Abstract
Background and aim
We recently showed that patients with inflammatory bowel disease (IBD) report significantly more sleep disturbances. To determine whether disrupted sleep can affect the severity of inflammation and the course of IBD, we used an animal model of colonic inflammation to determine the effects of acute and chronic intermittent sleep deprivation on the severity of colonic inflammation and tissue damage in colitis and recovery from this damage.
Methods
Acute sleep deprivation (ASD) consisted of 24 h of forced locomotor activity in a mechanical wheel rotating at a constant speed. Chronic intermittent sleep deprivation (CISD) consisted of an acute sleep deprivation episode, followed by additional sleep deprivation periods in the wheel for 6 h every other day throughout the 10 day study period. To induce colitis, mice were given 2% dextran sodium sulfate (DSS) in their daily drinking water for 7 days. The development and severity of colitis were monitored by measuring weight loss and tissue myeloperoxidase (MPO) activity daily and colon histology scores 10 days after initiation of colitis.
Results
ASD or CISD did not cause colonic inflammation in vehicle-treated mice. Changes in daily body weight, tissue MPO levels and colon histopathology score were similar between mice that were sleep deprived and controls. Daily DSS ingestion caused colitis in mice. ASD worsened colonic inflammation: tissue MPO levels in ASD/DSS-treated mice were significantly higher than in DSS-treated mice that were not sleep deprived. However, the worsening of colonic inflammation by ASD was not enough to exacerbate clinical manifestations of colitis such as weight loss. In contrast, the deleterious effects of CISD were severe enough to cause worsening of histological and clinical manifestations of colitis. The deleterious effects of sleep deprivation on severity of colitis appeared to be due to both increased colonic inflammation and a decrease in the ability of mice to recover from DSS-induced colonic injury.
Conclusion
Both acute and chronic intermittent sleep deprivation exacerbate colonic inflammation. Thus, sleep deprivation could be an environmental trigger that predisposes IBD patients to develop flare ups and a more severe disease course. These results provide a scientific rationale to conduct an interventional trial to determine whether improvement in sleep patterns will prevent IBD flare ups, modify the disease course, and improve quality of life.
Keywords: Inflammatory bowel disease, Sleep deprivation, Dextran sodium sulfate, Colitis, Myeloperoxidase, Sleep disturbance
1. Introduction
Inflammatory bowel disease (IBD) includes two life-long, relapsing disorders – Crohn’s disease (CD) and ulcerative colitis (UC) – that wax and wane with symptomatic periods (flare ups) interspersed with asymptomatic periods (remissions). During a flare up, patients suffer from disabling and embarrassing symptoms such as bloody diarrhea, abdominal pain, urgency, fecal incontinence, arthritis and/or arthralgia, fatigue, and fever [1]. During this active phase, there is evidence of active inflammation in the gut with tissue injury manifested histologically by neutrophil infiltration, crypt abscess, crypt destruction and, as seen endoscopically, by mucosal ulceration and friability [2].
IBD is believed to be caused by a complex interaction between genetic and environment factors which trigger initial presentation and subsequent relapses. Thus, identifying and removing the environmental triggers of inflammation could modify the disease course and disease severity. Indeed, several studies have suggested that ‘‘pro-inflammatory” day to day events may trigger IBD flare ups. For example, recent studies suggest that IBD flare ups are associated with the life style practices of patients, including improper diet, alcohol consumption, and stress [1–6]. There is also circumstantial evidence that sleep disturbances may be an important trigger for IBD flare ups [7–9].
The notion that sleep disturbance could be the trigger or predisposing factor for IBD flare ups is primarily supported by indirect experimental findings and clinical observations. IBD patients frequently identify a significant stressor prior to disease flare up, and sleep loss/sleep disturbance is considered a form of physiological stress [10,11]. In addition, there is increasing evidence that sleep deprivation dysregulates the immune system, and immune dysregulation is known to be a key pathophysiologic factor in IBD [12–14]. However, the clinical observations that disrupted sleep precede IBD symptoms and do not necessarily indicate that sleep disturbances activate or worsen gut inflammation since symptoms such as diarrhea and abdominal pain could be due to changes in intestinal transit and visceral sensitivity rather than intestinal inflammation. For example, it is known that sleep disturbance disrupts normal GI physiology, such as transit and intestinal function, and can aggravate GI symptoms in non-inflammatory disorders of the intestine such as irritable bowel syndrome (IBS) [14, 15].
Thus, disrupted sleep may worsen symptoms in IBD patients by affecting intestinal function and/or by increasing gut inflammation. Indeed, we have recently made the intriguing observation that patients with inactive IBD report significantly more sleep disturbances, including prolonged sleep latency, more frequent sleep fragmentation, higher rates of sleeping pill use, decreased day-time energy, increased tiredness and overall poor sleep quality compared to healthy controls [7–9]. We also found that sleep quality was inversely correlated with an IBD-related complaint disease severity score [7–9].
While these clinical studies indicate associations between sleep disturbance and IBD symptoms, they do not establish that disrupted sleep actually causes or worsens tissue inflammation, leading to a more severe IBD disease course. To establish such causality, further studies under well-controlled experimental conditions are needed. Accordingly, to establish proof of the concept that sleep deprivation exacerbates gut inflammation, we designed our sleep deprivation study using an animal model of colitis. Through this approach we were able to quantitatively study the effects of sleep deprivation on the severity of colonic inflammation and tissue injury.
2. Materials and methods
2.1. Animal housing and experimental protocol
Eight-week-old C57/BJ6 male mice were obtained from The Jackson Laboratory (JAX®). Animals were housed individually and maintained on a constant light–dark 12:12 cycle with free access to food and water. Following 2 weeks of entrainment, mice were weighed, split into three groups (n = 12 per group) with equal average body weight, and used for experiments to determine the effects of sleep deprivation on colitis. The experimental protocols were approved by the Northwestern University Institute Animal Care and Use Committee.
