Abstract
Persons with unrecognized HIV infection forgo timely clinical intervention and may unknowingly transmit HIV to partners. However, in the United States, unrecognized infection and late diagnosis are common. To understand barriers and facilitators to HIV testing and care, we conducted a qualitative study of 24 HIV infected persons attending a Southeastern HIV clinic who presented with clinically advanced illness. The primary barrier to HIV testing prior to diagnosis was perception of risk; consequently, most participants were diagnosed after the onset of clinical symptoms. While most patients were anxious to initiate care rapidly after diagnosis, some felt frustrated by the passive process of connecting to specialty care. The first visit with an HIV care provider was identified as critical in the coping process for many patients. Implications for the implementation of recent CDC HIV routine screening guidelines are discussed.
Keywords: HIV Infection, Voluntary Counseling and Testing, Delivery of Health Care, Southeastern United States, Social Support
Introduction
Unrecognized HIV infection has important public health consequences. In addition to limiting the benefits of early medical care including antiretroviral therapy and prophylaxis for opportunistic infections, persons with unrecognized infection may unknowingly transmit HIV to partners (MacKellar et al., 2006; Marks, Crepaz, Senterfitt, & Janssen, 2005). Despite these benefits, unrecognized infection and consequently, late diagnosis, are common among HIV infected adults. In the United States, 40% of those tested in 2004 were diagnosed with AIDS less than 1 year after the initial HIV diagnosis (Centers for Disease Control and Prevention, 2003b, 2006). Further, approximately 25% of all adults living with HIV/AIDS in the U.S. do not know their status, and as many as one-third may not be receiving care (Fleming & Wasserheit, 1999). Even more concerning is that the severity of immune suppression at first presentation may be worsening among some groups (Keruly & Moore, 2007).
Delayed presentation to care can be attributed to late diagnosis, delays between diagnosis and care, or both (Samet et al., 1998). Most of the research on delayed presentation to care has focused on descriptive characteristics of individuals who present late in the disease course, information often derived from medical records or clinical cohorts (Gay, Napravnik, & Eron, 2006; Keruly & Moore, 2007; Krawczyk et al., 2006a). While these data are informative about the population at risk, without context they limit causal inference and the subsequent design of public health interventions. Few have investigated the availability of voluntary counseling and testing services (VCT) and primary care, the barriers and facilitators to utilizing services, and the personal belief systems which influence the decision-making process. Although VCT and HIV specialty care is widely available in most U.S. settings and safe and effective therapy has extended the life expectancy of those diagnosed with HIV, many obstacles to timely presentation to care remain in the U.S.(Schackman et al., 2006).
Our objective was to understand barriers and facilitators to HIV testing and care among HIV infected persons attending a Southeastern HIV clinic who presented with clinically advanced illness. HIV positive patients in this region of the U.S. may face different challenges than the rest of the country, given the rural nature of the region and the degree to which heterosexual involvement is evident in the epidemic (Krawczyk, Funkhouser, Kilby, & Vermund, 2006b). Through detailed patient narratives, we describe the process of diagnosis and entering care among a unique group of HIV positive patients.
Methods
Study setting and population
The source population for the study was a large, university-based medical center that follows approximately 1,300 HIV infected patients per year and provides comprehensive HIV primary care services. Patients were eligible for the study if they (1) were at least 18 years old, (2) entered care between April 2006 and April 2008 and had not received HIV care elsewhere, (3) had a CD4+ T-lymphocyte cell count <350 cells/mm3 at entry, and (4) were cognitively competent to participate. By purposefully basing study inclusion on clinical criteria, patients with delayed diagnosis as well as patients who postponed entering care after a more distal diagnosis were included in the study.
Recruitment
Eligible patients were approached by a clinic-based research assistant on or after their second clinic visit, once laboratory results were available and the patient had the opportunity to discuss research participation. Interested patients met with the study interviewer before or after their clinic visit or at a separate appointment time. We employed a small purposeful study sample to collect in-depth narratives; data collection was guided by the achievement of theme saturation (Glaser & Strauss, 1967; Ulin, Robinson, & Tolley, 2005).
