotype [20], which suggested that chimeric human/bovine PIV3 vaccines could be developed to contain either a single BPIV3 gene substitution or multiple BPIV3 attenuating genes. In the present study, we evaluated one example of each of these types of chimeric human/bovine PIV3 vaccines in clinical trials. In rHPIV3-NB, the HPIV3 nucleocapsid (N) gene was replaced by its BPIV3 counterpart; the rest of the genome is HPIV3. In contrast, rB/HPIV3 consists of BPIV3 in which the HN and F genes were replaced by their HPIV3 counterparts. In previous studies, both rHPIV3-NB and rB/HPIV3 were found to be attenuated in non-human primates and to induce higher titers of antibody to the HPIV3 HN than were induced by BPIV3 [20], [23], [24], [25]. Here, we report the phase I evaluation of these vaccines in adults, children, and infants as young as 6 months of age.
2. Materials and methods
2.1. Vaccines
To generate recombinant rHPIV3-NB, cDNA encoding the genome of the HPIV3 JS strain was modified to replace the HPIV3 nucleoprotein (N) open reading frame (ORF) with the N ORF of the BPIV3 Kansas strain [23]. This cDNA was used to derive infectious virus as previously described [20]. Vaccine seed virus and clinical trials material (CTM) were manufactured in qualified Vero cells at Novavax, Inc. (Rockville, MD). rB/HPIV3 was derived entirely from plasmid cDNA in qualified Vero cells at the National Institutes of Health (NIH) [26], and CTM was manufactured in qualified Vero cells at Charles River Laboratories (Malvern, PA). The HPIV3-NB and B/HPIV3 CTMs were supplied to the clinical site as frozen concentrates with mean infectivity titers of 107.03 and 107.0 50%-tissue-culture-infectious-doses (TCID50) per mL, respectively. CTMs were stored at −70 °C and diluted to dose on-site using Leibovitz L15 medium. L15 medium was also used as the placebo.
2.2. Study population, study design, and clinical trial oversight
Both trials were conducted through the Center for Immunization Research, at the Johns Hopkins Bloomberg School of Public Health. Enrollment in the rHPIV3-NB trial was completed between April 2002 and October 2004, and enrollment in the rB/HPIV3 trial was completed between July 2007 and December 2010. The ClinicalTrials.gov identifier for the rB/HPIV3 trial is NCT00366782; the rHPIV3-NB trial was done earlier and does not have an identifier. Each vaccine was evaluated sequentially in (i) adults that were not screened for HPIV3 serostatus (but who all proved to be HPIV3 seropositive), (ii) HPIV3 seropositive children ages 15–59 months, and (iii) HPIV3 seronegative children ages 6–36 months. Studies in adults were conducted as open-label trials, with all subjects receiving vaccine. Studies in children were conducted as randomized, double-blind and placebo-controlled trials, with subjects randomized 2:1 to receive vaccine or placebo. Randomization was performed in blocks of 3 (2 vaccinees and 1 placebo recipient) to permit unblinding and analysis of adverse events throughout the study, which was done when each group of 3 subjects completed the initial follow-up period (approximately 28 days for HPIV3 seropositive children and approximately 56 days for HPIV3 seronegative children). Vaccines and placebo were each administered in a volume of 0.5 mL as nose drops (approximately 0.25 mL per nostril).
Both studies were designed to evaluate a single dose level of vaccine in adults and seropositive children, and to evaluate a 10-fold lower dose in seronegative children. In addition, each study had the contingency to subsequently increase the dose in seronegative children 10-fold if the infectivity of the vaccine virus was insufficient at the initial lower dose (insufficient infectivity was pre-specified as <90% of seronegative children infected, with infection defined as shedding of vaccine virus and/or a >4-fold rise in serum antibody titer). For rHPIV3-NB, the dose evaluated in adults and seropositive children was 105.0 TCID50, and the doses evaluated in seronegative children were 104.0 TCID50 and 105.0 TCID50. For rB/HPIV3, the dose evaluated in adults and seropositive children was 106.0 TCID50 and the dose evaluated in seronegative children was 105.0 TCID50; these higher doses were utilized based upon the infectivity of the rHPIV3-NB virus, as described in Section 3.
Written informed consent was obtained from study participants (adults) or from the parents or guardians of study participants (children) prior to enrollment. These studies were conducted in accordance with the principles of the Declaration of Helsinki and the Standards of Good Clinical Practice (as defined by the International Conference on Harmonization). The studies were performed under NIAID-held Investigational New Drug applications (BB-INDs #9876 and #13,108) and were reviewed by the US Food and Drug Administration. The clinical protocols, consent forms, and Investigators’ Brochures were developed by CIR and NIAID investigators and were reviewed and approved by the Western Institutional Review Board (WIRB) and the NIAID Regulatory Compliance and Human Subjects Protection Branch (RCHSPB). Clinical data were reviewed by CIR and NIAID investigators, and by a safety monitor (rHPIV3-NB) or by the Data Safety Monitoring Board of the NIAID Division of Clinical Research (rB/HPIV3).