2.2. Acute and chronic intermittent sleep disruption
The first group of animals was subjected to Acute Sleep Disruption (ASD), which consisted of a single 24 h sleep deprivation episode at the beginning of day 0. The second group was subjected to Chronic Intermittent Sleep Disruption (CISD), achieved by an acute 24 h episode at day 0, followed by 6 h of sleep deprivation every other day (days 2, 4, 6 and 8) during the last six hours of the light period (ZT 6–12). The last group remained in the home cage with No Sleep Disruption (NSD).
Both ASD and CISD were achieved by placing individual animals in rotating wheels. The wheels were made of stainless steel and were 13.5 in. in diameter and 3.75 in. in width. A constant speed was maintained at 1 round per minute by a motor [16]. Food and water were available to the animals throughout the sleep deprivation episodes as well as after their return to the home cage.
To determine whether forced movement rather than sleep disturbances was responsible for any effects, we induced sleep disruption in a selected group of mice [n = 5] by gentle handling of the animals for 6 h every other day during the 10-day course of the experiment.
2.3. Animal model of colitis
In order to determine whether sleep deprivation affects colitis, we used dextran sodium sulfate (DSS) to induce colitis in mice. DSS has been used repeatedly in modeling colitis in mice [17]. DSS disrupts the colonic mucosal barrier and leads to colonic inflammation, tissue damage, rectal bleeding and weight loss [17]. Colitis in these mice was induced by addition of DSS (2%) to their daily drinking water [17]. The effective DSS dosage was determined by daily measurements of the total water consumption of each animal. The severity of colitis was assessed using three sets of criteria: (1) clinical index (daily changes in body weight; diarrhea and rectal bleeding); (2) macroscopic evidence of colitis (shortening of colon length), and (3) microscopic evidence of colitis (tissue myeloperoxidase [MPO] activity as an index of acute inflammatory reaction with neutrophil infiltration into the colon; histopathological score as an index of tissue inflammation and injury). To investigate effects of sleep deprivation on tissue repair process, we chose an experimental paradigm that involved 7 days of treatment with DSS (induction), followed by a 3-day recovery period without DSS.
2.4. Experimental design
Each of the three groups of mice (NSD, ASD, CISD, n = 12 per group) were split into two subgroups, of which one (n = 4) served as an internal control, subjected to plain water (without DSS treatment), while the other one (n = 8) was challenged with DSS. On day 0 (Zeitgeber Time 0) of treatment, the animals’ water supply was changed to water containing 2% DSS for the DSS-treated NSD, ASD and CISD groups. The water supply was changed back to clean water on day 7.
Body weight and food or liquid consumption measurements were followed by a brief physical examination (stool consistency, overt blood or occult blood in stools) and were typically performed daily during the first two hours after the lights had been turned on.
On day 10 of the experiment, animals were sacrificed. The colon lengths were measured and colon tissues were harvested for measurement of myeloperoxidase (MPO) activity and histopathological assessment and scoring (ZT 24).
2.5. Assessment of diarrhea and rectal bleeding
Clinical assessment of disease activity was done using a validated scoring system previously reported [18]. Each animal was weighed daily to determine the percent change of weight during the DSS protocol. Fecal pellets were given a stool consistency score of 0 corresponding to a normal fecal pellet, 2 corresponding to a loose fecal pellet, or 4 corresponding to frank diarrhea. Occult blood was also scored using the Coloscreen occult blood card test and scored as 0 for negative and 2 for positive. Gross bleeding was determined as seen by wet blood on or around the anus (not dry blood in fecal material) and scored as 0 for negative and 4 for positive.
2.6. Tissue dissection and analysis
Colon tissue was removed from each animal after euthanasia and the colon length was measured from the end of the cecum to the anus. The colon was dived into three equal length pieces from distal to proximal end. Each section was then further separated into two pieces; one was used for MPO analysis while the other was kept in formaldehyde for tissue histopathology.
The colon tissues were fixed in 4% (wt/vol) paraformaldehyde in PBS and prepared for light microscopy. Tissue sections (7 µm) were stained with H&E to study histological changes as described previously [19, 20]. The determination of the histopathology score for intestinal inflammation of the colon was performed by a gastroenterological pathologist in a blinded manner using a validated scoring system as described previously [19, 20] with slight modifications (Table 1).
Table 1.
Histological criteria for the degree of gastrointestinal inflammation.
| Criteria | 0 | 1 (%) | 2 (%) | 3 (%) | 4 (%) |
|---|---|---|---|---|---|
| Goblet cell loss | 0 | 0–25 | 25–50 | 50–75 | 75–100 |
| Mucosal thickening | 0 | 0–25 | 25–50 | 50–75 | 75–100 |
| Inflammatory cells | 0 | 0–25 | 25–50 | 50–75 | 75–100 |
| Submucosal cell infiltration | 0 | 0–25 | 25–50 | 50–75 | 75–100 |
| Destruction architecture | 0 | 0–25 | 25–50 | 50–75 | 75–100 |
| Ulcers | 0 | 0–25 | 25–50 | 50–75 | 75–100 |
2.7. MPO measurement
Myeloperoxidase (MPO) is an enzyme stored in azurophilic granules of polymorphonuclear neutrophils and macrophages and released into the extracellular fluid during an inflammatory process. Thus, MPO activity serves as a sensitive marker for inflammation [20]. MPO activity was determined using a modified version of the method described by Bradley et al. [21]. Tissue samples were homogenized (50 mg/ml) in ice-cold 50 mM potassium phosphate buffer (pH 6.0) containing 0.5% hexadecyltrime-thylammonium bromide (Sigma, St. Louis, MO). Tissue samples were sonicated and then centrifuged at 12,000 rpm for 12 min at 4 °C. The supernatant was added to a solution of O-dianisidine (Sigma) and hydrogen peroxide. The absorbance of the colorimetric reaction was measured using a spectrophotometer. MPO is expressed in units of activity per milligram of tissue wet weight with 1 unit being the quantity of enzyme able to convert 1 µmol of hydrogen peroxide to water in 1 min at room temperature.