Data Collection
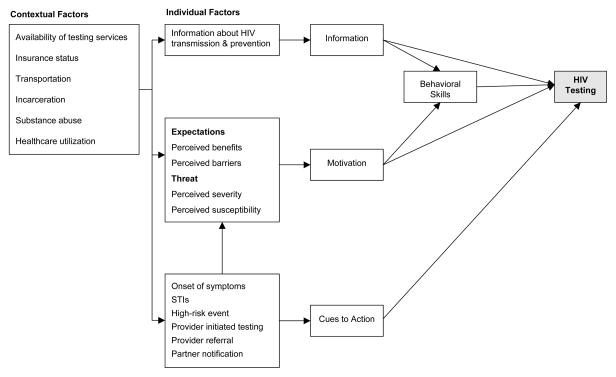
We identified in-person, qualitative interviews as the most appropriate method for our research question because we sought to understand the process, meaning, and context within which HIV positive persons make decisions about health care (Maxwell, 2004). A single female study interviewer explained the study and obtained written, informed consent at the start of the interview. In-depth, semi-structured, qualitative interviews were then conducted with study participants using an interview guide approach (Ulin et al., 2005). We developed a conceptual framework for VCT utilization based on constructs described in the Information-Motivation-Behavioral Skills (IMB) model and the Health Belief Model (HBM) to inform our interview guide (Figure 1) (Fisher & Fisher, 1992) (Janz & Becker, 1984). Interviews were audio recorded and averaged 43 minutes. Patients were compensated with a grocery gift card. At the completion of each interview, interview summaries were created to record non-verbal attributes, first impressions, and suggested improvements for future interviews.
Figure 1.
Conceptual framework of HIV testing utilization based on the Information-Motivation-Behavioral Skills model and the Health Belief Model (Fisher & Fisher, 1992; Janz & Becker, 1984).
Analysis
Interviews were recorded and transcribed verbatim. Analysis was ongoing and first involved single-case analysis, including the generation of memos and interview summaries, followed by cross-case analyses to identify emergent themes and patterns (Maxwell, 2004). A start list of deductive, descriptive codes was used to begin coding, however, coding was continuous and evolving to include interpretive, inductive codes (Maxwell, 2004; Miles & Huberman, 1984; Ulin et al., 2005). Interviews were broadly coded for themes, examined for narrative structure, and compared to the conceptual framework. Coding sorts and sub-themes of each code were evaluated to examine differences in the variation and context of narratives within the same code. Atlas/ti software (Berlin, Germany) was used for analysis.
Human subjects protection
All participants provided written informed consent and HIPAA authorization to access to medical information. This study was approved by the UNC Institutional Review Board.
Results
The study was conducted from April 2007 through April 2008. During this time, 89 eligible patients had visits in the clinic, of which 41 of 46 (89%) approached about the study were interested in participation. Of these 41, 24 (59%) interviews were completed, one person refused, and the remaining 16 people could not be re-located or wanted to be interviewed at another time. Most (67%) patients presented to care with less than 200 CD4 cells/mm3, and most initiated primary care quickly after diagnosis (Table 1).
Table 1.
Descriptive characteristics of 24 HIV positive patients initiating HIV primary care between April 2006 and April 2008 at the University of North Carolina outpatient infectious disease clinic.
| Characteristic | N (%) |
|---|---|
| Year of Diagnosis | |
| 1985 | 1 (4.2) |
| 2005 | 2 (8.3) |
| 2006 | 15 (62.5) |
| 2007 | 6 (25.0) |
| Gender | |
| Male | 19 (79.2) |
| Female | 5 (20.8) |
| Race | |
| African American | 12 (50.0) |
| Hispanic | 1 (4.2) |
| White | 11 (45.8) |
| Age (median, years) | 42.5 years (range: 26–62) |
| CD4+ T-cell count at entry to care (median, cells/ml) | 92 cells/ml (range: 17–332) |
| Time between diagnosis and entry to care (median, days) | 40.5 days (range: 0–22.7 years) |
| HIV testing method | |
| Provider-initiated | 16 (66.7) |
| Client-initiated | 3 (12.5) |
| Routine screening (e.g., blood donation, prison) | 5 (20.8) |
HIV-related information and experience
Prior to diagnosis, HIV-related knowledge varied. Most participants reported understanding fundamental HIV concepts, including modes of transmission and clinical impact. The media and family members and friends living with HIV were often cited as sources of information. Most (71%) knew someone with HIV prior to diagnosis; however, many held stereotypes about the types of people affected by HIV. There was a dichotomy of people who believed that an HIV diagnosis meant imminent death and those who believed that HIV was a manageable illness.
