Abstract
Tumor-specific CD8 T cell peripheral tolerance occurs through clonal deletion, suppression, and the induction of anergy and can limit the generation of anti-tumor immunity. Several groups have demonstrated that prostate cancer can render tumor-specific CD8 T cells anergic, suggesting reversing tumor-induced anergy may greatly augment anti-tumor immunity. Recent work has demonstrated that signaling through the OX40 (CD134) co-stimulatory receptor, a member of the TNFR super-family, can augment CD4 and CD8 T cell expansion, differentiation, and the generation of memory cells. However, whether OX40 ligation can reverse CD8 T cell anergy, and more specifically, tumor-induced CD8 T cell anergy, remains unclear. In the current study, we demonstrate that OX40 ligation can reverse CD8 T cell anergy to a prostate-specific self-antigen in non-tumor bearing hosts. Furthermore, OX40 engagement reversed tumor-specific CD8 T cell anergy and restored the proliferative capacity of tumor-reactive CD8 T cells, which attenuated tumor growth and enhanced the survival of tumor-bearing hosts. These data demonstrate that OX40 ligation can rescue the function of anergic self or tumor-reactive CD8 T cells in vivo and suggests that OX40-mediated therapy may provide a novel means of boosting anti-tumor immunity by restoring the responsiveness of previously anergic tumor-specific CD8 T cells.
Keywords: CD8 T cells, OX40, co-stimulation, anergy
Introduction
In order to prevent the onset of autoimmune disease, potentially auto-reactive CD8 T cells are eliminated or suppressed via the induction of central (thymic) and peripheral tolerance. CD8 T cell peripheral tolerance can be achieved through a variety of mechanisms including clonal deletion, immunosuppression, and the induction of anergy, which renders auto-reactive T cells non-responsive to TCR stimulation [1–4]. Anergy can be induced following the provision of strong TCR stimulation in the absence of co-stimulatory signals or as the result of continual TCR stimulation, such as in response to a systemically expressed self-Ag or as a consequence of a chronic infection [4, 5]. Importantly, tumors can also induce CD8 T cell anergy as a means of evading and/or suppressing tumor-specific responses [6–10]. Recent work in patients with prostate cancer has shown that tumors do limit T cell responsiveness via the induction of anergy [7, 9, 11]. Therefore, novel therapeutic strategies that reverse CD8 T cell anergy in vivo may augment tumor-specific immunity and promote tumor regression.
Extensive research has demonstrated that signaling through members of the TNFR super-family, including OX40 (CD134), 4-1BB (CD137), CD27, and GITR can greatly enhance T cell differentiation and survival [12–14]. Specifically, our laboratory and others have focused on the mechanisms by which signaling through OX40 enhances both CD4 and CD8 T cell responses. OX40 is typically expressed on both CD4 and CD8 T cells shortly (24–48 hours) after TCR stimulation. OX40 engagement via its endogenous ligand, OX40L, or a variety of OX40 agonists has been shown to enhance CD4 and CD8 T cell expansion, effector differentiation, survival, and, more recently, ameliorate regulatory T cell-mediated suppression [15–24]. OX40 expression has been detected on CD4 and CD8 T cells isolated from the tumor-draining lymph nodes and tumors of tumor-bearing mice and cancer patients; thus OX40 represents a potential target for cancer immunotherapy [25–27]. Indeed, OX40 ligation can significantly boost T cell-mediated anti-tumor immunity against a variety of tumor types including sarcoma, colon carcinoma, breast cancer, and prostate cancer [16, 28, 29]. Based upon the potent anti-tumor effects of OX40-mediated therapy observed in these pre-clinical studies, we recently initiated a phase I clinical trial with an anti-human OX40 mAb for the treatment of patients with cancer [12, 30].
In addition to enhancing CD4 and CD8 T cell priming to either soluble or tumor-associated antigens, OX40-mediated co-stimulation has been shown to overcome CD4 T cell peripheral tolerance induced by soluble peptide or a constitutively-expressed self-Ag [31, 32]. However, whether OX40 ligation can reverse CD8 T cell anergy to a self- or tumor-associated antigen is unknown. Therefore, the current study tests the hypothesis that OX40 ligation will restore the function of CD8 T cells that were rendered anergic to either a self- or tumor-associated Ag. Our results demonstrate an important role for OX40 ligation in the reversal of CD8 T cell anergy in vivo.
Results
Recognition of a prostate-specific self-Ag promotes CD8 T cell peripheral tolerance
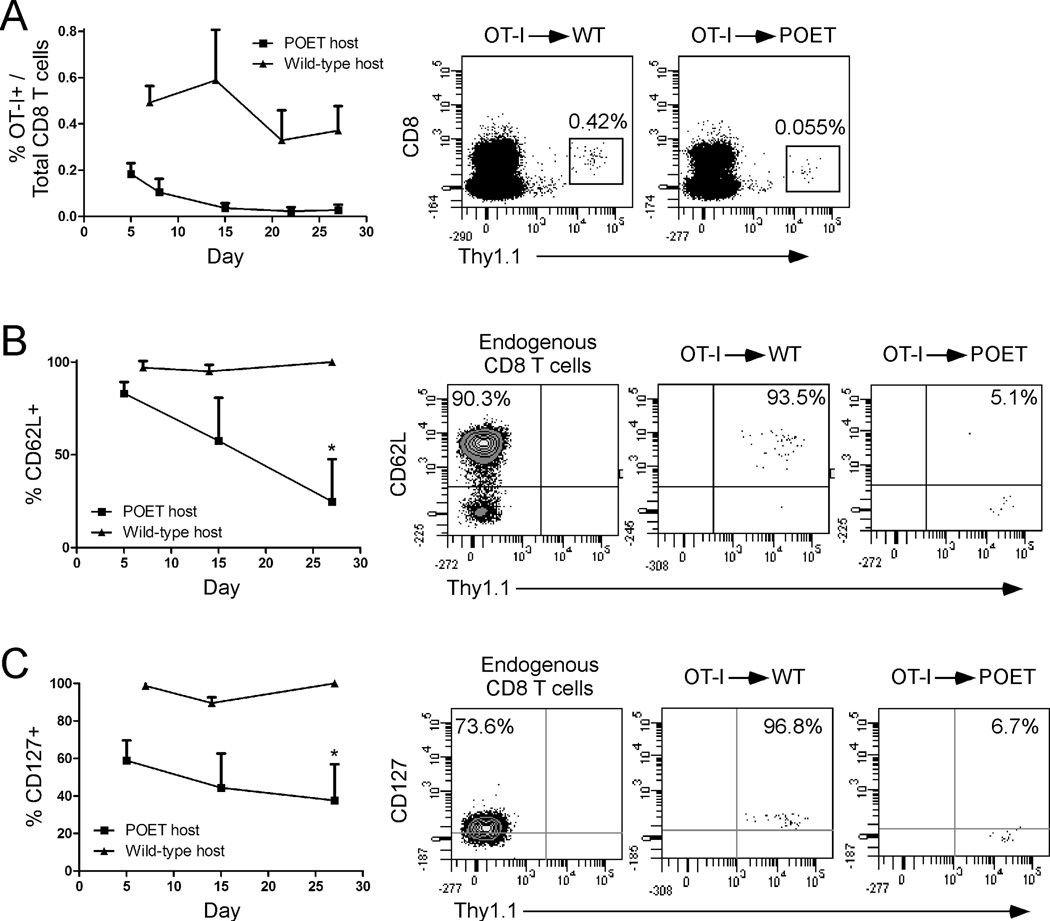
To test the hypothesis that OX40 ligation could restore the function of CD8 T cells that were rendered anergic to a self-Ag in vivo we first examined the fate of Ag-specific CD8 T cells undergoing peripheral tolerance in non-tumor bearing mice. Naïve OT-I CD8 T cells were adoptively transferred into wild-type or prostate ovalbumin-expressing transgenic (POET) mice, in which prostate-specific expression of membrane-bound OVA (mOVA) is driven in an androgen-dependent manner under the control of the rat probasin promoter [33]. Following adoptive transfer, low levels of donor OT-I T cells were detected (~0.2% of total CD8 T cells, day 5) and then underwent contraction until only a small stable pool of Ag-specific CD8 T cells remained in the peripheral blood (Fig. 1A) [33]. Similar low levels of donor CD8 T cells were detected in the lymph nodes, prostate-draining lymph nodes, and spleen, but not in peripheral organs, such as the prostate tissue indicating that the tolerized cells were not trafficking into non-lymphoid organs (data not shown). In contrast, CD8 T cells transferred into wild-type mice remained at a relatively stable level over time (~0.5% of total CD8 T cells, Fig. 1A). CD8 T cells transferred into wild-type mice retained a naïve CD62Lhigh phenotype (Fig. 1B), while cells transferred into POET hosts acquired an activated CD62Llow and CD127low (IL-7Rα) phenotype by 27 days post-transfer (PBL, spleen, and LN) (Figs. 1B, 1C, and data not shown), suggesting that these cells had encountered cognate Ag in vivo. Consistent with other models of CD8 T cell peripheral tolerance, CD8 T cells undergoing tolerance in POET recipients remained CD25neg (data not shown) [33–35].