For adults and HPIV3 seropositive children enrolled in these trials, clinical assessments and nasal washes were performed on study day 0 (the day of vaccination, with the nasal wash performed prior to inoculation), and on days 3–7 and 10 following inoculation. Following day 10, illness data (adverse events and reactogenicity events) were collected through day 28, with additional physical examinations performed and nasal washes obtained in the event of lower respiratory tract illness (LRI). All LRIs were defined as serious adverse events (SAEs), regardless of severity. As required by the FDA, serum alanine aminotransferase (ALT) was measured in adults and seropositive children before inoculation and 28 days after inoculation of rHPIV3-NB. For seronegative children, clinical assessments and nasal washes were performed on study days 0, 3–7, and on days 10, 12, 14, 17, 19, 21 ±1 day. As part of the rB/HPIV3 study, an additional clinical assessment and a nasal wash were performed on day 28, and a follow-up phone call was conducted approximately 6 months after vaccination to determine whether any SAEs had occurred after the acute observation period. Following the last scheduled nasal wash, illness data were obtained for seronegative children through day 56, with physical examinations performed and additional nasal washes obtained in the event of LRI. Titers of vaccine virus in nasal wash specimens were determined as described below. Fever, upper respiratory tract illness (URI [rhinorrhea or pharyngitis]), cough, LRI, and otitis media were defined as previously described [21]. When illnesses occurred, nasal washes were tested for adventitious agents by viral culture for the rHPIV3-NB study and by rRT-PCR (Fast-track Diagnostics, Luxembourg) for the rB/HPIV3 study.
Sera were obtained to measure antibodies to HPIV3 prior to inoculation, approximately 1 month after inoculation in adults and seropositive children, and approximately 2 months after inoculation in seronegative children.
2.3. Isolation, quantitation, and characterization of virus
Nasal washes were performed using a nasal bulb syringe and 15–20 mL of lactated Ringer's solution. Nasal washes were snap frozen on site and stored at −80 °C. An aliquot of each nasal wash was rapidly thawed, inoculated onto LLC-MK2 cells, and incubated at 32 °C for primary virus isolation [10], [11]. For all specimens that showed cytopathic effect or evidence of infection by hemadsorption, a second aliquot was titered by hemadsorption plaque assay with an agar overlay on LLC-MK2 cells at 32 °C [10], [11]. Titers of vaccine virus are expressed as the number of plaque-forming units (pfu) per mL of nasal wash fluid. The lower limit of detection was 0.6 log10 pfu/ml. To differentiate HPIV3 from B/HPIV3, RNA was extracted from virus isolates using Viral RNA Mini Kits (Qiagen, Valencia, CA), and cDNA was generated using Superscript First Strand Kit (Invitrogen, Carlsbad, CA). cDNA was amplified with virus-specific primers using the Clontech Advantage HF2 PCR Kit (Clontech, Mountain View, CA).
2.4. Immunologic assays
Sera from all subjects were tested for antibodies to HPIV3 (using the HPIV3 Washington/57 strain as the assay virus) by the hemagglutination-inhibition (HAI) antibody test [27], starting at a serum dilution of 1:4, with 2-fold dilution increments and endpoint titration.
2.5. Data analysis
Infection with vaccine virus was defined as either the isolation of vaccine virus or a >4-fold rise in antibody titer as measured by serum HAI to HPIV3 [27]. The mean peak titer of vaccine virus shed (log10 pfu/mL) was calculated for infected vaccinees only. The HAI reciprocal titers were transformed to log2 values for calculation of mean log2 titers, and Student's t test was used to compare HAI titers between groups. Rates of illness among vaccinees and placebo recipients were compared by the two-tailed Fisher's exact test.
3. Results
3.1. Study populations
As shown in Table 1, Table 2 and Fig. 1 , 112 subjects were enrolled in these studies. Each vaccine was administered to 15 adults (30 adults in total). Each vaccine was next evaluated in cohorts of HPIV3 seropositive children, 10 of whom received vaccine and 5 of whom received placebo (30 seropositive children in total). The rHPIV3-NB vaccine was subsequently evaluated in a cohort of 31 HPIV3 seronegative children (14 received 104.0 TCID50 of vaccine, 7 received 105.0 TCID50 of vaccine, and 10 received placebo). The rB/HPIV3 vaccine was evaluated in a cohort of 21 seronegative children (14 received 105.0 TCID50 of vaccine and 7 received placebo). In total, 52 seronegative children participated in these trials.
Table 1.