Perceived susceptibility
Most did not perceive themselves as having been susceptible to HIV infection. The lack of perceived vulnerability could be broadly divided into three themes: people who did not recognize their behavior as risky, people who viewed their behavior as very low risk, and people who felt like exposure to HIV was unlikely, regardless of behavior. Their narratives highlight the lack of susceptibility many participants felt prior to diagnosis and their surprise once diagnosed (Table 2). Several acknowledged engaging in risky behavior, but they felt it wasn’t risky enough to expose them to HIV. Others felt that regardless of their personal risk, HIV acquisition was unlikely. Together, these narratives indicate that most felt that their behavior did not place them at enough risk to warrant seeking testing, either because of beliefs about risk behavior or denial of the risks of their behaviors.
Table 2.
Illustrative participant quotations about the lack of perceived susceptibility to HIV infection.
| Participant Quotations |
|---|
|
Perceived benefits and barriers
Participants were probed about their beliefs prior to diagnosis about the value of seeking VCT services (perceived benefits) and about the material or psychological costs (perceived barriers). We separated benefits from cues to action, which are antecedent events that motivated participants to take action.
Benefits
Overall, few participants identified benefits of seeking an HIV test. Only 3 participants sought testing on their own, and all of them reported a cue to take action (the onset of symptoms or finding out a sexual partner was positive). In general, the facilitating factors to seek testing included being concerned about one’s health, wanting to take action in case one was positive, and being able to rule HIV out as a cause of illness.
Barriers
The lack of perceived susceptibility to HIV infection was the predominant barrier to testing. In addition to risk perception, participants listed numerous barriers to accessing VCT services including fear, substance abuse, and the presence or absence of symptoms. One participant explained, “I felt like … I ain’t got it so what’s the use of being tested? And then part of me was scared to find out.” While stigma was a predominant theme in the narratives about the trauma of discovering one’s HIV status, stigma was only mentioned explicitly by one person as a barrier to testing.
Cues to take action
All but one of the participants (who was diagnosed after blood donation) were eventually diagnosed because of an event that incited testing. Most participants (58%) were diagnosed because their provider initiated testing after the onset of symptoms. In some of these cases, HIV was a diagnosis of last resort, in others, HIV testing was recommended because of signs and symptoms consistent with HIV/AIDS (e.g. Kaposi’s sarcoma) or factors indicative of a higher risk of HIV infection (e.g. sexually transmitted disease). Several participants described screening events where testing was offered such as prison intake, a testing event at a residential substance abuse recovery program, and screening during clinical examinations. In these cases, participants were amenable to testing when it was readily available and free. Few (n=3) sought testing on their own; two participants sought testing after the onset of symptoms and another after finding out his ex-partner had recently died with HIV.
Connecting to medical care
Most were diagnosed due to symptomatic illness and consequently initiated medical care rapidly thereafter. However, some patients delayed medical care for 12 months or longer, and almost all participants reported having some fears or anxiety about initiating HIV care. Although the psychological impact of HIV was not the focus of this study, it was clear that the shock and devastation of diagnosis with advanced HIV had implications for connection to HIV specialty care with respect to deciding when and where to seek care.
Most patients were anxious to start care after diagnosis. The primary facilitator to enter care was avoidance of illness and death as well as wanting to take control of the situation and begin the journey toward health. When asked about any worries or fears about starting HIV care, a 26-year old man described his sense of urgency to schedule an appointment, “I felt like I was kind of on autopilot … I have to make this phone call …you know, because I didn’t want to wind up sick.”
Patients listed several barriers to initiating medical care. While not a predominant barrier to VCT, stigma was often at the forefront of patient’s minds after diagnosis when considering attending an HIV clinic. Patients were worried about being judged by healthcare professionals and being recognized in the clinic. Patients reported not wanting to go through the ups and downs of care, and a handful expressed a willingness to die rather than deal with the medical system. After diagnosis, six people reported feeling suicidal and of these, four made attempts. The desire to be remembered the way they were prior to getting sick was expressed by several patients. Transportation and financial concerns were rarely mentioned. Three patients delayed care for more than one year after diagnosis; their narratives suggest that the decision and process of engaging and maintaining care can be complex.
Some patients reported challenges when being transferred from one doctor or institution to HIV specialty care. This phenomenon was described by one man as “passing the buck” and often patients felt isolated and frustrated about their healthcare provider’s lack of HIV knowledge (Table 3, quotations 1–2). However, the first clinical visit for HIV care was identified as a turning point in the coping process for some. Up until their first visit in the HIV outpatient clinic, many who were diagnosed by non-HIV specialty physicians felt abandoned, confused, and terrified about the impending medical care to come. Some attempted to research HIV, often online, and felt overwhelmed by the findings. It was not until they met with an HIV specialist that their fears were reduced (Table 3, quotations 3–5).