Figure 1. Recognition of a prostate-specific self-Ag promotes CD8 T cell peripheral tolerance.
Naïve OT-I CD8 T cells were adoptively transferred into POET or wild-type hosts. At the indicated time points the A) total number of donor CD8 T cells in the peripheral blood and their expression of B) CD62L and C) CD127 was determined. Dot plots depict the phenotype of endogenous (Thy1.1−) and/or donor (Thy1.1+) CD8 T cells in the peripheral blood (day 27 post-transfer) of individual mice, while graphs depict the mean + SD of 15–20 (POET) or 4–6 (wild-type) mice per group from one of three independent experiments with similar results (*, p<0.05).
OX40 ligation reverses CD8 T cell anergy to a prostate-specific self-Ag
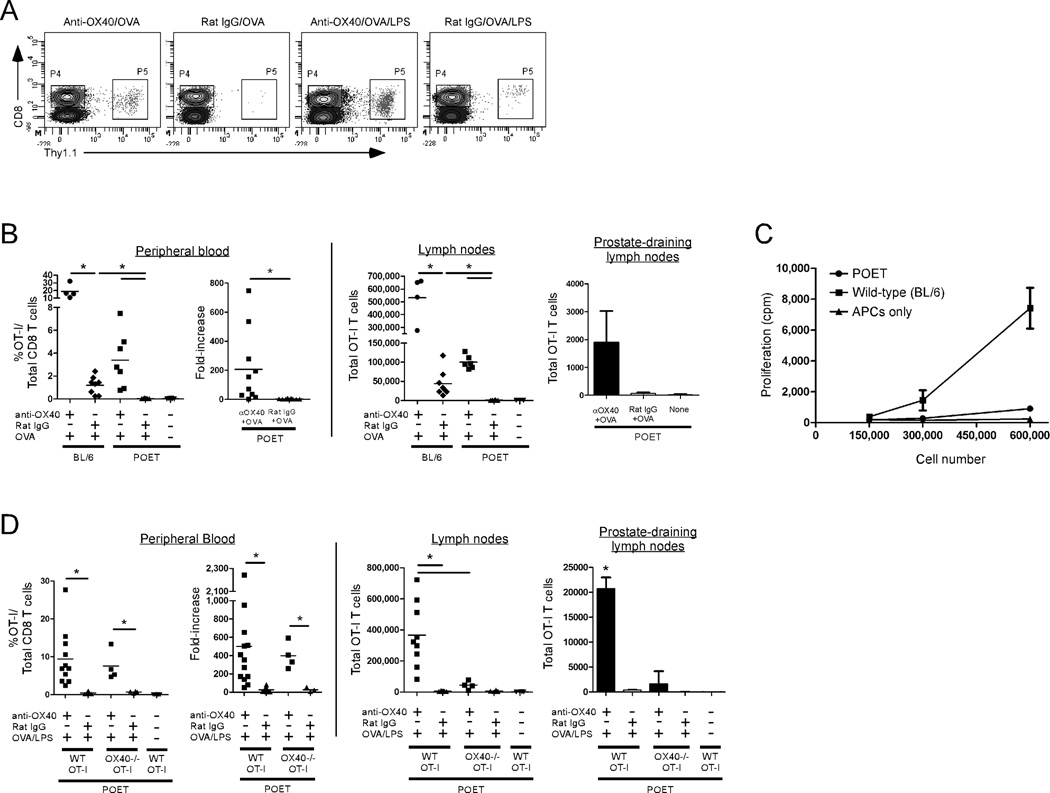
Since one of the hallmarks of anergy is the inability of T cells to proliferate following TCR-mediated stimulation, we determined whether the recognition of a prostate-specific self-Ag induced CD8 T cell anergy by assessing the proliferative response of donor CD8 T cells following Ag-specific re-stimulation in vivo. Twenty-eight days after adoptive transfer into wild-type or POET Tg hosts, donor CD8 T cells were re-stimulated with Ag (soluble OVA) along with an agonist anti-OX40 or control (rat IgG) Ab. Seven days later, the extent of donor CD8 T cell proliferation in the peripheral blood, lymph nodes, and prostate-draining lymph nodes was assessed. Ag-specific CD8 T cells expanded in wild-type (BL/6) hosts following TCR stimulation (rat IgG/OVA), which was enhanced in the presence of OX40 ligation (Fig. 2B). However, stimulation with Ag alone was not sufficient to promote CD8 T cell expansion in POET recipients (Figs. 2A, 2B). Similarly, donor cells harvested from wild-type, but not POET hosts, could proliferate in response to Ag-specific re-stimulation in vitro indicating that the cells were indeed rendered anergic in POET mice (Fig. 2C).
Figure 2. OX40 ligation reverses CD8 T cell anergy to a prostate-specific self-Ag in vivo.
Naïve wild-type or OX40-/- OT-I CD8 T cells were adoptively transferred into POET or wild-type mice (from Fig. 1). Twenty-eight days later, the donor cells were re-stimulated with anti-OX40 or rat IgG (control) Ab and soluble OVA in the presence or absence of LPS. Seven days later, the extent of donor cell expansion and accumulation in the peripheral blood, lymph nodes, and prostate-draining lymph nodes was determined. A) Representative plots depicting the detection of donor Thy1.1+CD8+ T cells in POET hosts by flow cytometry. B) The expansion and fold-increase (pre versus post re-stimulation) (PBL), and total number (LN, prostate-draining LN) of donor OT-I CD8 T cells was determined by flow cytometry following re-challenge with anti-OX40 or control (rat IgG) Abs and soluble OVA. C) Four days after re-stimulation with soluble OVA in vivo donor OT-I CD8 T cells were harvested from the LN of WT or POET recipients and the proliferative capacity of the indicated number of total LN cells was determined in vitro following Ag-specific re-stimulation with peptide-pulsed APCs. D) The donor CD8 T cell response was assessed as in (B) following re-challenge with anti-OX40 or control Abs, soluble OVA, and LPS. Graphs depict the results obtained from individual animals (PBL and lymph nodes) or pooled from 4–5 mice per group (prostate-draining LN) from one of two to three independent experiments with similar results (*, p<0.05).
In contrast, OX40 ligation plus Ag-specific TCR stimulation resulted in a significant increase in donor CD8 T cell accumulation in the peripheral blood and lymph nodes of POET hosts compared to control-treated cells (Fig. 2B), demonstrating that OX40 engagement could restore the proliferative capacity of the anergic CD8 T cells. Notably, the difference in the total expansion of donor OT-I T cells in wild-type versus POET hosts following anti-OX40/OVA treatment (Fig. 2B) reflects differences in the initial pool of responding cells as only a small pool (<0.06% of total CD8 T cells) of anergic donor OT-I T cells remains in POET hosts (see Fig. 1A). TCR-mediated signaling was critical for the OX40-dependent reversal of CD8 T cell anergy as anti-OX40 alone was not sufficient to promote the proliferation of Ag-specific CD8 T cells (data not shown). We also sought to exclude the possibility that the OX40-mediated reversal of anergy in POET hosts was due to the presence of any residual donor CD8 T cells that were not rendered anergic. Due to the low precursor frequency of anergic donor OT-I T cells in POET hosts (~0.05% of the total CD8 T cells), we were unable to obtain sufficient numbers of anergic cells for adoptive transfer. Instead, CFSE-labeled naïve OT-I T cells were adoptively transferred into wild-type hosts and then the cells were activated with soluble OVA. Four days later, the activated (dividing) cells (based upon the dilution of CFSE) were sorted and then transferred into POET hosts. Four weeks later, the extent of donor OT-I T cell anergy was assessed following Ag-specific stimulation with soluble OVA in the presence anti-OX40 or control Ab. Importantly, the donor OT-I T cells were unable to proliferate in response to Ag stimulation, while the addition of anti-OX40 restored the proliferative capacity of the anergic OT-I T cells (data not shown).