2.8. Statistical analysis
Changes in daily body weight were determined by group comparisons of total and relative daily body weight change. ANOVA was used to determine the significance of differences in body weight loss through the 10-day treatment period. Daily significances between groups were calculated using post hoc Tukey’s t-tests. Differences in colon lengths, MPO values, and histopathology scores were compared by t-tests between two groups; when more than two groups were compared, a one-way ANOVA followed by a post hoc Tukey’s t-test was used to evaluate the significance of differences. Significance was deemed to have been achieved if p values were less than 0.05.
3. Results
3.1. Effects of sleep deprivation on body weight, colon length and colon histology
Sleep deprivation episodes alone did not cause sustained body weight loss and did not induce colonic inflammation in vehicle-treated mice. Acute sleep deprivation caused a transient (day 0) decrease in food intake resulting in a transient and small drop in body weight in control mice. The drop in the body weight was not statistically significant. However, acute (ASD) or chronic (CISD) sleep deprivation did not affect the total food and water intake in mice who received water without DSS during the 10 days of the experiment period (days 1–10). Body weight averages in acute or chronic intermittent sleep deprived control mice were not different compared to non-sleep deprived mice throughout the 10 days. Acute and chronic intermittent sleep deprivation without DSS did not cause colonic inflammation. There was no difference in the colon length, tissue MPO levels, and histopathology scores between ASD or CISD and non-sleep deprived mice.
3.2. DSS-induced colitis
As expected, 2% DSS given daily in the drinking water caused moderately severe colitis in mice (Figs. 1–3). Mice receiving daily DSS in their drinking water began to lose weight after 3 days and continued to lose weight progressively throughout the 7 days of exposure to DSS, indicating the occurrence of colitis (NSD control vs. NSD + DSS; p = 0.0219, significance between groups reached on day 5–10). Mice stopped losing weight and began to gain weight during the 3 days of the recovery phase, indicating the reversal of colitis when DSS exposure ceased. Colitis induced by 2% DSS caused no deaths.
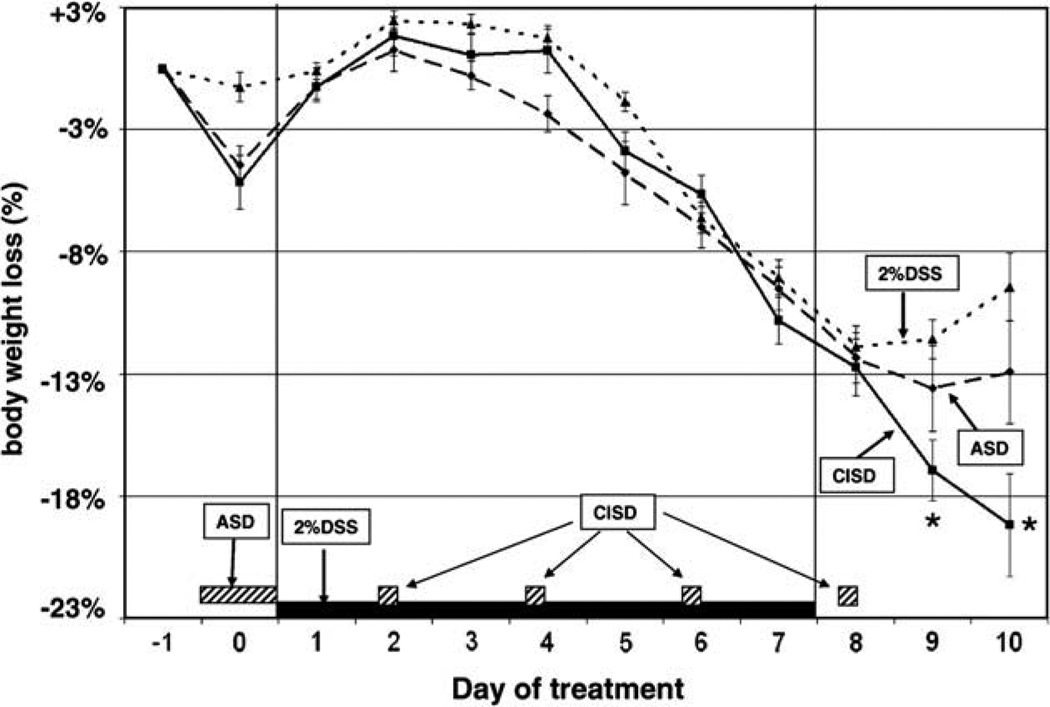
Fig. 1.
Effect of acute and chronic intermittent sleep deprivation on weight loss in 2% DDS-induced colitis mice. Weight loss relative to the animal’s base weight before induction of colitis was measured daily as a biomarker for colitis. Acute sleep deprivation (ASD) was achieved by forced wheel walking for 24 h prior to the administration of 2% DSS (as indicated by the dotted box). Chronic intermittent sleep deprivation (CISD) was a combination of an acute 24 h period of sleep deprivation followed by 6 h of forced activity in the wheel every other day after day 1 (as indicated by the dashed boxes on the x-axis). ASD or CISD increased weight loss caused by 2% DSS. Animals in the ASD & CISD groups received 7 days of 2% DSS treatment in their drinking water. There was a significant difference in weight loss in the CISD groups compared with controls (2% DSS alone) during the recovery period (days 9 and 10). Data are expressed as mean ± SEM. *p < 0.05 compared with the no sleep deprivation control group.