Clinical responses and vaccine virus shedding in adults and children following intranasal administration of rHPIV3-NB, rB/HPIV3, or placebo.
| Participants, virus given | Dose, log10 TCID50 | No. of participants | Participants infected, % | Virus isolation, nasal wash |
Participants with indicated illness, % |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Participants who shed virus, % | Duration of shredding, meana,b (± SD), days | Peak titer meanb (± SD), log10TCID50 | Fever | URIc | LRIc | Cough | OMc | Any respiratory or febrile illness | ||||
| Adults | ||||||||||||
| rHPIV3-NB | 5.0 | 15 | 0 | 0 | 0 | ≤0.6 | 0 | 7 | 0 | 0 | 0 | 7 |
| rB/HPIV3 | 6.0 | 15 | 20 | 20 | 6 (1.0) | 0.75 | 7 | 7 | 0 | 7 | 0 | 7 |
| Children | ||||||||||||
| HPIV3 seropositive | ||||||||||||
| rHPIV3-NB | 5.0 | 10 | 10 | 10 | 10.0 | 2.3 | 50 | 0 | 0 | 10 | 10 | 50 |
| rB/HPIV3 | 6.0 | 10 | 20 | 20 | 6.5 (0.7) | 2.9 (0.7) | 10 | 0 | 0 | 0 | 0 | 10 |
| Placebod | – | 5 | 0 | 0 | 0 | ≤0.6 | 20 | 20 | 0 | 20 | 0 | 40 |
| Placeboe | – | 5 | 0 | 0 | 0 | ≤0.6 | 20 | 0 | 0 | 0 | 0 | 20 |
| HPIV3 seronegative | ||||||||||||
| rHPIV3-NB | 4.0 | 14 | 71 | 71 | 15.4 (5.5) | 3.1 (1.7) | 21 | 43 | 0 | 14 | 0 | 64 |
| rHPIV3-NB | 5.0 | 7 | 86 | 86 | 14.7 (2.2) | 3.7 (1.2) | 14 | 29 | 0 | 0 | 0 | 29 |
| rB/HPIV3 | 5.0 | 14 | 100 | 86 | 8.2 (1.12) | 2.8 (1.2) | 43 | 57 | 7f | 14 | 14 | 79 |
| Placebod | – | 10 | 10g | 0 | 0 | ≤0.6 | 10 | 40 | 0 | 0 | 0 | 40 |
| Placeboe | – | 7 | 0 | 0 | 0 | ≤0.3 | 57 | 43 | 0 | 29 | 0 | 71 |
Duration of shedding was defined as the time between inoculation and the last day on which vaccine virus was recovered.
Means were calculated for subjects infected with vaccine virus (see Section 2 for definition).
LRI, lower respiratory tract illness; OM, otitis media; URI, upper respiratory tract illness.
Placebo recipients in studies of rHPIV3-NB vaccine.
Placebo recipients in studies of rB/HPIV3 vaccine.
At the time of this child's LRI, human metapneumovirus, bocavirus, and coronavirus 63 were detected in nasal wash specimens. Vaccine virus was not detected at the time of LRI.
One placebo recipient had an antibody response to HPIV3 but did not shed vaccine or wild-type virus. This most likely represents intercurrent infection with wild-type virus between the time of the last nasal wash specimen (day 21) and the postvaccination serum specimen (day 56).
Table 2.
Serum antibody responses in adults and children following intranasal administration of rHPIV3-NB, rB/HPIV3, or placebo.
| Participants, virus given | Dose, log10 TCID50 | # Participants | HAI antibody titer, mean (±SD) |
||
|---|---|---|---|---|---|
| Before | After | ≥4-fold increase, %a | |||
| Adults | |||||
| rHPIV3-NB | 5.0 | 15 | 6.9 (0.9) | 6.9 (0.9) | 0 |
| rB/HPIV3 | 6.0 | 15 | 6.1 (0.7) | 6.3 (0.5) | 0 |
| Children | |||||
| HPIV3 seropositive | |||||
| rHPIV3-NB | 5.0 | 10 | 6.1 (1.6) | 6.1 (1.7) | 0 |
| rB/HPIV3 | 6.0 | 10 | 6.3 (1.2) | 7.0 (1.2) | 10 |
| Placebob | – | 5 | 5.6 (1.0) | 5.6 (1.0) | 0 |
| Placeboc | – | 5 | 6.2 (0.8) | 6.4 (0.9) | 0 |
| HPIV3 seronegative | |||||
| rHPIV3-NB | 4.0 | 14 | 1.9 (0.9) | 4.6 (2.3) | 64 |
| rHPIV3-NB | 5.0 | 7 | 1.9 (08) | 4.3 (0.7) | 86 |
| rB/HPIV3 | 5.0 | 14 | 1.3 (0.7) | 5.6 (1.2) | 93 |
| Placebob | – | 10 | 1.2 (0.4) | 1.7 (1.6) | 10d |
| Placeboc | – | 7 | 1.0 | 2.9 (3.2) | 29e |
All antibody titers are expressed as mean reciprocal log2 values. Serum specimens were obtained before immunization and 4 weeks (for adults and HPIV3-seropositive children) or 8 weeks (for HPIV3-seronegative children) after immunization. HAI, hemagglutination-inhibition.
Percentage of participants who experience a ≥4-fold increase in the indicated antibody titer.
Placebo recipients in studies of rHPIV3-NB.
Placebo recipients in studies of rB/HPIV3
A single placebo recipient experienced a rise in serum HAI titer. This participant was inoculated in September and may have been infected with wild-type HPIV3 before collection of the postinoculation serum specimen in November.
