Abstract
A facile method for imparting hydrolytic degradability to poly(ethylene oxide) (PEO), compatible with current PEGylation strategies, is presented. By incorporating methylene ethylene oxide (MEO) units into the parent PEO backbone, complete degradation was defined by the molar incorporation of MEO, and the structure of the degradation byproducts was consistent with an acid-catalyzed vinyl-ether hydrolysis mechanism. The hydrolytic degradation of poly[(ethylene oxide)-co-(methylene ethylene oxide)] was pH-sensitive, with degradation at pH 5 being significantly faster than at pH 7.4 at 37 °C in PBS buffer while long-term stability could be obtained in either the solid-state or at pH 7.4 at 6 °C.
The non-degradability of poly(ethylene oxide) (PEO), commonly known as poly(ethylene glycol) (PEG), is a central limitation for its use in therapeutic applications.1 In this letter, we describe a simple strategy for incorporating hydrolytic degradability in PEO that is compatible with currently used PEGylation strategies. This approach is also well-suited for the synthesis of heterobifunctional PEG materials, e.g., α-azido-ω-hydroxy-PEO,2 and copolymerization routes toward multifunctional and branched PEGs.3,4
PEO provides an excellent biocompatible platform for a variety of applications.5 While PEO is a common component in cosmetics and an excipient in pharmaceutical formulations, PEO is finding increasing use in specialty biomedical applications, such as drug and peptide delivery.6 In fact, PEO is one of the most utilized synthetic materials in polymer therapeutics with a number of products already on the market or in clinical trials.7 The circulation half-life of PEO in vivo is a nonlinear function of molecular weight, with a precipitous increase in circulation half-life occurring above ca. 30 kg/mol.8 Due to slow clearance from the blood at molecular weights greater than 30 kg/mol, and a lack of biodegradablility in PEO,9 bioaccumulation is a concern.1,5 For this reason, PEO of lower molecular weight (< 30 kg/mol) is typically employed in therapeutic applications.
In order to allay concerns over bioaccumulation and to enable the use of higher molecular weight PEOs to achieve better steric shielding of PEGylated compounds and greater control over circulation half-life, several methods of including degradability in linear PEOs have been introduced.6,10–17 In addition, water-soluble PEO-replacements that combine biocompatibility with degradability have also been investigated.7,18–20
The most common method of incorporating degradability into PEO is via a step-growth polymerization of telechelic PEG-macromonomers.8,10,12,14–17,21–23 Esters,9,15,16,22,23 amides,12 and urethanes14 have all been utilized as hydrolytically degradable backbone moieties. A potentially versatile post-polymerization modification of PEO introducing hydrolytically-degradable hemiacetals along the PEO backbone was reported by Reid et al.24 However, PEO degradation occurred during hemiacetal formation at low molar incorporations of ca. 3%, limiting degradability and chain-end fidelity of these PEO-derivatives. Other degradation-stimuli have also been investigated with Lee et al. utilizing disulfide linkages that cleave under reductive conditions, such as those found within a cell.10 Degradable PEO-replacements prepared by step-growth synthesis have also been developed with Guan reporting methoxylated carbohydrate-derived polyester and polyamide derivatives.18,19 For polymer-based therapeutics, the breadth of the molecular weight distribution that results from a step-growth synthesis is a regulatory concern for in vivo applications. To address this issue, Guan developed a controlled chain-growth synthesis of these protein-resistant degradable carbohydrate-derived polyesters that leads to controlled molecular weights and low polydispersities (PDIs) of ca. 1.1.20
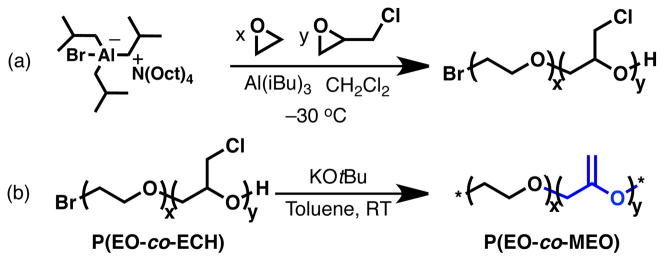
In designing the next generation of degradable PEO platforms, a number of requirements must be addressed. Control of molecular weight both before, and after degradation, retention of chain-end functionality for efficient PEGylation to high-value therapeutic and diagnostic agents, and the ability to store the degradable PEO before administration are all important characteristics of an ideal therapeutic degradable PEO platform. Most importantly, degradation must also occur under physiological conditions (pH 5.0–7.4). Toward this ultimate goal, we report a simple and effective strategy to render PEO hydrolytically-degradable through a combination of the chain-growth copolymerization of ethylene oxide and epichlorohydrin (ECH) to give a defined, linear copolymer followed by an efficient elimination reaction to generate degradable methylene ethylene oxide (MEO) repeat units within a PEO backbone (Scheme 1). Using this strategy, the molecular weight can be defined by the polymerization stoichiometry, and the number of degradable sites can be adjusted by the comonomer feed-ratio, which defines the average molecular weight after degradation. Precise definition of the post-degradation molecular weight is vital in order to avoid toxicity associated with PEO molecular weights lower than ca. 400 g/mol.25 With control over starting and final molecular weight, a greater degree of control over circulation half-life of PEGylated therapeutics can potentially be achieved.