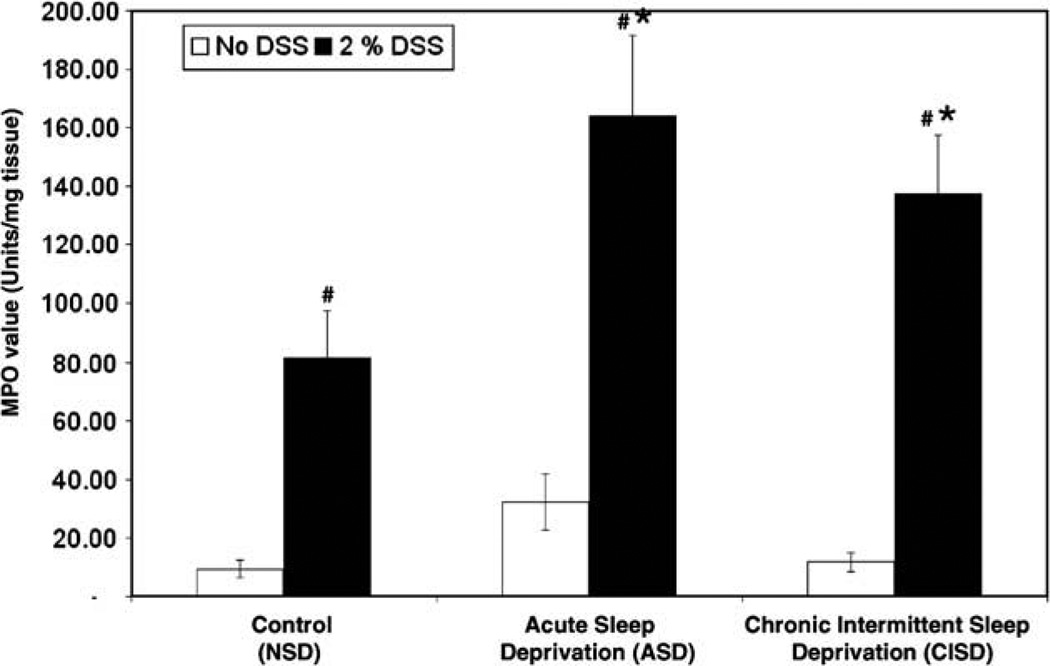
Fig. 3.
Effect of sleep deprivation on colonic inflammation assessed by myeloperoxidase activity (MPO) in the colon. A high MPO value indicates increased colonic inflammation. Both acute sleep deprivation (ASD) and chronic intermittent sleep deprivation (CISD) significantly increased MPO activity in the colon. Data are expressed as mean ± SEM. (#p < 0.05 compared with no DSS treatment group; *p < 0.5 compared with DSS treated alone, but no sleep deprivation (NSD) group).
Acute (ASD) sleep deprivation worsened colonic inflammation in DSS-induced colitis. As in the control groups, the acute sleep deprivation caused a transient, small weight loss (not statistically significant) in DSS-treated mice on day 0. All mice recovered and their weight went back up to the baseline level by experimental day 1. There was no significant difference in the total food intake between sleep deprived and non-sleep deprived DSS-treated groups during the 10 days. Acute sleep deprivation worsened the severity of colonic inflammation as evidenced by a marked increased in tissue MPO activity in sleep deprived mice. Tissue MPO levels in sleep deprived, DSS-treated mice were significantly higher than in non-sleep deprived mice (Fig. 3). However, the magnitude of the deleterious effects of acute sleep deprivation on colonic inflammation was not enough to worsen colitis to the degree where it was manifested either histologically (no significant further worsening of the histopathological score, Fig. 2C) or macroscopically (no significant further shortening of the colon length, Fig. 2B) or clinically (no significant further worsening of weight loss or diarrhea, Figs. 1 and 2A).
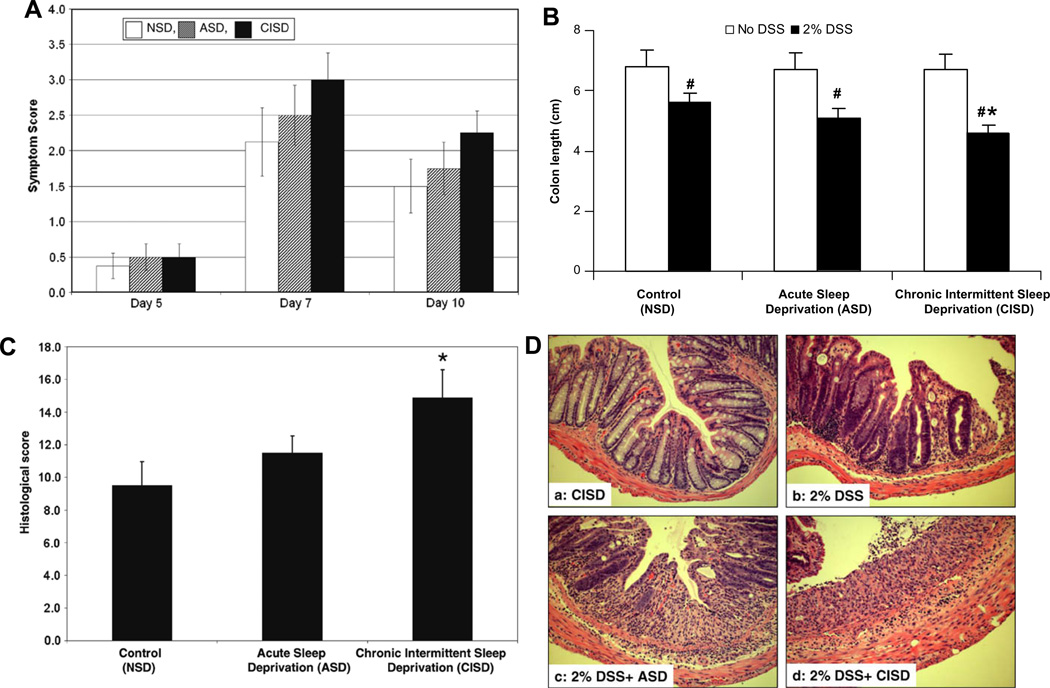
Fig. 2.