Two placebo recipients experienced rises in serum HAI titer. Wt HPIV3 was detected in nasal wash specimens obtained from each of these children.
Fig. 1.
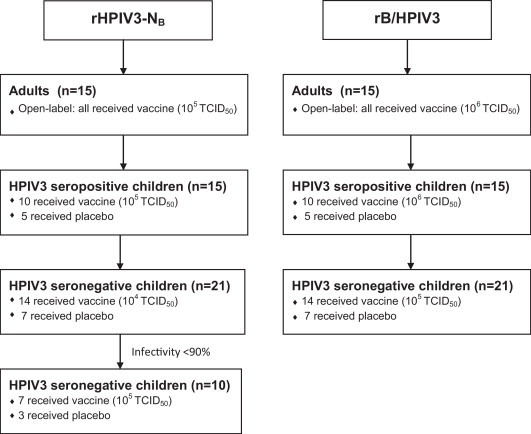
Enrollment of adults, HPIV3 seropositive, and HPIV3 seronegative children in phase I clinical trials of the rHPIV3-NB and the rB/HPIV3 vaccines. As described in Section 2, adults were enrolled in open-label trials and children were enrolled in placebo-controlled trials. For each vaccine, studies were performed sequentially in adults, HPIV3 seropositive children, and HPIV3 seronegative children. A dose-escalation study was performed in the seronegative cohorts of children receiving rHPIV3-NB because the vaccine was insufficiently infectious at a dose of 105 TCID50. No subjects withdrew from this study. Additional details are provided in the text.
3.2. Response of adults and HPIV3 seropositive children
In adults and HPIV3 seropositive children, rHPIV3-NB and rB/HPIV3 were well tolerated and, in most subjects, highly restricted in replication (Table 1). Vaccination-related SAEs did not occur. In each study, no more than 10% (rHPIV3-NB) or 20% (rB/HPIV3) of adults and HPIV3 seropositive children had any evidence of infection with vaccine virus (Table 1). All of the subjects who shed vaccine virus were asymptomatic, including one seropositive child who received rB/HPIV3 and shed vaccine virus on study days 3–7 with a relatively high peak titer of 104.4 TCID50/mL of nasal wash fluid. Replication of the vaccine viruses was highly restricted in all of the other of these HPIV3-experienced subjects. Illnesses (fever, rhinorrhea, cough and/or otitis media) occurred in some subjects who were not infected with the vaccine viruses; one of these individuals shed wt human parainfluenza virus type 1. ALT elevations were not observed in any subject. Antibody responses, as measured by >4-fold rises in serum HAI titer, were not detected in any of the recipients of the rHPIV3-NB vaccine. For the rB/HPIV3 vaccine, only the seropositive child with the relatively high level of vaccine virus shedding noted above developed an antibody response (Table 2). Taken together, these data suggest that these vaccines are poorly infectious and immunogenic in HPIV3-experienced populations.
3.3. Response of HPIV3 seronegative children
The rHPIV3-NB vaccine infected only 71% (10/14) of seronegative children at a dose level of 104.0 TCID50. As specified in the protocol (10-fold increase in dose if the vaccine was well-tolerated and infectivity <90%), a 105.0 TCID50 dose level was next evaluated in an additional group of seronegative children, and 6 of the 7 children (86%) who received this higher dose were infected. Based on these findings, the rB/HPIV3 virus was administered at an initial dose of 105.0 TCID50, which infected 100% (14 of 14) of the seronegative children (Table 1, Table 2).
When vaccine virus replication was compared between groups of seronegative children who received a 105.0 TCID50 of either vaccine, the mean peak titer of vaccine virus in nasal wash fluid was nearly 10-fold greater in rHPIV3-NB vaccinees than in rB/HPIV3 vaccinees (103.7 vs. 102.8 pfu/mL), but this difference was not statistically significant. However, the mean duration of vaccine virus shedding was significantly greater in seronegative recipients of rHPIV3-NB than in seronegative recipients of rB/HPIV3 (14.7 vs. 8.2 days, P = .009; Table 1 and Fig. 2 ).
Fig. 2.
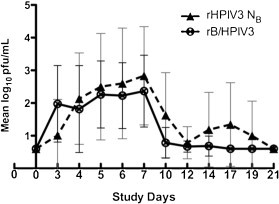
Quantitation of vaccine virus shedding in nasal washes from HPIV3 seronegative recipients of 105.0 TCID50 of either rHPIV3-NB or rB/HPIV3. Means and standard deviations of log10 titers are shown for those who shed vaccine virus. 0.6 log10 pfu/mL represents the limit of detection of the assay.
Upper respiratory tract and febrile illnesses were frequently observed in both vaccines and placebo recipients, but the rates of these illnesses were similar between groups (Table 1). One seronegative recipient of rB/HPIV3 met the protocol definition of LRI: rhonchi were heard on auscultation on study days 21 through 23. This child was afebrile and did not show other signs or symptoms of LRI. This LRI was the only vaccination-related SAE reported. While this child did shed vaccine virus on study days 3 through 7, vaccine virus was not detected in nasal wash specimens on subsequent days and in particular not at the time of the LRI (day 21); however, human metapneumovirus, bocavirus and coronavirus were detected in nasal washes obtained at that time, suggesting that a community-acquired infection was the most likely cause of the LRI (Table 1).