Table 3.
Participant quotations about referral from their primary care physician to HIV care after diagnosis and the importance of their first clinical visit for HIV care.
| Participant Quotations |
|---|
|
For some, the first visit was the first time they felt in control since diagnosis. It was a time to allay some of their fears and to learn the basics about HIV pathogenesis and immunologic monitoring, that death wasn’t imminent and that they had time to carefully consider decisions about therapy, and that although antiretroviral therapy may have side effects for some people, HIV can be a manageable disease. During the period from diagnosis until entry to care, patients without this basic information may be paralyzed, suicidal, and isolated as they come to terms with HIV.
Discussion
In the U.S. Southeast, a substantial proportion of people living with HIV are diagnosed or enter care late in the course of illness. In the hospital outpatient clinic where our study was conducted, 75% of all patients have an indication for antiretroviral therapy at their first clinic visit, and 50% have a CD4+ T-cell count less than 200 cells/mm3 (Gay et al., 2006). Likewise, in Birmingham, Alabama, 41% of patients presenting to an HIV/AIDS outpatient clinic had progressed to Centers for Disease Control and Prevention (CDC) defined AIDS (Krawczyk et al., 2006a). These findings and the knowledge of the unique HIV epidemic in the South raise special concerns about access to testing and medical services in the region (Krawczyk et al., 2006b).
Our study examined barriers and facilitators to HIV testing and medical care among a group of HIV-positive patients in the southeastern U.S. who entered care with moderate to advanced immunosuppression. Our findings suggest that lack of perceived susceptibility to HIV was the predominant barrier to early HIV testing. Predominantly, patients were tested and subsequently entered care due to the onset of clinical symptoms, consistent with other reports (Centers for Disease Control and Prevention, 2003a, 2003b; Samet, Freedberg, Savetsky, Sullivan, & Stein, 2001). Most felt an urgency to enter care after diagnosis, but encountered multiple barriers and sources of frustration before entering care. All of those in our study missed the benefits of early medical care and some may have unknowingly transmitted HIV to others.
Our study has several noteworthy limitations. First, clinic-based studies of delayed presentation to care are limited by only including patients who successfully entered care. HIV positive individuals who had not entered care are unobserved, and they may have different experiences than the patients in the study who all eventually entered care. Further, our qualitative study was comprised of a small purposeful sample that cannot be used to make generalizations or provide insight on to what extent these findings are unique to individuals who presented to care with advanced HIV infection. Despite these limitations, the results of the study have important implications for universal screening programs and linkage to care after diagnosis.
Our findings underscore the barrier that delayed diagnosis poses to HIV prevention. Recent CDC testing guidelines for the adoption of routine testing in all healthcare settings may have an impact on reducing the numbers of individuals who first test positive late in the course of disease (Branson et al., 2006). From a public health perspective, even small changes in the proportion of persons living with HIV/AIDS who are aware of their serostatus can have large impacts in preventing new infections (Pinkerton, Holtgrave, & Galletly, 2008). In our study, most participants accepted testing when it was offered; suggesting that routine screening may increase the numbers of people tested and de-stigmatize the testing process. However, for the program to have impact, people living with unrecognized HIV infection must have contact with the healthcare system. Given that in the Southeast, HIV infection is often a disease of the rural and poor, new strategies to improve health care access will be a necessary precursor for any increased screening to reach the groups most in need.
The fact that public health messages to encourage HIV testing did not reach the individuals in the study who were diagnosed recently, primarily in 2006 and 2007, is concerning. Southeastern residents live within a complex social and structural environment that may elevate their risk of HIV acquisition independent of personal behavior (Adimora, Schoenbach, & Doherty, 2006). Health and economic disparities drive HIV transmission in the Southeast. High rates of sexually transmitted diseases increase the likelihood of HIV acquisition and transmission (Reif, Geonnotti, & Whetten, 2006). The loss of African American men from their communities, either from excess mortality or incarceration, disrupts partnerships and promotes concurrency (Adimora et al., 2006; Doherty, Leone, & Aral, 2007). Stigma, trust in providers, marriage rates, and injection and non-injection drug use are also associated with the epidemic (Reif et al., 2006; Whetten & Reif, 2006). These factors result in frequent heterosexual transmission and a non-urban epidemic (Krawczyk et al., 2006b). It is worrisome that public awareness campaigns for VCT and routine contact with the healthcare system were ineffective in our study population.