Effect of sleep deprivation on severity of 2% DSS-induced colitis. (A) Disease symptom scores determined by stool consistency, overt blood and occult blood in stools are shown for days 5, 7 and 10 of DSS treatment. However, ASD and CISD did not show significant differences in DSS-induced colitis symptoms. (B) The colon length of each mouse was measured from the end of the cecum to the anus. Chronic intermittent sleep deprivation (CISD) significantly exacerbated the shortening of the colon induced by 2% DSS (#p < 0.05 compared with no DSS treatment group; *p < 0.05 compared with DSS treated alone group). (C) Histology scores as indicators of colonic inflammation and tissue injury induced by DSS. All animals were treated with 2% DSS. Scores were determined as detailed in Section 2. Compared with control animals (2% DSS treated), the score in the chronically sleep deprived mice (CISD group) was significantly higher, indicating a more severe colitis as a consequence of the sleep deprivation. Data are expressed as mean ± SEM. *p < 0.05 compared with non-deprived DSS-treated mice (no sleep deprivation, NSD + DSS, n = 8/group). (D) Representative sections of the distal colon on day 10 after the administration of the DSS. (a) Chronic intermittent sleep deprivation (CISD alone) did not significantly affect the histology of the colon. (b) Two percentage of DSS caused colitis with an inflammatory reaction and tissue injury with loss of crypts and mucosal ulceration. (c and d) Acute sleep deprivation (ASD) and chronic intermittent sleep deprivation (CISD) exacerbated the damage caused by 2% DSS.
3.3. Chronic, intermittent sleep deprivation (CISD) worsened colonic inflammation and tissue injury in DSS-induced colitis
As in the control groups, the CISD caused a transient, small weight loss (not statistically significant) in DSS-treated mice on day 0. All mice recovered and their weight went back up to baseline levels on experimental day 1. There was no significant difference in the total food intake between sleep deprived and non-sleep deprived DSS-treated groups during the 10-day experimental period. CISD worsened the severity of colonic inflammation as evidenced by a marked increased in tissue MPO activity in sleep deprived mice. Tissue MPO levels in chronic, intermittent sleep deprived, DSS-treated mice were significantly higher than in non-sleep deprived mice (Fig. 3). The magnitude of the deleterious effects of chronic, intermittent sleep deprivation on colonic inflammation was significantly greater than the effects of acute sleep deprivation. Indeed, the deleterious effects were severe enough to worsen colitis to the degree that it was manifested histologically (further significant worsening of the histopathological score compared to NSD and DSS-treated mice, Fig. 2C) and macroscopically (further significant shortening of the colon length compared to NSD- and DSS-treated mice, Fig. 2B). However, CISD did not induce further significant worsening of weight loss, diarrhea and bleeding compared to NSD- and DSS-treated mice (Figs. 1 and 2A). Similar to the non-sleep deprived group, ASD and CISD mice began to lose weight 3 days after exposure to daily DSS and continued to lose weight progressively during the 7 days of DSS exposure (CISD control vs. CISD + DSS; p = 0.0062). The magnitude of weight loss in ASD and CISD mice was similar to normal-sleep mice during the 7-day exposure to DSS (Fig. 1). Acute sleep deprivation showed no significant differences compared to the NSD group (NSD + DSS vs. ASD + DSS, p = 0.2352) or the CISD group (CISD + DSS vs. ASD + DSS, p = 0.9171). However, the chronically sleep deprived mice did not recover or maintain body weight as normal-sleep mice did after DSS exposure ceased. In fact, chronically sleep deprived mice continued to lose weight even during the recovery period, indicating that the colitis remained severe to the end of the study period (Fig. 1, NSD + DSS vs. CISD + DSS; p = 0.0275). The relative body weight loss was statistically significant at days 9 and 10 between the NSD and CISD groups.
DSS treatment caused diarrhea with liquid stools between days 3 and 5 in all mice. All mice also had occult blood positive stools on treatment days 5–7 (Fig. 2A). CISD worsened the severity of the clinical manifestations of colitis. Gross rectal bleeding occurred after 5 days of exposure to DSS in most chronically deprived mice and continued even throughout the last 3 days of the recovery period.
As expected, colitis caused shortening of the colon length. DSS-treated mice had significantly shorter colon lengths (5.5 ± 0.3 cm) than control mice (6.8 ± 0.2 cm; Fig. 2B, p = 0.001). The colon length in the acute sleep deprivation group was 5.1 ± 0.3 cm. Chronic, intermittent sleep deprivation exaggerated the shortening of the colon induced by DDS to 4.7 ± 0.2 cm (Fig. 2B, p = 0.001 vs. NSD + DSS).
DSS (2%) caused moderately severe colitis (Fig. 2D) with a significantly increased histology score of 9.5 ± 1.6 (p = 0.001 compared to the control group; Fig. 2C). ASD did not show a significant worsening of tissue injury compared to the non-sleep deprived group (ASD + DSS vs. NSD + DSS, p = 0.632), but chronic intermittent sleep deprived mice had a significant increase in histology score (CISD + DSS = 14.7 ± 2.1 vs. NSD + DSS = 9.5 ± 1.6, Fig. 2C, p = 0.010). Representative sections of the colon on day 10 both with and without administration of DSS are shown in Fig. 2D.