HPIV3 was detected in nasal washes obtained from two placebo recipients in our rB/HPIV3 trial: virus was isolated from nasal wash specimens obtained from one placebo recipient on study days 4 and 5, and from the second placebo recipient on study days 27 and 28. Sequencing confirmed that these were wt HPIV3 isolates.
A single dose of 105.0 TCID50 of either vaccine induced serum antibody responses in nearly all seronegative vaccinees: 86% of rHPIV3-NB recipients and 93% of rB/HPIV3 recipients had >4-fold rises in HAI antibody titer to HPIV3 following vaccination (Table 2). Of note, the mean reciprocal log2 serum HAI antibody titer in recipients of a single dose of 105.0 TCID50 was significantly higher in rB/HPIV3 vaccinees than in rHPIV3-NB vaccinees (5.6 vs. 4.3, P = .01, Table 2). Significant differences in pre-vaccination titers were not observed between the two groups.
4. Discussion
The use of antigenically related animal viruses for immunization against human pathogens has led to the eradication of smallpox and more recently, to the development of Rotateq®, a live-attenuated bovine-human reassortant rotavirus vaccine. We and others previously evaluated BPIV3 as a live-attenuated vaccine against HPIV3 and found that it was well-tolerated and highly infectious in HPIV3 seronegative children and infants as young as 2 months of age [10], [21]. In the majority of children over 6 months of age, experimental BPIV3 vaccines also elicited serum HAI antibody responses to the BPIV3 HN glycoprotein, the major viral protective antigen; however, very few of these children developed high titers of antibody to the HPIV3 HN. This result was consistent with cross-neutralization studies that demonstrated only 25% antigenic relatedness between BPIV3 and HPIV3 HN and F [28], [29], and suggested that inclusion of the HN and F glycoproteins from HPIV3 would be important for induction of antibodies against these protective antigens. In this study, we describe the evaluation of two experimental chimeric bovine-human PIV3 vaccines. Each of these vaccines contains the HN and F glycoproteins of HPIV3. rHPIV3-NB is a single gene substitution recombinant in which the only contribution from BPIV3 is the N gene and its encoded protein, whereas rB/HPIV3 contains all of the internal genes from BPIV3, and only the F and HN genes and encoded proteins from HPIV3.
rHPIV3-NB and rB/HPIV3 were previously evaluated in rhesus monkeys [20], [23], [25]. Replication of these chimeric viruses in non-human primates was comparable: each replicated in the upper respiratory tract to a level that was intermediate between the parent wt BPIV3 strain and wt HPIV3, and each was highly restricted in the lower respiratory tract, similar to BPIV3 [20], [23], [25]. Interestingly, however, replication of these two experimental vaccines differed in HPIV3 seronegative children. In our study, the mean peak titer of virus detected in nasal wash fluid was greater for rHPIV3-NB than for rB/HPIV3 (103.7 vs. 102.8 pfu/mL), and the mean duration of shedding was significantly greater for rHPIV3-NB than for rB/HPIV3 (14.7 vs. 8.2 days). At a dose of 105.0 TCID50, rHPIV3-NB was slightly less restricted in replication in HPIV3 seronegative children than the parental BPIV3 (mean peak titer 103.2 TCID50; mean duration shedding 10.7 days [21]). While replication of both rHPIV3-NB and rB/HPIV3 was restricted compared to what we have previously observed in children naturally infected with wt HPIV3 [11], this study underscores the need for careful stepwise evaluation in susceptible pediatric populations to uncover subtle differences that may not be apparent when these viruses are evaluated in non-human primates.
Despite being more restricted in replication, the rB/HPIV3 vaccine induced significantly higher titers of HAI antibody than the rHPIV3-NB vaccine (5.6 vs. 4.3 reciprocal mean log2 titer). The reasons for this apparent difference are unclear. One potential explanation for this observation might be that the BPIV3-specific accessory P gene products encoded by the rB/HPIV3 vaccine are less able to inhibit human interferon induction and signaling than are the HPIV3-specific P gene products encoded by the rHPIV3-NB vaccine. Greater interferon production and signaling in response to the rB/HPIV3 vaccine might have adjuvant effects resulting in a stronger antibody response, as has been noted in bovines infected with bovine RSV mutants that differed in their ability to control the host interferon response [30]. In any case, the combination of greater restriction in replication with increased antibody response makes the rB/HPIV3 vaccine a more promising candidate than the rHPIV3-NB vaccine. The titer of antibody achieved in HPIV3 seronegative children after a single dose of rB/HPIV3 vaccine was only slightly lower than that measured in seropositive children who had presumably experienced prior infection with wt HPIV3 (Table 2), and, since more than one dose of this vaccine would likely be administered [31], it is possible than antibody titers comparable to infection with wt HPIV3 might be achieved following completion of a vaccination series with rB/HPIV3.