Although few participants reported significant delays between HIV diagnosis and entering care, their narratives highlight shortcomings in our system to link newly diagnosed individuals to primary care. As HIV screening becomes increasingly incorporated in non-traditional testing settings, specialized case-management programs to rapidly and effectively link patients to care could bridge the gap between HIV diagnosis and primary care, and allay patient concerns during this unsettling time (Gardner et al., 2005). At least 25% of participants in this study considered or attempted suicide after diagnosis, consistent with other reports (Cooperman & Simoni, 2005; Sherr et al., 2008; Stevens & Hildebrandt, 2006). A study of HIV positive women in New York found that of those who made a suicide attempt after their diagnosis, 86% reported that their diagnosis was very much or partly related to their suicide attempt (Cooperman & Simoni, 2005). It is unclear if the HIV diagnosis acted an “accelerator” or “inducer” of suicide ideation in our study, but this finding warrants further examination to understand risk factors for suicide and whether active referral with follow-up is the optimal standard of care (Marzuk, 1991; Simoni, Nero, & Weinberg, 1998).
Fortunately, the issue of late diagnosis and delayed presentation to care is receiving increased attention among public health and medical professionals. As evidenced by this study, a rote or passive approach to increasing HIV testing and the subsequent linkage to care may miss segments of the population, some of whom are at high risk. A reliance on personal awareness of risk to initiate testing did not work for this group of people who entered care with moderate to advanced immunosuppression. Many felt disillusioned after diagnosis as they encountered difficulties when trying to navigate the health care system. Focusing research efforts toward these issues may help to avert late diagnosis and delayed entry to care in the future.
Acknowledgments
The authors are grateful to all of the patients who participated in the study and to Blair Turner and Ben Kirkley for assistance with recruitment. We are also appreciative of Marie Leak, Crystal Shakleford, and Kim Hayes for transcribing the interviews. Paul Mihas at the Odum Institute provided helpful instruction on qualitative research methods. This work was supported in part by a grant from the University of North Carolina Center for AIDS Research (P30 AI50410) and the U.S. Centers for Disease Control and Prevention Grant for Public Health Dissertations (1R36PS000848-01). The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the U.S. Centers for Disease Control and Prevention.
References
- Adimora AA, Schoenbach VJ, Doherty IA. HIV and African Americans in the southern United States: sexual networks and social context. Sex Transm Dis. 2006;33(7 Suppl):S39–45. doi: 10.1097/01.olq.0000228298.07826.68. [DOI] [PubMed] [Google Scholar]
- Branson BM, Handsfield HH, Lampe MA, Janssen RS, Taylor AW, Lyss SB, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1–17. quiz CE11–14. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Advancing HIV prevention: new strategies for a changing epidemic--United States, 2003. MMWR Morb Mortal Wkly Rep. 2003a;52(15):329–332. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Late versus early testing of HIV--16 Sites, United States, 2000–2003. MMWR Morb Mortal Wkly Rep. 2003b;52(25):581–586. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. HIV/AIDS Surveillance Report. 2006. Cases of HIV infection and AIDS in the United States and Dependent Areas, 2005. [Google Scholar]
- Doherty IA, Leone PA, Aral SO. Social determinants of HIV infection in the Deep South. Am J Public Health. 2007;97(3):391. doi: 10.2105/AJPH.2006.104208. author reply 391–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JD, Fisher WA. Changing AIDS-risk behavior. Psychol Bull. 1992;111(3):455–474. doi: 10.1037/0033-2909.111.3.455. [DOI] [PubMed] [Google Scholar]
- Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75(1):3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner LI, Metsch LR, Anderson-Mahoney P, Loughlin AM, del Rio C, Strathdee S, et al. Efficacy of a brief case management intervention to link recently diagnosed HIV-infected persons to care. Aids. 2005;19(4):423–431. doi: 10.1097/01.aids.0000161772.51900.eb. [DOI] [PubMed] [Google Scholar]