Scheme 1.
Synthesis of poly[(ethylene oxide)-co-(methylene ethylene oxide)]-P(EO-co-MEO).
Scheme 1 depicts the synthesis of poly[(ethylene oxide)-co-(methylene ethylene oxide)] from a copolyether precursor poly[(ethylene oxide)-co-(epichlorohydrin)] (P(EO-co-ECH)), which was prepared by the trialkylaluminum-activated copolymerization of ethylene oxide (EO) and epichlorohydrin (ECH) using trioctylammonium bromide as initiator (Scheme 1a).2,26–31 To demonstrate the versatility of this strategy, a range of P(EO-co-ECH) copolymers were synthesized with 0.0–10.0 mol% ECH in the comonomer feed, and overall number-averaged molecular weights (Mn) of ca. 10 kg/mol. Copolymerizations were carried out in methylene chloride and initiated at –30 °C under an argon atmosphere. Significantly, the incorporation of ECH repeat units closely matched the feed ratio with polydispersities for the random copolymers being between 1.2 and 1.4.
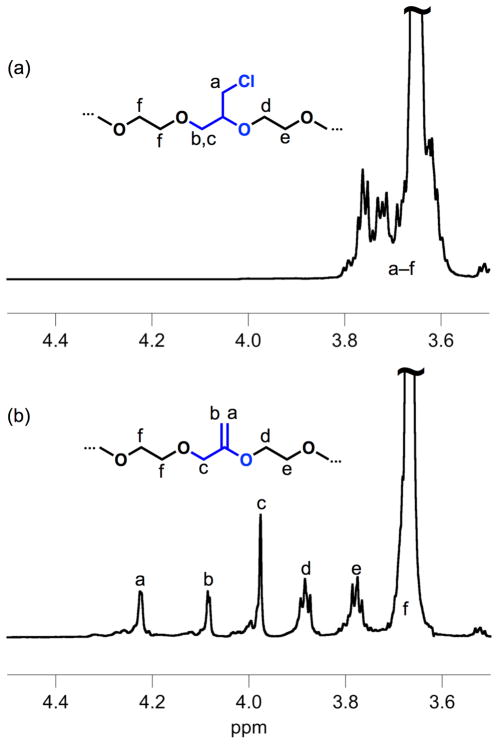
While elimination can be performed on numerous functionalities, the backbone chlorides provided by epichlorohydrin repeat-units provide the most direct substrate for MEO repeat-unit synthesis (Scheme 1b). The P(EO-co-ECH) copolymers were therefore treated with potassium tert-butoxide in toluene and the heterogeneous mixture stirred for two days at room temperature.32 The transformation of P(EO-co-ECH) to the unsaturated P(EO-co-MEO) can be observed by 1H NMR spectroscopy as shown in Figure 1. While the 1H NMR spectrum of P(EO-co-ECH) (Figure 1a) presents many overlapping resonances at 3.6–3.8 ppm for the ECH and neighboring EO units, it is noteworthy that elimination of ECH to MEO repeat-units results in a dramatic change in the chemical shift of resonances associated with the presence of a vinyl ether (Figure 1b). For these materials, all peaks could be unambiguously assigned to protons within, and adjacent to MEO repeat units.
Figure 1.
1H NMR spectra of P(EO-co-ECH) with 9 mol% ECH (a), and the resultant P(EO-co-MEO) after elimination (b).