The tolerability of these vaccines was difficult to assess completely in these small phase I clinical trials. Importantly, LRI and/or other SAEs associated with vaccine virus shedding were not observed. Fever and upper respiratory illnesses were frequently detected in both vaccinees and placebo recipients, and a variety of adventitious viral agents were isolated from several subjects. These types of illnesses often occur in infants and preschoolers and can confound the assessment of reactogenicity of a live-attenuated respiratory virus vaccine. In particular, assessing the causal relationship between vaccine administration and a mild illness such as rhinorrhea will require studies in larger numbers of children: for example, the association between the live-attenuated influenza vaccine FluMist and mild upper respiratory symptoms was not apparent until large-scale studies were completed [32]. If the rB/HPIV3 vaccine undergoes further clinical development, additional information regarding the safety and reactogenicity of this vaccine will need to be obtained.
In summary, we have shown that two chimeric bovine-human PIV3 viruses are restricted in replication in HPIV3 seronegative children. Of the two vaccines, rB/HPIV3 appears to be both more restricted in replication and more immunogenic, making it the preferred candidate for further clinical development. Currently, a live-attenuated HPIV3 vaccine (cp45 and the recombinant version, rcp45) has been shown to be highly attenuated, infectious and immunogenic in phase I and II clinical trials in HPIV3-naïve infants and children [11], [33], [34]. rB/HPIV3 represents a potential additional live-attenuated investigational HPIV3 vaccine that could be evaluated in phase II/III studies. Alternatively, rB/HPIV3 could be used to express the surface glycoproteins of related paramyxoviruses that are important pediatric pathogens, such as RSV or human metapneumovirus [35]. Evaluation of a similar chimeric B/HPIV3 vaccine virus expressing the RSV F protein from an added gene is currently in progress [36].
Acknowledgements
This study was supported by the Intramural Research Program of the National Institutes of Allergy and Infectious Diseases (NIAID), National Institutes of Health. Clinical trials were conducted as part of contracts between NIAID and the Johns Hopkins Bloomberg School of Public Health (N01-A1-15444 and HHSN272200900010C).
We thank Roberta Casey, Barbara Burns, Karen Loehr, Milena Gatto, Racine Harris, Amy Hoffman, Susan DiLorenzo and Nicole Yoder for expert clinical and technical assistance and Romeo Paredes and Jason Morsell for data management and administrative assistance. We would also like to thank the Regulatory Compliance and Human Subjects Protection Program within the NIAID Division of Clinical Research and the NIAID Data Safety Monitoring Board for their support. We are grateful to the physicians and staff of Primary Care Pediatrics, Dundalk Pediatrics, Bright Oaks Pediatrics, The Pediatric Group, and the Harriet Lane Clinic and to the families who participated in this study. We are grateful to Mario Skiadopoulos for his contributions to the preclinical development of these investigational vaccines.
References
- 1.Chanock R.M., Parrott R.H. Acute respiratory disease in infancy and childhood: present understanding and prospects for prevention. Pediatrics. 1965;36:21–39. [PubMed] [Google Scholar]
- 2.Glezen W.P., Denny F.W. Epidemiology of acute lower respiratory disease in children. N Engl J Med. 1973;288:498–505. doi: 10.1056/NEJM197303082881005. [DOI] [PubMed] [Google Scholar]
- 3.Counihan M.E., Shay D.K., Holman R.C., Lowther S.A., Anderson L.J. Human parainfluenza virus-associated hospitalizations among children less than five years of age in the United States. Pediatr Infect Dis J. 2001;20(7):646–653. doi: 10.1097/00006454-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Weinberg G.A., Hall C.B., Iwane M.K., Poehling K.A., Edwards K.M., Griffin M.R. Society for Pediatric Research; San Francisco, CA: 2004. Parainfluenza virus infection of young children: burden of hospitalization and seasonal patterns of infection. p. 1824. [Google Scholar]
- 5.Weinberg G.A. Parainfluenza viruses: an underappreciated cause of pediatric respiratory morbidity. Pediatr Infect Dis J. 2006;25(5):447–448. doi: 10.1097/01.inf.0000218037.83110.c4. [DOI] [PubMed] [Google Scholar]
- 6.Weinberg G.A., Hall C.B., Iwane M.K., Poehling K.A., Edwards K.M., Griffin M.R. Parainfluenza virus infection of young children: estimates of the population-based burden of hospitalization. J Pediatr. 2009;154(5):694–699. doi: 10.1016/j.jpeds.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 7.Glezen W.P., Frank A.L., Taber L.H., Kasel J.A. Parainfluenza virus type 3: seasonality and risk of infection and reinfection in young children. J Infect Dis. 1984;150(6):851–857. doi: 10.1093/infdis/150.6.851. [DOI] [PubMed] [Google Scholar]