Since previous studies have demonstrated a potent synergistic effect on CD4 T cells when OX40 ligation is combined with an adjuvant (LPS) [22, 36], we examined whether the addition of LPS would further augment the OX40-mediated reversal of CD8 T cell anergy. Donor CD8 T cells were adoptively transferred into POET hosts (as described above) and then, 28 days later, the anergic CD8 T cells were re-challenged in vivo with anti-OX40 or rat IgG, soluble OVA, and LPS. Seven days after re-stimulation, the extent of donor CD8 T cell expansion in the peripheral blood, lymph nodes, and prostate-draining lymph nodes was assessed. Anti-OX40/OVA/LPS treatment induced a potent proliferative response and greatly augmented the expansion and survival of the anergic CD8 T cells compared to control-treated cells (Fig. 2D). The efficacy of anti-OX40 required direct ligation of OX40 on the responding CD8 T cells as significantly reduced numbers of OX40-/- OT-I T cells were detected in the lymph nodes and prostate-draining lymph nodes compared to wild-type OT-I T cells (Fig. 2D). Anti-OX40/OVA/LPS treatment also led to a more robust response than anti-OX40/OVA stimulation alone (note change in scale, total cell number, Figs. 2D vs. 2B) likely due to the pro-inflammatory signals induced by the presence of LPS. One of the pro-inflammatory signals induced by LPS, IL-12, did not appear to be required for synergistic effects of anti-OX40/OVA/LPS signaling as blocking IL-12 with an anti-IL-12 mAb had no effect on the extent of OT-I T cell expansion (data not shown).
OX40 engagement promotes the differentiation of anergic CD8 T cells into effector CTL
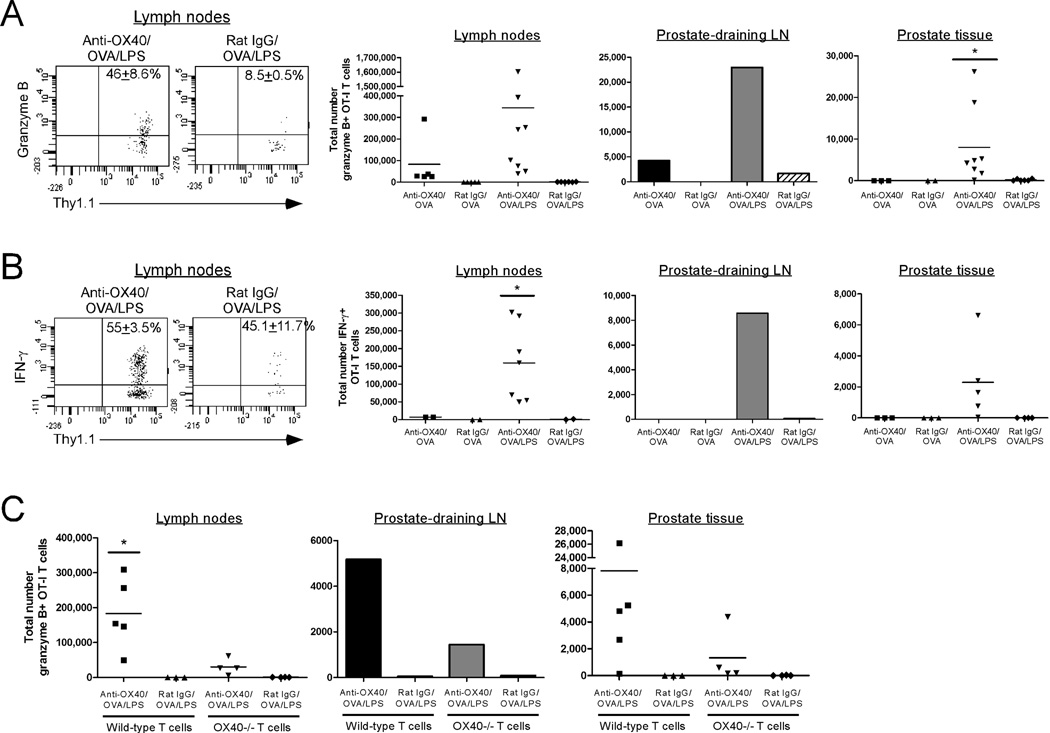
We and others have demonstrated that OX40 ligation can augment the differentiation of naïve CD8 T cells into effector CTL [16, 37, 38]. In particular, OX40 ligation greatly augments granzyme B (grzB) expression in CD8 T cells, which strongly correlates with increased cytolytic activity [16, 38]. Since OX40 ligation restored the proliferative capacity of anergic CD8 T cells in POET hosts (Fig. 2), we sought to determine whether OX40-mediated co-stimulation would also facilitate their differentiation into effector CTL. Anergic OT-I CD8 T cells in POET hosts were re-stimulated with anti-OX40 or rat IgG along with OVA in the presence or absence of LPS and then 7 days later, the lymph nodes, prostate-draining lymph nodes, and prostate tissue were harvested and the expression of grzB and IFN-γ was assessed.
OX40 ligation in combination with OVA/LPS induced a striking increase in the percent and number of grzB+ OT-I T cells as compared to rat IgG/OVA/LPS alone (Fig. 3A). Importantly, anti-OX40/OVA/LPS-mediated signaling also led to a significant increase in the localization and accumulation of grzB+ donor CD8 T cells in the prostate-draining lymph nodes and prostate tissue itself as compared to control-treated cells (Fig. 3A), demonstrating that OX40 ligation in the presence of LPS promotes trafficking of effector cells into the target organ. Similarly, anti-OX40/OVA/LPS treatment significantly enhanced the accumulation of IFN-γ+ OT-I CD8 T cells in the peripheral lymph nodes, prostate-draining lymph nodes, and prostate tissue (Fig. 3B). It should be noted that the potent effects of OX40 ligation required OX40-specific signaling on the responding donor CD8 T cells as the accumulation of granzyme B+ OX40-/- OT-I T cells was greatly reduced as compared to wild-type cells (Fig. 3C). Consistent with our previous data, anti-OX40 treatment partially enhanced the response of OX40-/- OT-I T cells (Fig. 3C), which has been shown to occur via OX40 ligation of the host CD4 T cells [16].
Figure 3. OX40 engagement augments the differentiation of anergic CD8 T cells.
Naive OT-I CD8 T cells were adoptively transferred into POET mice. Twenty-eight days later, the donor cells were re-stimulated with anti-OX40 or control Abs and soluble OVA in the presence or absence of LPS. Seven days later the extent of A) granzyme B or B) IFN-γ production by the donor CD8 T cells in the lymph nodes, prostate-draining lymph nodes, and prostate was assessed. C) Wild-type or OX40-/- OT-I T cells were adoptively transferred into POET mice. Twenty-eight days later, the donor cells were re-stimulated with anti-OX40 or control Abs, soluble OVA, and LPS. Seven days later the extent of granzyme B production by the donor CD8 T cells was assessed as in (A). Dot plots depict the expression of the indicated molecules on donor CD8 T cells isolated from the lymph nodes of POET mice (mean ± SD), while graphs show the results obtained from individual animals (lymph nodes, prostate tissue) or pooled from 4–5 mice per group (prostate-draining LN) from one of two to three independent experiments with similar results (*, p<0.05).