Tissue MPO activity is a marker of tissue neutrophil infiltration and an index of acute inflammatory reactions. As expected, MPO levels were significantly increased in DSS-treated mice (NSD = 8 ± 3 vs. NSD + DSS = 82 ± 18 units/mg, p = 0.006). Both acute and chronic intermittent sleep deprivation significantly worsened DSS-induced colonic inflammation (Fig. 3). ASD significantly increased the MPO to a level of 165 ± 23 units/mg (ASD + DSS vs. NSD + DSS, p = 0.010). CISD also significantly increased the MPO level to 138 ± 18 units/mg (CISD + DSS vs. NSD + DSS, p = 0.011, Fig. 3). There was no significant difference in acute colonic inflammation between acute and chronic intermittent sleep deprivation.
3.4. Effects of different modes of inducing sleep deprivation
To make sure that the observed effects of sleep deprivation were not due to forced movement in wheel running mice, in selected mice we induced sleep deprivation by gentle handling of mice for 6 h every other day during the 10-day experimental period. Chronic intermittent gentle handling also caused sleep deprivation but did not cause excess physical activity. Compared with the effect of wheel running, the gentle handling caused similar worsening of colitis with exaggerated body weight loss and colon inflammation in DSS-treated mice.
4. Discussion
The primary finding of this study is that sleep deprivation exacerbates DSS-induced colitis in mice, and the magnitude of the deleterious effects of sleep deprivation on the severity of colitis appears to depend on the duration and pattern of disrupted sleep. The DSS-induced colitis model, which was originally reported by Okayasu et al. [17], has been extensively used to study both therapeutic interventions and the effects of various factors as triggers for exacerbation of colitis because it accurately models many aspects of human colitis [18,22–24]. Therefore, we used DSS-induced colitis to study the effects of sleep deprivation on colitis and found that both acute and chronic intermittent sleep deprivation worsen the severity of colonic inflammation. To our knowledge, this report is the first to demonstrate that sleep deprivation worsens the severity of colitis.
We found that neither acute nor chronic intermittent sleep deprivation has any sustained deleterious effects on food intake, weight gain or colon histology. Neither acute nor chronic, intermittent sleep deprivation caused colonic inflammation or histological colonic tissue injury. However, both sleep deprivation paradigms worsened DSS-induced colonic inflammation as evidenced by marked increase in tissue MPO activity. MPO is an enzyme stored in azurophilic granules of polymorphonuclear neutrophils and macrophages and released into extracellular fluid during an inflammatory process. Thus, MPO activity serves as a sensitive marker for the severity of neutrophil infiltration in inflammatory disorders [19–21]. Tissue neutrophil infiltration is the first step of mucosal inflammation that leads to tissue injury, macroscopic mucosal ulceration and eventually clinical manifestation of colitis. Indeed, several studies in humans demonstrated a strong correlation between severity of ulcerative colitis and levels of colonic neutrophil infiltration assessed by measuring another azurophilic granule of neutrophil, calprotectin, in the stool [25]. We found that acute sleep deprivation did not worsen other aspects of DSS-induced colitis. It did not worsen DSS-induced shortening of colon length or histological score or clinical symptom index. These findings suggest that the deleterious effects of acute sleep deprivation on colonic inflammation were not enough to worsen colitis to the degree that it is manifested either macroscopically (further shortening of the colon) or clinically (further worsening of weight loss). This is not surprising because many patients with inactive ulcerative colitis who have no symptoms have elevated stool calprotectin levels [25]. It should be noted that patients with inactive ulcerative colitis who have elevated stool calprotectin have a higher risk of flare ups during the following 6 months than those with normal stool calprotectin. Thus, increased levels of inflammation due to acute sleep deprivation may still have clinical importance.
Unlike acute sleep deprivation, the deleterious effects of chronic, intermittent sleep deprivation on DSS-induced colonic inflammation was severe enough to be manifested both macroscopically and clinically. Thus, our data provide a “dose–response” effect of sleep deprivation on the severity of colitis – the deleterious effects of sleep deprivation increase by increasing the duration of sleep deprivation.
Several clinical observations have suggested that sleep deprivation is an important environmental predisposing factor for exacerbation of IBD disease. Using a validated sleep questionnaire, we [7, 8] and others [15] found strong links between bowel problems and sleep disruption. Yet these studies have relied on the subjective judgment of the subjects and run the risk of being biased because IBD patients with sleep problems may be more likely to report them, more likely to participate in a study, or more likely to remember episodes of insomnia that occurred just prior to and during a flare up. To overcome the design weaknesses of these clinical studies, we used a more objective method of studying disrupted sleep and again found abnormal sleep patterns in patients with inactive IBD [9]. However, even polysomnography cannot eliminate potential bias in data collection. For example, acquisition of polysomnography data on actual sleep time is limited; typically data for only a single day are obtained and are often modulated by a first-night-effect (people adapting to foreign environments). Furthermore, frequent disrupted sleep in IBD patients could be, as noted above, a consequence rather than a cause of a more severe IBD course. Also, more severe IBD symptoms in sleep deprived patients could be a consequence of changes in intestinal function and associated irritable bowel syndrome in IBD patients and not due to worsening of inflammation. To overcome these shortcomings, we studied the effects of sleep deprivation in an animal model of colitis and showed worsening of gut inflammation. Our findings in an animal model of colitis could have a major clinical impact in the management of patients with colitis because chronic intermittent sleep deprivation has become a common social problem in western industrial societies. Indeed, over the past 25 years in modern industrial societies, total sleep time has steadily decreased [13]. It is estimated that 10% or more of the general population experiences poor sleep quality [12, 13]. This situation is even worse for IBD patients where there are even more complaints of sleep disturbances [7, 9].