Having developed a viable, large scale synthesis of the statistical copolymer, the degradability of P(EO-co-MEO) was initially established by treatment with a 1% aqueous solution of tri-fluoroacetic acid (TFA) for 24 h at room temperature followed by neutralization, extraction, and drying. To determine the molecular weight of the degradation byproducts, gel permeation chromatography (GPC) calibrated with PEO standards was used. Representative GPC traces of P(EO91.0%-co-MEO9.0%) (sample 4, Table 1) before and after degradation are shown in supporting information (Figure S1) with the molecular weights relative to PEO standards before, and after TFA treatment listed in Table 1. As expected, copolymers containing increased incorporation of MEO repeat-units degraded to lower molecular weight PEO derivatives. Polydispersities increased for all samples, but did not exceed 2.0; the expected value for degraded high molecular weight copolymers with randomly interspersed degradable units.
Table 1.
Characterization of P(EO-co-ECH) precursors and P(EO-co-MEO) copolymers.
| P(EO-co-MEO) | Degraded | Feed %ECH(b) | Final %MEO(c) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Mn (a) (kg/mol) | PDI(a) | Mn (a) (kg/mol) | PDI(a) | |||
| 1 | 10.5 | 1.25 | 7.03 | 1.57 | 1.0 | 0.7 |
| 2 | 9.20 | 1.33 | 3.60 | 1.93 | 2.0 | 1.1 |
| 3 | 10.0 | 1.26 | 2.13 | 1.74 | 5.0 | 4.5 |
| 4 | 9.00 | 1.34 | 0.85 | 1.61 | 10.0 | 9.0 |
Molecular weights and polydispersities measured by GPC using PEO standards.
Mole percent of epichlorohydrin (ECH) in polymerization feed ratio.
Mole percent of methylene ethylene oxide (MEO) units as measured by 1H NMR spectroscopy.
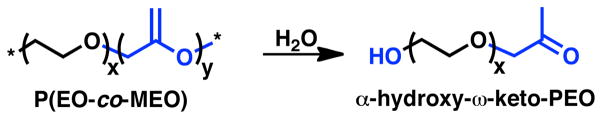
Hydrolytic degradation of P(EO-co-MEO) is expected to occur through the acid-catalyzed hydrolysis of vinyl-ether groups within the MEO repeat-units and result in release of non-acidic α-hydroxy-ω-keto-PEO oligomers into solution as shown in Scheme 2.33 The detailed structure of the degradation byproducts was investigated using electrospray ionization mass spectrometry (ESI-MS) of P(EO98.9%-co-MEO1.1%) (Table 1, entry 2) that had been treated with 1M HCl, neutralized, extracted into methylene chloride and dried. A section of the mass spectrogram (300–800 amu) of the degradation byproducts is shown in supporting information (Figure S2). Two series of products are present as a result of hydrolytic degradation. The predominant product of degradation is the expected α-hydroxy-ω-keto-PEO that resulted from the classical vinyl-ether hydrolysis mechanism.33 A minor component of degradation was dihydroxy-terminal PEO attributable to the end-group segments that are naturally present in the P(EO-co-MEO) starting copolymer. The well-defined nature of this degradation process further illustrates the potential of this strategy.
Scheme 2.
Hydrolytic degradation of P(EO-co-MEO) results in α-hydroxy-ω-keto-PEO byproducts.