- Gay CL, Napravnik S, Eron JJ., Jr Advanced immunosuppression at entry to HIV care in the southeastern United States and associated risk factors. Aids. 2006;20(5):775–778. doi: 10.1097/01.aids.0000216380.30055.4a. [DOI] [PubMed] [Google Scholar]
- Glaser B, Strauss A. The Discovery of Grounded Theory. Chicago: Aldine; 1967. [Google Scholar]
- Inungu JN. Potential barriers to seeking human immunodeficiency virus testing among adults in the United States: data from the 1998 National Health Interview Survey. AIDS Patient Care STDS. 2002;16(6):293–299. doi: 10.1089/10872910260066723. [DOI] [PubMed] [Google Scholar]
- Janz NK, Becker MH. The Health Belief Model: a decade later. Health Educ Q. 1984;11(1):1–47. doi: 10.1177/109019818401100101. [DOI] [PubMed] [Google Scholar]
- Keruly JC, Moore RD. Immune status at presentation to care did not improve among antiretroviral-naive persons from 1990 to 2006. Clin Infect Dis. 2007;45(10):1369–1374. doi: 10.1086/522759. [DOI] [PubMed] [Google Scholar]
- Krawczyk CS, Funkhouser E, Kilby JM, Kaslow RA, Bey AK, Vermund SH. Factors associated with delayed initiation of HIV medical care among infected persons attending a southern HIV/AIDS clinic. South Med J. 2006a;99(5):472–481. doi: 10.1097/01.smj.0000215639.59563.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk CS, Funkhouser E, Kilby JM, Vermund SH. Delayed access to HIV diagnosis and care: Special concerns for the Southern United States. AIDS Care. 2006b;18(7):35–44. doi: 10.1080/09540120600839280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Quintero C, Shtarkshall R, Neumark YD. Barriers to HIV-testing among Hispanics in the United States: analysis of the National Health Interview Survey, 2000. AIDS Patient Care STDS. 2005;19(10):672–683. doi: 10.1089/apc.2005.19.672. [DOI] [PubMed] [Google Scholar]
- MacKellar DA, Valleroy LA, Behel S, Secura GM, Bingham T, Celentano DD, et al. Unintentional HIV exposures from young men who have sex with men who disclose being HIV-negative. Aids. 2006;20(12):1637–1644. doi: 10.1097/01.aids.0000238410.67700.d1. [DOI] [PubMed] [Google Scholar]
- Marks G, Crepaz N, Senterfitt JW, Janssen RS. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for HIV prevention programs. J Acquir Immune Defic Syndr. 2005;39(4):446–453. doi: 10.1097/01.qai.0000151079.33935.79. [DOI] [PubMed] [Google Scholar]
- Maxwell JA. Qualitative Research Design: An Interactive Approach. Sage Publications; 2004. [Google Scholar]
- Miles M, Huberman A. Qualitative Data Analysis: A sourcebook of new methods. Beverly Hills, CA: Sage; 1984. [Google Scholar]
- Pinkerton SD, Holtgrave DR, Galletly CL. Infections prevented by increasing HIV serostatus awareness in the United States, 2001 to 2004. J Acquir Immune Defic Syndr. 2008;47(3):354–357. doi: 10.1097/QAI.0b013e318160d57e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif S, Geonnotti KL, Whetten K. HIV Infection and AIDS in the Deep South. Am J Public Health. 2006;96(6):970–973. doi: 10.2105/AJPH.2005.063149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samet JH, Freedberg KA, Savetsky JB, Sullivan LM, Stein MD. Understanding delay to medical care for HIV infection: the long-term non-presenter. Aids. 2001;15(1):77–85. doi: 10.1097/00002030-200101050-00012. [DOI] [PubMed] [Google Scholar]
- Sanchez T, Finlayson T, Drake A, Behel S, Cribbin M, Dinenno E, et al. Human immunodeficiency virus (HIV) risk, prevention, and testing behaviors--United States, National HIV Behavioral Surveillance System: men who have sex with men, November 2003-April 2005. MMWR Surveill Summ. 2006;55(6):1–16. [PubMed] [Google Scholar]
- Schackman BR, Gebo KA, Walensky RP, Losina E, Muccio T, Sax PE, et al. The lifetime cost of current human immunodeficiency virus care in the United States. Med Care. 2006;44(11):990–997. doi: 10.1097/01.mlr.0000228021.89490.2a. [DOI] [PubMed] [Google Scholar]
- Ulin P, Robinson E, Tolley E. Qualitative Methods in Public Health: A Field Guide for Applied Research. San Francisco, CA: Jossey-Bass; 2005. [Google Scholar]
- Whetten K, Reif S. Overview: HIV/AIDS in the deep south region of the United States. AIDS Care. 2006;18(Suppl 1):S1–5. doi: 10.1080/09540120600838480. [DOI] [PubMed] [Google Scholar]