The mechanism through which sleep deprivation worsens colonic inflammation is not known and requires further studies. However, there are several potential candidates. For example, both human and animal studies suggest that stress can activate inflammatory cascades in the gut that might lead to IBD flare ups [5, 6, 10, 11, 26, 27], and sleep deprivation is a potent stressor. However, in the absence of both proper controls for stress and measurement of stress hormones in the current study, we cannot directly assess the role of stress in the sleep deprivation-induced worsening of colitis. Furthermore, forced movement that was used by us to induce sleep deprivation could be the major source of stress, and thus it could be an important contributing factor to our observed worsening of colitis. One limitation of the study is that there was no “control” for the forced movement for sleep deprivation. However, we attempted to assess the impact of forced movement on our outcome measures by inducing sleep deprivation by gentle handing. We found that sleep deprivation induced by gentle handling had a similar effect on severity of colitis as did forced movement. Thus, although it is difficult to rule out with certainty that sleep deprivation procedures are not responsible for our observed findings, our gentle handling data makes this possibility less likely. Nonetheless, a possible contribution of stress to sleep deprivation-induced worsening of colitis cannot be excluded. We believe that sleep deprivation may worsen DSS-induced colitis through both stress-dependent and stress-independent mechanisms.
One possible mechanism for sleep deprivation-induced worsening of colitis is promotion of mucosal immune dysregulation. IBD is thought to result from a dysregulated mucosal immune response to normal gut flora in a genetically susceptible host [1, 2, 28, 29]. There is increasing evidence that sleep deprivation has detrimental effects on the immune system [12, 13] and this may worsen DSS-induced colitis through dysregulation of the immune system. Indeed, many studies indicate that short-term sleep deprivation increases markers of inflammation and increases plasma TNF, IL-1 and IL-6 [30–32]. In addition, the number of CD3+, CD4+ and CD8+ cells have been found to be increased, while NK-cell responses are reduced in subjects with chronic insomnia [33, 34]. In addition, insomnia is associated with low levels of protective factors such as IFN-γ and a low IFN-γ/IL-4 ratio, and insomnia results in a shift in the balance between Th1 and Th2 cells [35]. Our data suggest that sleep deprivation worsens acute inflammation and hampers tissue repair and recovery. We found that both acute and chronic intermittent sleep deprivation increases tissue MPO levels in DSS-treated mice, supporting their pro-inflammatory impact. Furthermore, chronic intermittent sleep deprivation appears to blunt the ability of mice to recover from colitis. These sleep deprived mice, unlike non-sleep deprived mice, could not regain their lost weight during the last 3 days of the recovery phase. Further studies are now needed to elucidate the effects of sleep deprivation on intestinal mucosal immune regulation in colitis.
In summary, we present the first objective and quantitative evidence that sleep deprivation can exacerbate the severity of colitis and delay recovery. Such a finding in an animal model provides a scientific rationale for a clinical trial to determine whether sleep management can improve IBD disease course by preventing IBD flare ups and by prolonging remission in patients with ulcerative colitis. While data from such a trial will be necessary for any definitive recommendations regarding the care and treatment of IBD patients, the results from these animal studies serve to alert physicians that perhaps more attention should be paid to the management of sleep disturbances in patients with IBD, particularly for those who report disrupted or poor quality sleep.
Acknowledgments
This work was supported in part by a grant from the Department of Internal Medicine of Rush University Medical Center.
References
- 1.Keighley MR, Stockbrugger RW. Inflammatory bowel disease. Aliment Pharmacol Ther. 2003;18(Suppl 3):66–70. doi: 10.1046/j.0953-0673.2003.01727.x. [DOI] [PubMed] [Google Scholar]
- 2.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 3.Hart A, Kamm MA. Review article: mechanisms of initiation and perpetuation of gut inflammation by stress. Aliment Pharmacol Ther. 2002;16:2017–2028. doi: 10.1046/j.1365-2036.2002.01359.x. [DOI] [PubMed] [Google Scholar]
- 4.Mawdsley JE, Rampton DS. Psychological stress in IBD: new insights into pathogenic and therapeutic implications. Gut. 2005;54:1481–1491. doi: 10.1136/gut.2005.064261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mawdsley JE, Rampton DS. The role of psychological stress in inflammatory bowel disease. Neuroimmunomodulation. 2006;13:327–336. doi: 10.1159/000104861. [DOI] [PubMed] [Google Scholar]
- 6.Mawdsley JE, Macey MG, Feakins RM, Langmead L, Rampton DS. The effect of acute psychologic stress on systemic and rectal mucosal measures of inflammation in ulcerative colitis. Gastroenterology. 2006;131:410–419. doi: 10.1053/j.gastro.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 7.Ranjbaran Z, Keefer L, Farhadi A, Stepanski E, Sedghi S, Keshavarzian A. Impact of sleep disturbances in inflammatory bowel disease. J Gastroenterol Hepatol. 2007;22:1748–1753. doi: 10.1111/j.1440-1746.2006.04820.x. [DOI] [PubMed] [Google Scholar]