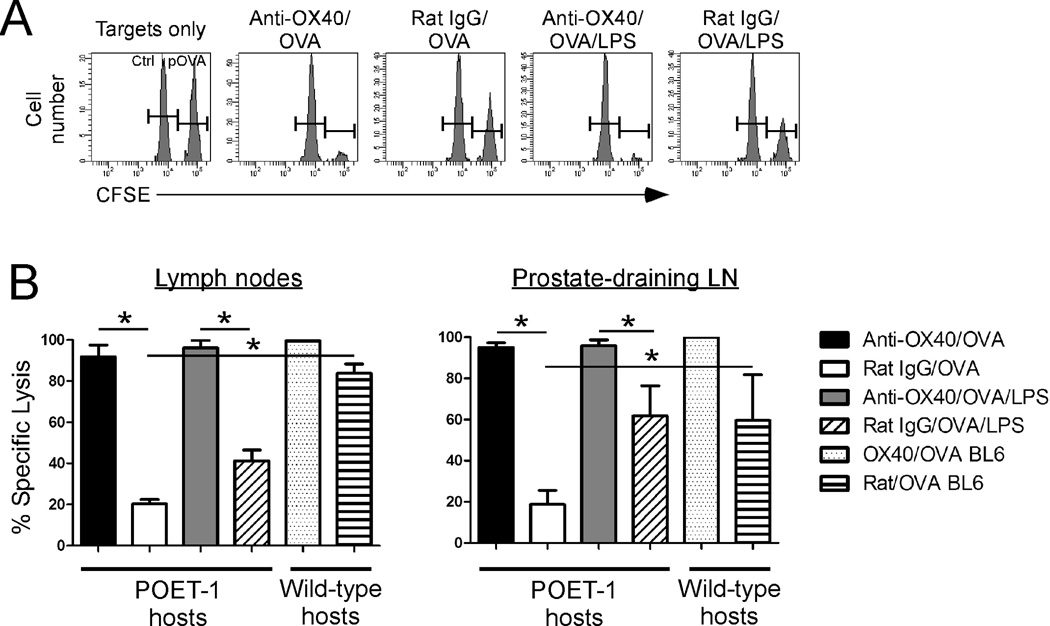
In addition to monitoring the extent of cytokine production, we also used an in vivo cytolytic assay to test whether OX40 ligation enhanced the cytolytic function of the anergic CD8 T cells. A pool of anergic donor CD8 T cells was established in POET mice (as in Fig. 1) and then the donor cells were re-stimulated with anti-OX40 or rat IgG and soluble OVA in the presence or absence of LPS. Six days later, mice received a mixture of cognate peptide-pulsed or non-pulsed splenocytes that were differentially labeled with high or low levels of the fluorescent label, CFSE, respectively. The next day, the peripheral and prostate-draining lymph nodes were harvested and the extent of target cell lysis, shown by a loss of peptide-pulsed (CFSEhigh) cells, was determined by flow cytometry (Fig. 4). Consistent with the previously observed OX40-mediated increase in granzyme B expression (Fig. 3A), OX40 ligation significantly augmented the cytolytic function of the anergic CD8 T cells compared to control-Ig treated cells, both in terms of the ratio of target cells eliminated (Figs. 4A) and in the extent of specific lysis (Fig. 4B).
Figure 4. OX40 ligation restores the cytolytic function of anergic CD8 T cells.
Naive OT-I CD8 T cells were adoptively transferred into wild-type or POET mice. Twenty-eight days later, the donor cells were re-stimulated with anti-OX40 or control Abs and soluble OVA in the presence or absence of LPS. Seven days later, a 1:1 mixture of peptide-pulsed (CFSEhigh) and non-pulsed (CFSElow) target cells was injected into recipient mice and then, four hours later, the extent of target cell lysis in the lymph nodes or prostate-draining lymph nodes was determined. A) Representative histograms depicting the detection of target cells in the lymph nodes of individual POET mice. B) Data represent the extent of specific lysis in the lymph nodes or prostate-draining lymph nodes. Graphs depict the mean ± SD of 3–4 mice per group (lymph nodes) or pooled from 3-4 mice per group (prostate-draining lymph nodes) from one of two independent experiments with similar results (*, p<0.05).
Since OX40 ligation reversed anergy and augmented the differentiation of CD8 T cells isolated from the lymph nodes and prostate tissue (Figs. 3, 4), we sought evidence of autoimmunity by assessing the extent of lymphocyte infiltration and tissue-specific damage in the prostate. Twenty-eight days after their adoptive transfer into POET hosts, anergic CD8 T cells were re-stimulated with anti-OX40 or rat IgG Abs, soluble OVA, and LPS. Seven days later, the prostate was harvested for histological analysis. Although OX40 ligation did not appear to induce autoimmune prostatitis, OX40-mediated therapy did result in increased turnover of prostate epithelial cells as well as increased lymphocyte infiltration in the prostate tissue (Figs. 5A, 5B, respectively) as compared to control-Ig treated mice. These data demonstrate that OX40-specific signaling could reverse CD8 T cell anergy to a prostate tissue-specific self-Ag and promote the differentiation of Ag-specific CD8 T cells into cytolytic effector cells capable of trafficking into the target organ, the prostate.
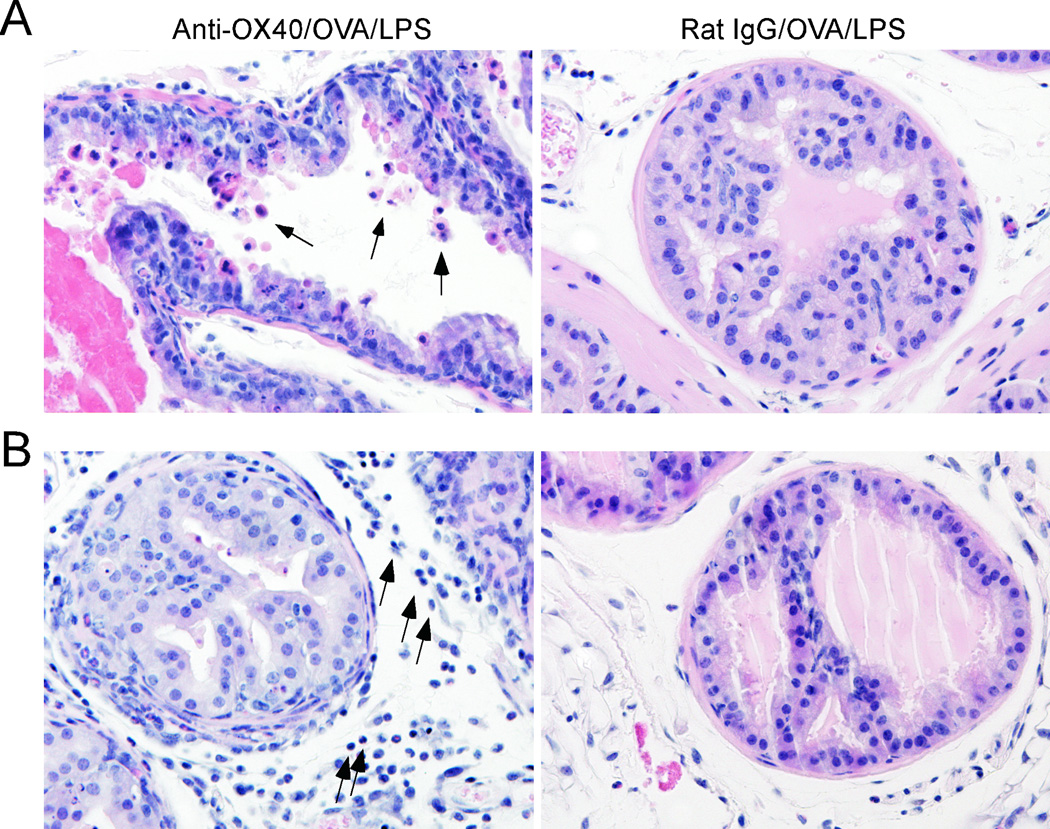
Figure 5. OX40-mediated signaling augments lymphocyte infiltration into the prostate.
OT-I CD8 T cells were adoptively transferred into POET mice. Twenty-eight days later, the donor cells were re-stimulated with anti-OX40 or control Abs along with OVA/LPS. Seven days later, the mice were sacrificed and the prostate was harvested for histology. A) OX40-mediated reversal of Ag-specific CD8 T cell anergy induces turnover of prostate epithelial cells (arrows). B) Accumulation of lymphocytes in the prostate tissue (arrows) following OX40 engagement. Data are representative of 2–4 mice per group from one of two independent experiments with similar results. Original magnification: x400.
OX40 ligation reverses tumor-specific CD8 T cell anergy
Thus far, we have demonstrated that OX40 ligation can reverse CD8 T cell anergy to a prostate-specific self-Ag. Given the striking increase in the accumulation of effector CD8 T cells in both the lymph nodes and prostate (Figs. 2–5), we sought to determine whether OX40-mediated signaling could also reverse tumor-specific CD8 T cell anergy in vivo. TRAMP-C1 prostate tumor cells modified to express membrane-bound OVA as a surrogate tumor-associated Ag [16] were injected into POET mice. Approximately 21 days later, tumor-bearing mice (~40–50 mm2 tumors) received Ag-specific OT-I CD8 T cells. Previous work from our laboratory has demonstrated that the adoptive transfer of tumor-reactive CD8 T cells into tumor-bearing POET mice fails to induce complete tumor regression [16]. Additional studies revealed that the failure to control tumor growth was not due to the outgrowth of an Ag-loss variant or resistance to CD8 T cell-mediated lysis (data not shown). Rather, it appeared that the inability to mediate complete tumor regression might be due to the induction of CD8 T cell anergy.