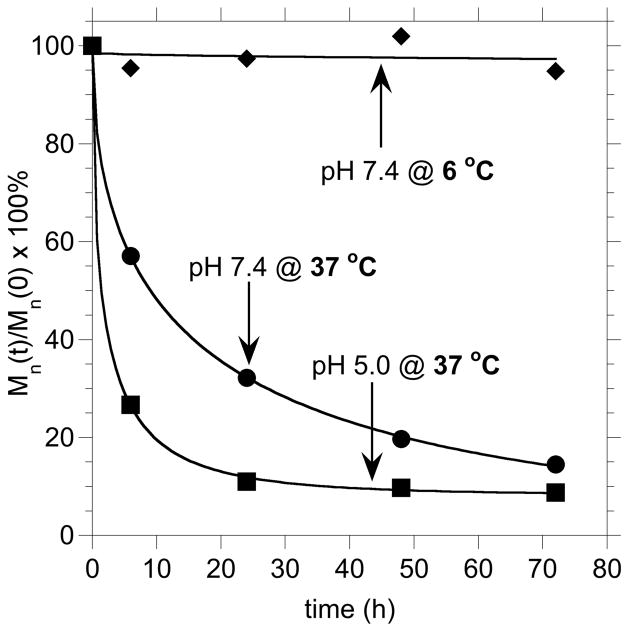
In order to further investigate the acid-catalyzed degradation behavior of these materials and examine their potential for biomedical applications,34,35 we compared the rate of hydrolysis in two buffer solutions at pH 5.0 and 7.4 at 37 °C to simulate in vivo degradation conditions. The relative number-average molecular weights relative to PEO standards as a function of degradation time (Mn(t)/Mn(0)×100%) are shown in Figure 2. From an application viewpoint it was critical that the P(EO91%-co-MEO9%) (Table 1, entry 4) was observed to degrade at a faster rate at pH 5.0 than at pH 7.4 at 37 °C with complete hydrolysis occurring within 6–24 hours at pH 5.0, whereas at pH 7.4, degradation was not complete after 72 hours of hydrolysis. In order to investigate storage conditions, samples were held as dry powders at –20 °C, and in pH 7.4 buffer solution at 6 °C. Significantly, no detectable degradation occurred over the course of three months when stored as a dry powder, and no appreciable degradation occurred within 72 hours in pH 7.4 buffer at 6 °C (Figure 2). These studies clearly demonstrate the long-term stability of these materials and suggests that the introduction of hydrolytic degradability to PEG would enable the use of higher molecular weight derivatives leading to improved biodistribution. The increased rate of P(EO-co-MEO) degradation at lower pH also allows passive targeting for PEGylated therapeutic agents and associated release near sites of inflammation or disease.
Figure 2.
Relative molecular weight measured by SEC with respect to PEO standards as a function of time (Mn(t)/Mn(0)×100%) in pH 7.4, and pH 5.0 buffers at 37 °C and at 6 °C in pH 7.4 buffer for the degradation of P(EO-co-MEO) copolymers.
To address the non-degradability of PEO, we present a versatile synthetic strategy for the next generation of degradable PEO platforms. Through post-polymerization modification, methylene ethylene oxide, MEO repeat units, are introduced to the PEO backbone and provide for controlled and tunable degradation of the resulting copolymers. Introducing hydrolytic degradability in PEO has implications beyond materials for healthcare, and provides new robust synthetic routes to advanced materials for nanolithography, and in the fabrication of advanced porous and functional membrane materials.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health as a Program of Excellence in Nanotechnology (HHSN268201000046C) (EDP, CJH, and NAL), by the MRSEC Program of the National Science Foundation under Award No. DMR 1121053 (CJH, SAB, and AL), and through a Samsung fellowship (BFL). PL would like to acknowledge the Wenner-Gren foundations and the Bengt Lundqvist memorial foundation for financial support.
Footnotes
Supporting Information. Additional characterization and experimental procedures are available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Ulery BD, Nair LS, Laurencin CT. J Polym Sci B Polym Phys. 2011;49:832–864. doi: 10.1002/polb.22259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gervais M, Labbé A, Carlotti S, Deffieux A. Macromolecules. 2009;42:2395–2400. [Google Scholar]
- 3.Obermeier B, Wurm F, Mangold C, Frey H. Angew Chem Int Ed. 2011;50:7988–7997. doi: 10.1002/anie.201100027. [DOI] [PubMed] [Google Scholar]
- 4.Mangold C, Wurm F, Frey H. Polym Chem. 2012;3:1714–1721. [Google Scholar]
- 5.Webster R, Didier E, Harris P, Siegel N, Stadler J, Tilbury L, Smith D. Drug Metab Dispos. 2006;35:9–16. doi: 10.1124/dmd.106.012419. [DOI] [PubMed] [Google Scholar]
- 6.Alconcel SNS, Baas AS, Maynard HD. Polym Chem. 2011;2:1442–1448. [Google Scholar]