- 8.Karron R.A., O’Brien K.L., Froehlich J.L., Brown V.A. Molecular epidemiology of a parainfluenza type 3 virus outbreak on a pediatric ward. J Infect Dis. 1993;167(6):1441–1445. doi: 10.1093/infdis/167.6.1441. [DOI] [PubMed] [Google Scholar]
- 9.Reed G., Jewett P.H., Thompson J., Tollefson S., Wright P.F. Epidemiology and clinical impact of parainfluenza virus infections in otherwise healthy infants and young children <5 years old. J Infect Dis. 1997;175(4):807–813. doi: 10.1086/513975. [DOI] [PubMed] [Google Scholar]
- 10.Karron R.A., Makhene M., Gay K., Wilson M.H., Clements M.L., Murphy B.R. Evaluation of a live attenuated bovine parainfluenza type 3 vaccine in 2-to-6-month-old infants. Pediatr Infect Dis J. 1996;15:650–654. doi: 10.1097/00006454-199608000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Karron R.A., Belshe R.B., Wright P.F., Thumar B., Burns B., Newman F. A live human parainfluenza type 3 virus vaccine is attenuated and immunogenic in young infants. Pediatr Infect Dis J. 2003;22(5):394–405. doi: 10.1097/01.inf.0000066244.31769.83. [DOI] [PubMed] [Google Scholar]
- 11a.Karron R.A., Casey R., Thumar B., Surman S., Murphy B.R., Collins P.L. The cDNA-derived investigational human parainfluenza virus type 3 vaccine rcp45 is well-tolerated, infectious, and immunogenic in infants and young children. Pediatr Infect Dis J. 2011;30(10):e186–e189. doi: 10.1097/INF.0b013e31822ea24f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith C.B., Purcell R.H., Bellanti J.A., Chanock R.M. Protective effect of antibody to parainfluenza type 1 virus. N Engl J Med. 1966;275(21):1145–1152. doi: 10.1056/NEJM196611242752101. [DOI] [PubMed] [Google Scholar]
- 13.Tremonti L.P., Lin J.L., Jackson G.G. Neutralizing activity in nasal secretions and serum in resistance of volunteers to parainfluenza virus type 2. J Immunol. 1968;101:572–577. [PubMed] [Google Scholar]
- 14.Clements M.L., Makhene M.K., Karron R.A., Murphy B.R., Steinhoff M.C., Subbarao K. Effective immunization with live attenuated influenza A virus can be achieved in early infancy. J Infect Dis. 1996;173:44–51. doi: 10.1093/infdis/173.1.44. [DOI] [PubMed] [Google Scholar]
- 15.Wright P.F., Karron R.A., Belshe R.B., Thompson J., Crowe J.E., Jr., Boyce T.G. Evaluation of a live cold-passaged, temperature-sensitive, respiratory syncytial virus vaccine candidate in infancy. J Infect Dis. 2000;182(5):1331–1342. doi: 10.1086/315859. [DOI] [PubMed] [Google Scholar]
- 16.Homa F.L., Brideau R.J., Lehman D.J., Thomsen D.R., Olmsted R.A., Wathen M.W. Development of a novel subunit vaccine that protects cotton rats against both human respiratory syncytial virus and human parainfluenza virus type 3. J Gen Virol. 1993;74:1995–1999. doi: 10.1099/0022-1317-74-9-1995. [DOI] [PubMed] [Google Scholar]
- 17.Spriggs M.K., Murphy B.R., Prince G.A., Olmsted R.A., Collins P.L. Expression of the F and HN glycoproteins of human parainfluenza virus type 3 by recombinant vaccinia viruses: contributions of the individual proteins to host immunity. J Virol. 1987;61:3416–3423. doi: 10.1128/jvi.61.11.3416-3423.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ray R., Glaze B.J., Moldoveanu Z., Compans R.W. Intranasal immunization of hamsters with envelope glycoproteins of human parainfluenza virus type 3. J Infect Dis. 1988;157(4):648–654. doi: 10.1093/infdis/157.4.648. [DOI] [PubMed] [Google Scholar]
- 19.Ambrose M.W., Wyde P.R., Ewasyshyn M., Bonneau A.M., Caplan B., Meyer H.L. Evaluation of the immunogenicity and protective efficacy of a candidate parainfluenza virus type 3 subunit vaccine in cotton rats. Vaccine. 1991;9(7):505–511. doi: 10.1016/0264-410x(91)90037-7. [DOI] [PubMed] [Google Scholar]
- 20.Skiadopoulos M.H., Schmidt A.C., Riggs J.M., Surman S.R., Elkins W.R., St Claire M. Determinants of the host range restriction of replication of bovine parainfluenza virus type 3 in rhesus monkeys are polygenic. J Virol. 2003;77(2):1141–1148. doi: 10.1128/JVI.77.2.1141-1148.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karron R.A., Wright P.F., Hall S.L., Makhene M., Thompson J., Burns B.A. A live attenuated bovine parainfluenza type 3 virus vaccine is safe, infectious, immunogenic, and phenotypically stable in infants and children. J Infect Dis. 1995;171:1107–1114. doi: 10.1093/infdis/171.5.1107. [DOI] [PubMed] [Google Scholar]
- 22.Sato M., Wright P.F. Current status of vaccines for parainfluenza virus infections. Pediatr Infect Dis J. 2008;27(10 Suppl.):S123–S125. doi: 10.1097/INF.0b013e318168b76f. [DOI] [PubMed] [Google Scholar]