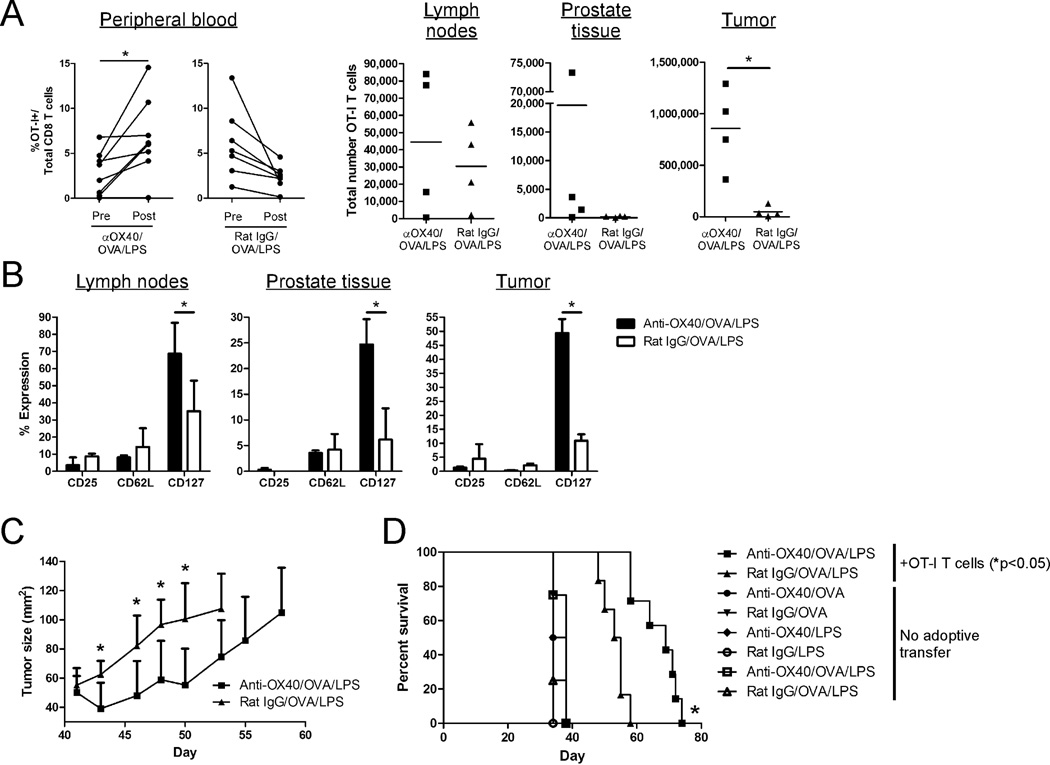
To test this hypothesis, 3 weeks after their adoptive transfer into tumor-bearing POET hosts, the donor CD8 T cells were re-stimulated with anti-OX40 or rat IgG along with OVA/LPS. The tumor-reactive donor CD8 T cells were rendered anergic as Ag-specific challenge in the absence of OX40 engagement did not induce proliferation (Fig. 6A, peripheral blood). In contrast, OX40 ligation restored the proliferative capacity of the anergic Ag-specific CD8 T cells (Fig. 6A, peripheral blood) and led to a significant accumulation of tumor-reactive CD8 T cells at the tumor site (Fig. 6A, tumor). OX40-stimulated tumor-specific CD8 T cells also exhibited a significant increase in CD127 expression (Fig. 6B), which has been associated with the ability of OX40 ligation to enhance the generation of memory CD8 T cells [37, 39]. No differences were observed in the expression of CD25 or CD62L (Fig. 6B). Most importantly, the OX40-mediated reversal of tumor-specific CD8 T cell anergy inhibited tumor growth (Fig. 6C) and enhanced the survival of tumor-bearing hosts (Fig. 6D), which was dependent upon the presence of the donor OT-I T cells. Taken together these data demonstrate that treatment with an OX40 agonist can restore the anti-tumor function of anergic tumor-reactive CD8 T cells in vivo.
Figure 6. OX40 ligation reverses tumor-specific CD8 T cell anergy and enhances the survival of tumor-bearing hosts.
TRAMP-C1-mOVA tumor cells were injected into male POET mice on day 0. Approximately twenty-one days later, tumor-bearing mice (~40 mm2 tumors) received 5×105 naïve OT-I CD8 T cells. Approximately 17 days later, donor cells in tumor-bearing mice were re-stimulated with anti-OX40 or control Abs, soluble OVA, and LPS. A) Seven days later, the extent of donor cell expansion (peripheral blood) and accumulation (lymph nodes, prostate tissue, and tumor) was determined. B) The expression of CD25, CD62L, and CD127 on the donor CD8 T cells (from (A)) was determined. C) Tumor growth was monitored every 2–3 days following re-stimulation. D) The overall survival of tumor-bearing mice that received no adoptive transfer or donor OT-I T cells (from (C)) was assessed. Graphs depict the results obtained from (A) individual animals or (B, C) mean ± SD of 4–5 mice per group from one of three independent experiments with similar results (*, p<0.05).
Discussion
Many of the same mechanisms used by the immune system to prevent the onset of autoimmune disease are often exploited by tumors to limit the generation of potent anti-tumor responses. In particular, several recent studies have demonstrated that tumors can promote the induction of CD8 T cell anergy, or hypo-responsiveness, in both tumor-bearing mice and cancer patients [6, 7, 9, 10]. Thus, it is of critical importance to develop novel therapies that can reverse tumor-specific anergy and thereby augment tumor regression in vivo. Although several studies have demonstrated that signaling through the OX40 co-stimulatory receptor can prevent the induction of peptide-specific CD4 T cell anergy as well as augment the priming of low-avidity tumor-specific CD8 T cells [31, 32, 40, 41], whether OX40 ligation can reverse CD8 T cell anergy remains unclear. Therefore, the goal of the current study was to test the hypothesis that OX40 ligation could reverse CD8 T cell anergy induced by a self-Ag or tumor in vivo.
The initial recognition of a prostate-specific self-Ag in POET hosts led to a limited proliferative response by donor Ag-specific CD8 T cells, which was followed by the establishment of a stable pool of anergic cells (Fig. 1). It should be noted that in contrast to the classical methods of anergy induction such as in vitro stimulation with anti-CD3 or peptide immunization in vivo [4], our model system provided a unique opportunity to test the ability of OX40 ligation to reverse Ag-specific CD8 T cell anergy to a self-Ag in vivo in an otherwise normal animal. Importantly, treatment with an OX40 agonist not only restored the proliferative capacity of anergic CD8 T cells, but also promoted their differentiation into cytolytic effector cells (Figs. 2–4). Although previous studies from our laboratory and others have demonstrated that OX40-mediated signaling can augment the priming of naïve CD8 T cells [16, 38], to our knowledge this is the first study to demonstrate that OX40 ligation can reverse CD8 T cell anergy to a self-Ag in vivo.
Although OX40 ligation in the presence of TCR stimulation was sufficient to restore the proliferative capacity and differentiation of anergic CD8 T cells, anti-OX40/OVA-stimulated cells were unable to traffic into the prostate tissue (Fig. 3A and data not shown). Since previous studies have shown a potent synergy between OX40 ligation and LPS [22, 36], we examined whether the addition of LPS would further enhance the efficacy of OX40-mediated therapy and promote lymphocyte infiltration into the prostate. The combination of OX40 ligation and OVA/LPS further enhanced the expansion and differentiation of the anergic CD8 T cells compared to control (rat/OVA/LPS) or anti-OX40/OVA-treated cells (Figs. 2–4). More importantly, anti-OX40/OVA/LPS-mediated therapy enabled the anergic CD8 T cells to traffic into the prostate gland, which was associated with increased turnover of the prostate epithelial cells (Figs. 3, 5A). Though the addition of LPS clearly enhances the efficacy of OX40-mediated therapy, the mechanisms by which LPS augments the response remains unclear.
Previous studies have demonstrated that LPS does not directly stimulate T cell activation [42], but rather, enhances T cell responses by stimulating the production of type I interferons (IFN-α, β) by APCs [43]. Although the precise role of IFN-α/β-dependent signaling in modulating the efficacy of combined anti-OX40/LPS-mediated therapy remains to be determined, the role of type I interferons is of particular interest since IFN-α is used for the treatment of various cancers, including renal cell carcinoma and malignant melanoma [44]. In addition to type I interferons, LPS stimulation of APCs can also trigger the production of pro-inflammatory cytokines, including IL-12 and TNF-α [2, 3]. It is unlikely that the synergy between anti-OX40 and LPS requires IL-12-dependent signaling since treatment with a blocking anti-IL-12 mAb did not abrogate the ability of anti-OX40/LPS treatment to reverse CD8 T cell anergy (data not shown). In contrast, TNF-α has been shown to up-regulate OX40 expression on CD4 T cells stimulated in vitro [45]. Thus, it is possible that the adjuvant effects of LPS are modulated through increased TNF-α production by APCs, which in turn augments and/or sustains OX40 expression on the responding CD8 T cells, thereby enhancing the efficacy of OX40-mediated therapy in vivo. Future studies will be needed to explore whether the combination of agonist anti-OX40 mAb and IFN-α or TNF-α can augment the anti-tumor response in both pre-clinical models and ultimately, cancer patients.