- 8.Ranjbaran Z, Keefer L, Stepanski E, Farhadi A, Keshavarzian A. The relevance of sleep abnormalities to chronic inflammatory conditions. Inflamm Res. 2007;56:51–57. doi: 10.1007/s00011-006-6067-1. [DOI] [PubMed] [Google Scholar]
- 9.Keefer L, Stepanski EJ, Ranjbaran Z, Benson LM, Keshavarzian A. An initial report of sleep disturbance in inactive inflammatory bowel disease. J Clin Sleep Med. 2006;2:409–416. [PubMed] [Google Scholar]
- 10.North CS, Alpers DH, Helzer JE, Spitznagel EL, Clouse RE. Do life events or depression exacerbate inflammatory bowel disease? A prospective study. Ann Intern Med. 1991;114:381–386. doi: 10.7326/0003-4819-114-5-381. [DOI] [PubMed] [Google Scholar]
- 11.Levenstein S, Prantera C, Varvo V, Scribano ML, Andreoli A, Luzi C, et al. Stress and exacerbation in ulcerative colitis: a prospective study of patients enrolled in remission. Am J Gastroenterol. 2000;95:1213–1220. doi: 10.1111/j.1572-0241.2000.02012.x. [DOI] [PubMed] [Google Scholar]
- 12.Zisapel N. Sleep and sleep disturbances: biological basis and clinical implications. Cell Mol Life Sci. 2007;64:1174–1186. doi: 10.1007/s00018-007-6529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bryant PA, Trinder J, Curtis N. Sick and tired: does sleep have a vital role in the immune system? Nat Rev Immunol. 2004;4:457–467. doi: 10.1038/nri1369. [DOI] [PubMed] [Google Scholar]
- 14.Orr WC, Chen CL. Sleep and the gastrointestinal tract. Neurol Clin. 2005;23:1007–1024. doi: 10.1016/j.ncl.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Zimmerman J. Extraintestinal symptoms in irritable bowel syndrome and inflammatory bowel diseases: nature, severity, and relationship to gastrointestinal symptoms. Dig Dis Sci. 2003;48:743–749. doi: 10.1023/a:1022840910283. [DOI] [PubMed] [Google Scholar]
- 16.Kim Y, Laposky AD, Bergmann BM, Turek FW. Repeated sleep restriction in rats leads to homeostatic and allostatic responses during recovery sleep. Proc Natl Acad Sci USA. 2007;104:10697–10702. doi: 10.1073/pnas.0610351104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 18.Abdelbaqi M, Chidlow JH, Matthews KM, Pavlick KP, Barlow SC, Linscott AJ, et al. Regulation of dextran sodium sulfate induced colitis by leukocyte beta 2 integrins. Lab Invest. 2006;86:380–390. doi: 10.1038/labinvest.3700398. [DOI] [PubMed] [Google Scholar]
- 19.Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 20.Rath HC, Herfarth HH, Ikeda JS, Grenther WB, Hamm TE, Jr, Balish E, et al. Normal luminal bacteria, especially Bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human beta2 microglobulin transgenic rats. J Clin Invest. 1996;98:945–953. doi: 10.1172/JCI118878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 22.Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- 23.Pizarro TT, Arseneau KO, Bamias G, Cominelli F. Mouse models for the study of Crohn’s disease. Trends Mol Med. 2003;9:218–222. doi: 10.1016/s1471-4914(03)00052-2. [DOI] [PubMed] [Google Scholar]
- 24.Elson CO, Sartor RB, Tennyson GS, Riddell RH. Experimental models of inflammatory bowel disease. Gastroenterology. 1995;109:1344–1367. doi: 10.1016/0016-5085(95)90599-5. [DOI] [PubMed] [Google Scholar]
- 25.Costa F, Mumolo MG, Ceccarelli L, Bellini M, Romano MR, Sterpi C. Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn’s disease. Gut. 2005;54:364–368. doi: 10.1136/gut.2004.043406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernstein CN, Walker JR, Graff LA. On studying the connection between stress and IBD. Am J Gastroenterol. 2006;101:782–785. doi: 10.1111/j.1572-0241.2006.00474.x. [DOI] [PubMed] [Google Scholar]
- 27.Sperber AD, Tarasiuk A. Disrupted sleep in patients with IBS–a wake-up call for further research? Nat Clin Pract Gastroenterol Hepatol. 2007;4:412–413. doi: 10.1038/ncpgasthep0847. [DOI] [PubMed] [Google Scholar]
- 28.van Dieren JM, Kuipers EJ, Samsom JN, Nieuwenhuis EE, van der Woude CJ. Revisiting the immunomodulators tacrolimus, methotrexate, and mycophenolate mofetil: their mechanisms of action and role in the treatment of IBD. Inflamm Bowel Dis. 2006;12:311–327. doi: 10.1097/01.MIB.0000209787.19952.53. [DOI] [PubMed] [Google Scholar]
- 29.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 30.Vgontzas AN, Bixler EO, Lin HM, Prolo P, Trakada G, Chrousos GP. IL-6 and its circadian secretion in humans. Neuroimmunomodulation. 2005;12:131–140. doi: 10.1159/000084844. [DOI] [PubMed] [Google Scholar]
- 31.Opp MR. Cytokines and sleep. Sleep Med Rev. 2005;9:355–364. doi: 10.1016/j.smrv.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Uthgenannt D, Schoolmann D, Pietrowsky R, Fehm HL, Born J. Effects of sleep on the production of cytokines in humans. Psychosom Med. 1995;57:97–104. doi: 10.1097/00006842-199503000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Irwin M, Clark C, Kennedy B, Christian Gillin J, Ziegler M. Nocturnal catecholamines and immune function in insomniacs, depressed patients, and control subjects. Brain Behav Immun. 2003;17:365–372. doi: 10.1016/s0889-1591(03)00031-x. [DOI] [PubMed] [Google Scholar]
- 34.Savard J, Laroche L, Simard S, Ivers H, Morin CM, et al. Chronic insomnia and immune functioning. Psychosom Med. 2003;65:211–221. doi: 10.1097/01.psy.0000033126.22740.f3. [DOI] [PubMed] [Google Scholar]
- 35.Sakami S, Ishikawa T, Kawakami N, Haratani T, Fukui A, Kobayashi F, et al. Coemergence of insomnia and a shift in the Th1/Th2 balance toward Th2 dominance. Neuroimmunomodulation. 2002;10:337–343. doi: 10.1159/000071474. [DOI] [PubMed] [Google Scholar]