- 23.Bailly J.E., McAuliffe J.M., Durbin A.P., Elkins W.R., Collins P.L., Murphy B.R. A recombinant human parainfluenza virus type 3 (PIV3) in which the nucleocapsid N protein has been replaced by that of bovine PIV3 is attenuated in primates. J Virol. 2000;74(7):3188–3195. doi: 10.1128/jvi.74.7.3188-3195.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bailly J.E., McAuliffe J.M., Skiadopoulos M.H., Collins P.L., Murphy B.R. Sequence determination and molecular analysis of two strains of bovine parainfluenza virus type 3 that are attenuated for primates. Virus Genes. 2000;20(2):173–182. doi: 10.1023/a:1008130917204. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt A.C., McAuliffe J.M., Huang A., Surman S.R., Bailly J.E., Elkins W.R. Bovine parainfluenza virus type 3 (BPIV3) fusion and hemagglutinin-neuraminidase glycoproteins make an important contribution to the restricted replication of BPIV3 in primates. J Virol. 2000;74(19):8922–8929. doi: 10.1128/jvi.74.19.8922-8929.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Surman S.R., Collins P.L., Murphy B.R., Skiadopoulos M.H. An improved method for the recovery of recombinant paramyxovirus vaccine candidates suitable for use in human clinical trials. J Virol Methods. 2007;141(1):30–33. doi: 10.1016/j.jviromet.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 27.Clements M.L., Belshe R.B., King J., Newman F., Westblom T.U., Tierney E.L. Evaluation of bovine, cold-adapted human, and wild-type human parainfluenza type 3 viruses in adult volunteers and in chimpanzees. J Clin Microbiol. 1991;29:1175–1182. doi: 10.1128/jcm.29.6.1175-1182.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coelingh K.L.V., Winter C.C., Murphy B.R. Conserved epitopes of the hemagglutinin-neuraminidase proteins of human and bovine parainfluenza type 3 viruses: nucleotide sequence analysis of variants selected with monoclonal antibodies. J Virol. 1986;60:90–96. doi: 10.1128/jvi.60.1.90-96.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Wyke Coelingh K.L., Winter C.C., Tierney E.L., Hall S.L., London W.T., Kim H.W. Antibody responses of humans and nonhuman primates to individual antigenic sites of the hemagglutinin-neuraminidase and fusion glycoproteins after primary infection or reinfection with parainfluenza type 3 virus. J Virol. 1990;64(8):3833–3843. doi: 10.1128/jvi.64.8.3833-3843.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valarcher J.F., Furze J., Wyld S., Cook R., Conzelmann K.K., Taylor G. Role of alpha/beta interferons in the attenuation and immunogenicity of recombinant bovine respiratory syncytial viruses lacking NS proteins. J Virol. 2003;77(15):8426–8439. doi: 10.1128/JVI.77.15.8426-8439.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy B.R., Collins P.L. Live-attenuated virus vaccines for respiratory syncytial and parainfluenza viruses: applications of reverse genetics. J Clin Invest. 2002;110(1):21–27. doi: 10.1172/JCI16077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ambrose C.S., Yi T., Falloon J. An integrated, multistudy analysis of the safety of Ann Arbor strain live attenuated influenza vaccine in children aged 2–17 years. Influenza Other Respir Viruses. 2011 doi: 10.1111/j.1750-2659.2011.00243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karron R.A., Wright P.F., Newman F.K., Makhene M., Thompson J., Samorodin R. A live human parainfluenza type 3 virus vaccine is attenuated and immunogenic in healthy infants and children. J Infect Dis. 1995;172:1445–1450. doi: 10.1093/infdis/172.6.1445. [DOI] [PubMed] [Google Scholar]
- 34.Belshe R.B., Newman F.K., Tsai T.F., Karron R.A., Reisinger K., Roberton D. Phase 2 evaluation of parainfluenza type 3 cold passage mutant 45 live attenuated vaccine in healthy children 6–18 months old. J Infect Dis. 2004;189(3):462–470. doi: 10.1086/381184. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt A.C., Wenzke D.R., McAuliffe J.M., St Claire M., Elkins W.R., Murphy B.R. Mucosal immunization of rhesus monkeys against respiratory syncytial virus subgroups A and B and human parainfluenza virus type 3 by using a live cDNA-derived vaccine based on a host range-attenuated bovine parainfluenza virus type 3 vector backbone. J Virol. 2002;76(3):1089–1099. doi: 10.1128/JVI.76.3.1089-1099.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gomez M., Mufson M.A., Dubovsky F., Knightly C., Zeng W., Losonsky G. Phase-I study MEDI-534: of a live, attenuated intranasal vaccine against respiratory syncytial virus and parainfluenza-3 virus in seropositive children. Pediatr Infect Dis J. 2009;28(7):655–658. doi: 10.1097/INF.0b013e318199c3b1. [DOI] [PubMed] [Google Scholar]