Numerous reports have indicated that progressively growing tumors can render T cells anergic through a variety of mechanisms, including down-regulation of the TCR-associated CD3ς chain, increased expression of TGF-β, or through metabolic suppression [7, 9, 11, 46–48]. Regardless of the precise mechanism of tumor-induced anergy in this model system, our results demonstrated that anti-OX40/OVA/LPS treatment restored the proliferative capacity of anergic tumor-reactive CD8 T cells leading to reduced tumor growth and increased long-term survival of tumor bearing hosts compared to control Ig-treated mice (Fig. 6). It should be noted that although anti-OX40/OVA/LPS-mediated therapy also enhanced trafficking of CD8 T cells into the tumor site (Fig. 6B), it was not sufficient to completely eradicate the tumors (Fig. 6D). Since the mice had relatively large (~50 mm2) and established (>40 days post-tumor implantation) tumors prior to treatment, it is possible that the cytolytic activity of the tumor-infiltrating CTL was partially suppressed by the presence of additional tumor-derived factors, such as TGF-β, which is known to block the cytolytic function of CTL [48]. We are currently investigating whether TGF-β blockade will further enhance the anti-tumor efficacy of OX40/LPS-mediated therapy in vivo.
Direct ligation of the OX40 co-stimulatory receptor on Ag-specific CD8 T cells is required to promote their maximal expansion and differentiation (Fig. 3C) [16, 38]. OX40 signaling can also abrogate the suppressive activity of FoxP3+CD25+CD4+ regulatory T cells (Treg) [15, 24, 49, 50], although some reports suggest that OX40 ligation does directly affect the suppressive activity of Treg, but rather augments anti-tumor immunity by increasing the ratio of Teff/Treg at the tumor site [51]. Whether anti-OX40 also affects the suppressive activity of Treg in normal or tumor-bearing POET mice remains to be determined, however our data clearly demonstrates that direct OX40 engagement on the anergic donor CD8 T cells was necessary for their optimal expansion and differentiation in vivo (Figs. 2D, 3C).
In summary, these data demonstrate a novel role for OX40 ligation in reversing CD8 T cell anergy to a self- or tumor-specific Ag. OX40-mediated therapy restored the proliferative capacity of anergic CD8 T cells in non-tumor bearing hosts and enhanced their differentiation into effector CTL. Furthermore, OX40 ligation in the presence of cognate Ag and LPS was able to restore the function of anergic tumor-reactive CD8 T cells and increase the survival of tumor-bearing hosts. It will be of interest to explore whether the combination of OX40 ligation along with TCR stimulation with autologous or allogeneic tumor and adjuvants, such as IFN-α or various TLR ligands, will similarly restore the function of anergic tumor-reactive CD8 T cells and promote tumor regression in cancer patients.
Materials and Methods
Mice
Wild-type C57BL/6 mice were purchased from Jackson Labs (Bar Harbor, ME). OT-I Thy1.1 TCR Tg, (Prostate ovalbumin expressing transgenic) POET-1 Tg, and OX40-/- OT-I TCR Tg mice were kindly provided by Dr. Charles Surh (The Scripps Research Institute, La Jolla, CA), Dr. Timothy Ratliff (Purdue Cancer Center, West Layfayette, IN), and Dr. Michael Croft (La Jolla Institute for Allergy and Immunology, La Jolla, CA), respectively. All mice were bred and maintained under specific pathogen-free conditions in the Providence Portland Medical Center animal facility. Experimental procedures were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Adoptive transfer and re-stimulation of anergic OT-I T cells
Single cell suspensions were prepared from the lymph nodes and spleens of OT-I Thy1.1 TCR Tg mice. Cell suspensions were depleted of CD4+, CD11b+, CD45R+, DX5+, and Ter-119+ cells using the MACS CD8α+ T cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany). OT-I T cells were purified by negative selection per the manufacturer’s instructions and had a naïve phenotype (CD25-negative, CD44low, CD62Lhi, and CD69-negative) as indicated by flow cytometry (data not shown). 5×105 naïve OT-I T cells were injected i.v. in 200 µl of PBS into recipient mice.
At the indicated time points, recipient mice received 500 µg of soluble ovalbumin (Sigma, St. Louis, MO), 50 µg of anti-OX40 (OX86) or isotype control rat IgG Ab (Sigma), and/or 10 µg bacterial lipopolysaccharide (LPS) (Sigma) s.c. Mice received an additional dose (50 µg) of anti-OX40 or control Ab one day later.
Lymphocyte isolation and analysis
Peripheral (axillary, brachial, and inguinal) or prostate-draining (peri-aortic lumbar) lymph nodes were harvested and processed to obtain single cell suspensions. ACK lysing buffer (Lonza, Walkersville, MD) was added for 5 min at RT to lyse red blood cells. Cells were then rinsed with RPMI 1640 medium (Lonza) containing 10% FCS (10% cRPMI) (Gemini Bio-Products, Calabasas, CA) supplemented with 1M HEPES, non-essential amino acids, sodium pyruvate, and pen-strep glutamine (Lonza).
To obtain peripheral blood lymphocytes, peripheral blood was collected via the tail vein into tubes containing 50 µl heparin (Hospira, Lake Forest, IL). One ml of flow cytometry buffer (0.5% FBS, 0.02% NaN3 in PBS) was added, the cells were thoroughly mixed, and then 700 µl of Ficoll-Paque (GE Healthcare, Piscataway, NJ) was added prior to centrifugation. Lymphocytes were collected from the interface and then washed with flow cytometry buffer prior to staining.
For analysis by flow cytometry, cells were incubated for 30 min on ice with a combination of the following antibodies: Thy1.1-FITC, Thy1.1-Pacific Blue, CD122-PE, CD8α-PE-Texas Red, CD25-APC, CD62L-PE-Cy7, CD127-Alexa Fluor-750, CD4-Pacific Blue, CD4-Pacific Orange, and Granzyme B-PE. Dead cells were excluded by adding 300 µl of 7-AAD (5 µg/ml) (Invitrogen, Carlsbad, CA) to each sample 5 min prior to analysis. All antibodies were obtained from eBioscience, BD Pharmingen, or Invitrogen. For intracellular granzyme B staining, cells were fixed in 1% paraformaldehyde for 20 min on ice, rinsed 1X with flow cytometry buffer, and then permeabilized with 1X Permwash (BD Pharmingen, San Diego, CA) for 20 min on ice. Cells were analyzed with an LSR II flow cytometer using FACSDiva software (BD Biosciences, Mountain View, CA).
To measure antigen-specific cytokine production, lymphocytes were incubated in 10% cRPMI with 5 µg/ml of the OVA (SIINFEKL) peptide (Anaspec, San Jose, CA) and 1 µl/ml of brefeldin A containing Golgi-Plug solution (BD PharMingen) for 5 h at 37 °C. After washing, cells were stained to detect CD4, CD8, and Thy1.1 as described above. Cells were then permeabilized and stained with anti-IFN-γ-APC mAb using the Cytofix/Cytoperm Plus kit (BD PharMingen) according to the manufacturer’s instructions.
In vivo cytolytic assays
Syngeneic spleen cells were labeled by incubation for 10 minutes at 37 °C with either 5 µM CFSE in PBS (CFSEhigh cells) or 0.5 µM CFSE in PBS (CFSElow cells) and washed twice with HBSS. CFSEhigh cells were pulsed with OVA peptide at 5 µg/ml for 1 h at 37 °C. CFSElow cells were pulsed with no peptide and served as an internal control. A mixture of 5×106 CFSEhigh peptide-pulsed cells plus 5×106 CFSElow non-pulsed cells were injected i.v. into recipient mice. Peripheral or prostate-draining lymph nodes were harvested 16 hrs later and single cell suspensions were analyzed for detection and quantification of CFSE-labeled cells by flow cytometry. The percent specific lysis was calculated as 100-(100*((CFSElow/CFSEhigh control group) / (CFSElow/CFSEhigh experimental group)).
Proliferation Assay
Donor OT-I T cells were adoptively transferred into wild-type (BL/6) or POET Tg hosts. Four weeks later, donor cells were re-stimulated with soluble OVA in vivo. Four days later, cells were harvested from the lymph nodes of recipient mice and the indicated numbers of cells were stimulated for 72 h with irradiated syngeneic splenocytes that were pulsed with pOVA (SIINFEKL). During the last 18 h of culture, the plates were pulsed with 1 µCi of [3H]thymidine. The cells were harvested on a Tomtec 96-well plate harvester and thymidine incorporation was counted on a Trilux 1450 microbeta liquid scintillation counter (Wallac, Gaithersburg, MD).