- 7.Duncan R. Nat Rev Drug Discov. 2003;2:347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 8.Caliceti P, Veronese FM. Adv Drug Deliv Rev. 2003;55:1261–1277. doi: 10.1016/s0169-409x(03)00108-x. [DOI] [PubMed] [Google Scholar]
- 9.Knop K, Hoogenboom R, Fischer D, Schubert US. Angew Chem Int Ed. 2010;49:6288–6308. doi: 10.1002/anie.200902672. [DOI] [PubMed] [Google Scholar]
- 10.Lee Y, Koo H, Jin GW, Mo H, Cho MY, Park JY, Choi JS, Park JS. Biomacromolecules. 2005;6:24–26. doi: 10.1021/bm049658l. [DOI] [PubMed] [Google Scholar]
- 11.Wang N, Dong A, Radosz M, Shen Y. J Biomed Mater Res. 2008;84A:148–157. doi: 10.1002/jbm.a.31466. [DOI] [PubMed] [Google Scholar]
- 12.Iha RK, van Horn BA, Wooley KL. J Polym Sci Part A: Polym Chem. 2010;48:3553–3563. [Google Scholar]
- 13.Knorr V, Ogris M, Wagner E. Pharm Res. 2008;25:2937–2945. doi: 10.1007/s11095-008-9700-6. [DOI] [PubMed] [Google Scholar]
- 14.Luo YL, Nan YF, Xu F, Chen YS, Zhao P. J Biomater Sci Polym Ed. 2010;21:1143–1172. doi: 10.1163/092050609X12459333183584. [DOI] [PubMed] [Google Scholar]
- 15.Tizzotti M, Labeau MP, Hamaide T, Drockenmuller E, Charlot A, Fleury E. J Polym Sci Part A: Polym Chem. 2010;48:2733–2742. [Google Scholar]
- 16.Harris JM. U.S. Patent 6,214,966. 2001 Apr 10;
- 17.Ulbrich K, Strohalm J, Kopecek J. Makromol Chem. 1986;187:1131–1144. [Google Scholar]
- 18.Metzke M, Bai JZ, Guan Z. J Am Chem Soc. 2003;125:7760–7761. doi: 10.1021/ja0349507. [DOI] [PubMed] [Google Scholar]
- 19.Metzke M, Guan Z. Biomacromolecules. 2008;9:208–215. doi: 10.1021/bm701013y. [DOI] [PubMed] [Google Scholar]
- 20.Urakami H, Guan Z. Biomacromolecules. 2008;9:592–597. doi: 10.1021/bm701180r. [DOI] [PubMed] [Google Scholar]
- 21.Aimetti AA, Machen AJ, Anseth KS. Biomaterials. 2009;30:6048–6054. doi: 10.1016/j.biomaterials.2009.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mero A, Schiavon O, Pasut G, Veronese FM, Emilitri E, Ferruti P. J Bioact Compat Pol. 2009;24:220–234. [Google Scholar]
- 23.Wang N, Dong A, Tang H, Van Kirk EA, Johnson PA, Murdoch WJ, Radosz M, Shen Y. Macromol Biosci. 2007;7:1187–1198. doi: 10.1002/mabi.200700065. [DOI] [PubMed] [Google Scholar]
- 24.Reid B, Tzeng S, Warren A, Kozielski K, Elisseeff J. Macromolecules. 2010;43:9588–9590. doi: 10.1021/ma1020648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Working PK, Newman MS, Johnson J, Cornacoff JB. Safety of Poly(ethylene glycol) and Poly(ethylene glycol) Derivatives. In: Harris JM, Zalipsky S, editors. Poly(ethylene glycol) Chemistry and Biological Applications; ACS Symposium Series 680; Washington DC: American Chemical Society; 1997. pp. 45–57. [Google Scholar]
- 26.Billouard C, Carlotti S, Desbois P, Deffieux A. Macromolecules. 2004;37:4038–4043. [Google Scholar]
- 27.Carlotti S, Billouard C, Gautriaud E, Desbois P, Deffieux A. Macromol Symp. 2005;226:61–68. [Google Scholar]
- 28.Rejsek V, Sauvanier D, Billouard C, Desbois P, Deffieux A, Carlotti S. Macromolecules. 2007;40:6510–6514. [Google Scholar]
- 29.Labbé A, Carlotti S, Billouard C, Desbois P, Deffieux A. Macromolecules. 2007;40:7842–7847. [Google Scholar]
- 30.Carlotti S, Labbé A, Rejsek V, Doutaz S, Gervais M, Deffieux A. Macromolecules. 2008;41:7058–7062. [Google Scholar]
- 31.Gervais M, Brocas AL, Cendejas G, Deffieux A, Carlotti S. Macromolecules. 2010;43:1778–1784. [Google Scholar]
- 32.Iizawa T, Nishikubo T, Ichikawa M, Sugawara Y, Okawara M. J Polym Sci Polym Chem Ed. 1985;23:1893–1906. [Google Scholar]
- 33.Jones DM, Wood NF. J Chem Soc. 1964:5400–5403. [Google Scholar]
- 34.Roos A, Boron WF. Physiol Rev. 1981;61:296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- 35.Tannock I, Rotin D. Cancer Res. 1989;49:4373–4384. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.