Tumor challenge
TRAMP-C1 prostate tumor cells were modified to express membrane-bound OVA (mOVA) as previously described [16]. 2.5x106 TRAMP-C1-mOVA (TC1-OVA) cells were injected s.c. (day 0) into male POET Tg mice. When tumors reached ~40–50 mm2 (approximately 21–24 days after tumor inoculation), mice received either 5×105 wild-type or OX40-/- OT-I Thy1.1 T cells. Approximately 23 days after CD8 T cell adoptive transfer, donor cells in tumor-bearing mice were re-challenged with soluble OVA, anti-OX40 or control Ab, and/or LPS s.c. as described above. Tumor growth (area) was assessed every 2–3 days with micro-calipers and mice were sacrificed when tumors reached >150 mm2.
Isolation of tissue infiltrating lymphocytes
Prostate tissue or tumor infiltrating lymphocytes (TIL) were harvested by dissection of tissue into small fragments followed by digestion in 1 mg/ml collagenase (Invitrogen), 100 µg/ml hyaluronidase (Sigma), and 20 mg/ml DNase (Sigma) in PBS for 60 min. Following filtration through nylon mesh, donor OT-I T cells were stained as described above and analyzed by flow cytometry.
Histology
Prostates were harvested and fixed overnight in 10% (v/v) formalin solution (Sigma, St. Louis, MO) and processed for paraffin embedding. Paraffin-embedded tissue was cut using a microtome and sections were placed onto saline-coated Superfrost slides for processing (Fisher Scientific, Pittsburgh, PA). After de-paraffinizing tissue, serial sections were stained with eosin and then counterstained with Mayer’s hematoxylin (Sigma).
Statistical Analysis
Statistical significance was determined by unpaired student t-test (for comparison between 2 groups), one-way ANOVA (for comparison among 3 or more groups), or Kaplan-Meier survival (for tumor survival studies) using GraphPad InStat or Prism software (GraphPad, San Diego, CA); a p-value of <0.05 was considered significant.
Acknowledgements
We thank Drs. Carl Ruby and Walter Urba for helpful discussions and critical reading of the manuscript, Mr. Dan Haley for his expertise with flow cytometry, and Dr. Christopher Corless for assistance with analysis of histology. This work was supported by an American Cancer Society – Sam E. and Kathleen Henry post-doctoral fellowship, a Prostate Cancer Foundation Young Investigator Award (W.L.R.), and grants from the M.J. Murdock Charitable Trust and the NIH (A.D.W.).
Footnotes
Financial/Commercial Disclosures
Dr. Weinberg has patents pending for the use of OX40 agonists in patients with cancer.
References
- 1.Redmond WL, Sherman LA. Peripheral tolerance of CD8 T lymphocytes. Immunity. 2005;22:275–284. doi: 10.1016/j.immuni.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 3.Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol. 2001;19:47–64. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 5.Walker LS, Abbas AK. The enemy within: keeping self-reactive T cells at bay in the periphery. Nature Rev Immunol. 2002;2:11–19. doi: 10.1038/nri701. [DOI] [PubMed] [Google Scholar]
- 6.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adler AJ. Mechanisms of T cell tolerance and suppression in cancer mediated by tumor-associated antigens and hormones. Curr Cancer Drug Targets. 2007;7:3–14. doi: 10.2174/156800907780006931. [DOI] [PubMed] [Google Scholar]
- 8.Anderson MJ, Shafer-Weaver K, Greenberg NM, Hurwitz AA. Tolerization of tumor-specific T cells despite efficient initial priming in a primary murine model of prostate cancer. J Immunol. 2007;178:1268–1276. doi: 10.4049/jimmunol.178.3.1268. [DOI] [PubMed] [Google Scholar]
- 9.Bronte V, Kasic T, Gri G, Gallana K, Borsellino G, Marigo I, Battistini L, Iafrate M, Prayer-Galetti T, Pagano F, Viola A. Boosting antitumor responses of T lymphocytes infiltrating human prostate cancers. J Exp Med. 2005;201:1257–1268. doi: 10.1084/jem.20042028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pardoll D. Does the immune system see tumors as foreign or self? Annu Rev Immunol. 2003;21:807–839. doi: 10.1146/annurev.immunol.21.120601.141135. [DOI] [PubMed] [Google Scholar]
- 11.Ebelt K, Babaryka G, Figel AM, Pohla H, Buchner A, Stief CG, Eisenmenger W, Kirchner T, Schendel DJ, Noessner E. Dominance of CD4(+) lymphocytic infiltrates with disturbed effector cell characteristics in the tumor microenvironment of prostate carcinoma. Prostate. 2008;68:1–10. doi: 10.1002/pros.20661. [DOI] [PubMed] [Google Scholar]
- 12.Redmond WL, Weinberg AD. Targeting OX40 and OX40L for the treatment of autoimmunity and cancer. Crit Rev Immunol. 2007;27:415–436. doi: 10.1615/critrevimmunol.v27.i5.20. [DOI] [PubMed] [Google Scholar]
- 13.Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003;3:609–620. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 14.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 15.Piconese S, Valzasina B, Colombo MP. OX40 triggering blocks suppression by regulatory T cells and facilitates tumor rejection. J Exp Med. 2008;205:825–839. doi: 10.1084/jem.20071341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Redmond WL, Gough MJ, Charbonneau B, Ratliff TL, Weinberg AD. Defects in the Acquisition of CD8 T Cell Effector Function after Priming with Tumor or Soluble Antigen Can Be Overcome by the Addition of an OX40 Agonist. J Immunol. 2007;179:7244–7253. doi: 10.4049/jimmunol.179.11.7244. [DOI] [PubMed] [Google Scholar]
- 17.Biagi E, Dotti G, Yvon E, Lee E, Pule M, Vigouroux S, Gottschalk S, Popat U, Rousseau R, Brenner M. Molecular transfer of CD40 and OX40 ligands to leukemic human B cells induces expansion of autologous tumor-reactive cytotoxic T lymphocytes. Blood. 2005;105:2436–2442. doi: 10.1182/blood-2004-07-2556. [DOI] [PubMed] [Google Scholar]
- 18.Hendriks J, Xiao Y, Rossen JW, van der Sluijs KF, Sugamura K, Ishii N, Borst J. During viral infection of the respiratory tract, CD27, 4-1BB, and OX40 collectively determine formation of CD8+ memory T cells and their capacity for secondary expansion. J Immunol. 2005;175:1665–1676. doi: 10.4049/jimmunol.175.3.1665. [DOI] [PubMed] [Google Scholar]
- 19.Bansal-Pakala P, Halteman BS, Cheng MH, Croft M. Costimulation of CD8 T cell responses by OX40. J Immunol. 2004;172:4821–4825. doi: 10.4049/jimmunol.172.8.4821. [DOI] [PubMed] [Google Scholar]
- 20.Weinberg AD. OX40: targeted immunotherapy--implications for tempering autoimmunity and enhancing vaccines. Trends Immunol. 2002;23:102–109. doi: 10.1016/s1471-4906(01)02127-5. [DOI] [PubMed] [Google Scholar]
- 21.Rogers PR, Song J, Gramaglia I, Killeen N, Croft M. OX40 promotes Bcl-xL and Bcl-2 expression and is essential for long-term survival of CD4 T cells. Immunity. 2001;15:445–455. doi: 10.1016/s1074-7613(01)00191-1. [DOI] [PubMed] [Google Scholar]
- 22.Evans DE, Prell RA, Thalhofer CJ, Hurwitz AA, Weinberg AD. Engagement of OX40 enhances antigen-specific CD4(+) T cell mobilization/memory development and humoral immunity: comparison of alphaOX-40 with alphaCTLA-4. J Immunol. 2001;167:6804–6811. doi: 10.4049/jimmunol.167.12.6804. [DOI] [PubMed] [Google Scholar]
- 23.Gramaglia I, Jember A, Pippig SD, Weinberg AD, Killeen N, Croft M. The OX40 costimulatory receptor determines the development of CD4 memory by regulating primary clonal expansion. J Immunol. 2000;165:3043–3050. doi: 10.4049/jimmunol.165.6.3043. [DOI] [PubMed] [Google Scholar]
- 24.Vu MD, Xiao X, Gao W, Degauque N, Chen M, Kroemer A, Killeen N, Ishii N, Chang Li X. OX40 costimulation turns off Foxp3+ Tregs. Blood. 2007;110:2501–2510. doi: 10.1182/blood-2007-01-070748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kjaergaard J, Tanaka J, Kim JA, Rothchild K, Weinberg A, Shu S. Therapeutic efficacy of OX-40 receptor antibody depends on tumor immunogenicity and anatomic site of tumor growth. Cancer Res. 2000;60:5514–5521. [PubMed] [Google Scholar]
- 26.Morris A, Vetto JT, Ramstad T, Funatake CJ, Choolun E, Entwisle C, Weinberg AD. Induction of anti-mammary cancer immunity by engaging the OX-40 receptor in vivo. Breast Cancer Res Treat. 2001;67:71–80. doi: 10.1023/a:1010649303056. [DOI] [PubMed] [Google Scholar]
- 27.Vetto JT, Lum S, Morris A, Sicotte M, Davis J, Lemon M, Weinberg A. Presence of the T-cell activation marker OX-40 on tumor infiltrating lymphocytes and draining lymph node cells from patients with melanoma and head and neck cancers. Am J Surg. 1997;174:258–265. doi: 10.1016/s0002-9610(97)00139-6. [DOI] [PubMed] [Google Scholar]
- 28.Ruby CE, Montler R, Zheng R, Shu S, Weinberg AD. IL-12 Is Required for Anti-OX40-Mediated CD4 T Cell Survival. J Immunol. 2008;180:2140–2148. doi: 10.4049/jimmunol.180.4.2140. [DOI] [PubMed] [Google Scholar]
- 29.Weinberg AD, Rivera MM, Prell R, Morris A, Ramstad T, Vetto JT, Urba WJ, Alvord G, Bunce C, Shields J. Engagement of the OX-40 receptor in vivo enhances antitumor immunity. J Immunol. 2000;164:2160–2169. doi: 10.4049/jimmunol.164.4.2160. [DOI] [PubMed] [Google Scholar]
- 30.Weinberg AD, Thalhofer C, Morris N, Walker JM, Seiss D, Wong S, Axthelm MK, Picker LJ, Urba WJ. Anti-OX40 (CD134) administration to nonhuman primates: immunostimulatory effects and toxicokinetic study. J Immunother (1997) 2006;29:575–585. doi: 10.1097/01.cji.0000211319.00031.fc. [DOI] [PubMed] [Google Scholar]
- 31.Bansal-Pakala P, Jember AG, Croft M. Signaling through OX40 (CD134) breaks peripheral T-cell tolerance. Nat Med. 2001;7:907–912. doi: 10.1038/90942. [DOI] [PubMed] [Google Scholar]
- 32.Lathrop SK, Huddleston CA, Dullforce PA, Montfort MJ, Weinberg AD, Parker DC. A signal through OX40 (CD134) allows anergic, autoreactive T cells to acquire effector cell functions. J Immunol. 2004;172:6735–6743. doi: 10.4049/jimmunol.172.11.6735. [DOI] [PubMed] [Google Scholar]
- 33.Lees JR, Charbonneau B, Swanson AK, Jensen R, Zhang J, Matusik R, Ratliff TL. Deletion is neither sufficient nor necessary for the induction of peripheral tolerance in mature CD8+ T cells. Immunology. 2006;117:248–261. doi: 10.1111/j.1365-2567.2005.02293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Redmond WL, Marincek BC, Sherman LA. Distinct requirements for deletion versus anergy during CD8 T cell peripheral tolerance in vivo. J Immunol. 2005;174:2046–2053. doi: 10.4049/jimmunol.174.4.2046. [DOI] [PubMed] [Google Scholar]
- 35.Hernandez J, Aung S, Redmond WL, Sherman LA. Phenotypic and functional analysis of CD8(+) T cells undergoing peripheral deletion in response to cross-presentation of self-antigen. J Exp Med. 2001;194:707–717. doi: 10.1084/jem.194.6.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maxwell JR, Weinberg A, Prell RA, Vella AT. Danger and OX40 receptor signaling synergize to enhance memory T cell survival by inhibiting peripheral deletion. J Immunol. 2000;164:107–112. doi: 10.4049/jimmunol.164.1.107. [DOI] [PubMed] [Google Scholar]
- 37.Ruby CE, Redmond WL, Haley D, Weinberg AD. Anti-OX40 stimulation in vivo enhances CD8+ memory T cell survival and significantly increases recall responses. Eur J Immunol. 2007;37:157–166. doi: 10.1002/eji.200636428. [DOI] [PubMed] [Google Scholar]
- 38.Lee SW, Park Y, Song A, Cheroutre H, Kwon BS, Croft M. Functional dichotomy between OX40 and 4-1BB in modulating effector CD8 T cell responses. J Immunol. 2006;177:4464–4472. doi: 10.4049/jimmunol.177.7.4464. [DOI] [PubMed] [Google Scholar]
- 39.Song A, Tang X, Harms KM, Croft M. OX40 and Bcl-xL promote the persistence of CD8 T cells to recall tumor-associated antigen. J Immunol. 2005;175:3534–3541. doi: 10.4049/jimmunol.175.6.3534. [DOI] [PubMed] [Google Scholar]
- 40.Murata S, Ladle BH, Kim PS, Lutz ER, Wolpoe ME, Ivie SE, Smith HM, Armstrong TD, Emens LA, Jaffee EM, Reilly RT. OX40 costimulation synergizes with GM-CSF whole-cell vaccination to overcome established CD8+ T cell tolerance to an endogenous tumor antigen. J Immunol. 2006;176:974–983. doi: 10.4049/jimmunol.176.2.974. [DOI] [PubMed] [Google Scholar]
- 41.Lustgarten J, Dominguez AL, Cuadros C. The CD8+ T cell repertoire against Her-2/neu antigens in neu transgenic mice is of low avidity with antitumor activity. Eur J Immunol. 2004;34:752–761. doi: 10.1002/eji.200324427. [DOI] [PubMed] [Google Scholar]
- 42.Tough DF, Sun S, Sprent J. T cell stimulation in vivo by lipopolysaccharide (LPS) J Exp Med. 1997;185:2089–2094. doi: 10.1084/jem.185.12.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khoruts A, Mondino A, Pape KA, Reiner SL, Jenkins MK. A natural immunological adjuvant enhances T cell clonal expansion through a CD28-dependent, interleukin (IL-2-independent mechanism. J Exp Med. 1998;187:225–236. doi: 10.1084/jem.187.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer. 2004;4:11–22. doi: 10.1038/nrc1252. [DOI] [PubMed] [Google Scholar]
- 45.Horai R, Nakajima A, Habiro K, Kotani M, Nakae S, Matsuki T, Nambu A, Saijo S, Kotaki H, Sudo K, Okahara A, Tanioka H, Ikuse T, Ishii N, Schwartzberg PL, Abe R, Iwakura Y. TNF-alpha is crucial for the development of autoimmune arthritis in IL-1 receptor antagonist-deficient mice. J Clin Invest. 2004;114:1603–1611. doi: 10.1172/JCI20742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koneru M, Schaer D, Monu N, Ayala A, Frey AB. Defective proximal TCR signaling inhibits CD8+ tumor-infiltrating lymphocyte lytic function. J Immunol. 2005;174:1830–1840. doi: 10.4049/jimmunol.174.4.1830. [DOI] [PubMed] [Google Scholar]
- 47.Whiteside TL. Down-regulation of zeta-chain expression in T cells: a biomarker of prognosis in cancer? Cancer Immunol Immunother. 2004;53:865–878. doi: 10.1007/s00262-004-0521-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomas DA, Massague J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8:369–380. doi: 10.1016/j.ccr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 49.Takeda I, Ine S, Killeen N, Ndhlovu LC, Murata K, Satomi S, Sugamura K, Ishii N. Distinct roles for the OX40-OX40 ligand interaction in regulatory and nonregulatory T cells. J Immunol. 2004;172:3580–3589. doi: 10.4049/jimmunol.172.6.3580. [DOI] [PubMed] [Google Scholar]
- 50.Valzasina B, Guiducci C, Dislich H, Killeen N, Weinberg AD, Colombo MP. Triggering of OX40 (CD134) on CD4(+)CD25+ T cells blocks their inhibitory activity: a novel regulatory role for OX40 and its comparison with GITR. Blood. 2005;105:2845–2851. doi: 10.1182/blood-2004-07-2959. [DOI] [PubMed] [Google Scholar]
- 51.Gough MJ, Ruby CE, Redmond WL, Dhungel B, Brown A, Weinberg AD. OX40 agonist therapy enhances CD8 infiltration and decreases immune suppression in the tumor. Cancer Res. 2008;68:5206–5215. doi: 10.1158/0008-5472.CAN-07-6484. [DOI] [PubMed] [Google Scholar]