Abstract
Toluene, a widely used and commonly abused organic solvent, produces various behavioral disturbances, including motor incoordination and cognitive impairment. Toluene alters the function of a large number of receptors and ion channels. Blockade of N-methyl-d-aspartate (NMDA) receptors has been suggested to play a critical role in toluene-induced behavioral manifestations. The present study determined the effects of various toluene doses on motor coordination, recognition memory, body temperature, and intracranial self-stimulation (ICSS) thresholds in mice. Additionally, the effects of sarcosine on the behavioral and physiological effects induced by toluene were evaluated. Sarcosine may reverse toluene-induced behavioral manifestations by acting as an NMDA receptor co-agonist and inhibiting the effects of the type I glycine transporter (GlyT1). Mice were treated with toluene alone or combined with sarcosine pretreatment and assessed for rotarod performance, object recognition memory, rectal temperature, and ICSS thresholds. Toluene dose-dependently induced motor incoordination, recognition memory impairment, and hypothermia and lowered ICSS thresholds. Sarcosine pretreatment reversed toluene-induced changes in rotarod performance, novel object recognition, and rectal temperature but not ICSS thresholds. These findings suggest that the sarcosine-induced potentiation of NMDA receptors may reverse motor incoordination, memory impairment, and hypothermia but not the enhancement of brain stimulation reward function associated with toluene exposure. Sarcosine may be a promising compound to prevent acute toluene intoxications by occupational or intentional exposure.
Keywords: toluene, motor coordination, recognition memory, body temperature, intracranial self-stimulation
Introduction
Toluene is a widely used solvent. In addition to occupational exposure, a particular type of intoxication is caused by its intentional inhalation for recreational purposes, such as glue sniffing (Anderson and Loomis, 2003; Flanagan and Fisher, 2008; Howard et al., 2011). Toluene is frequently abused for its euphoric and hallucinating effects (Garland and Howard, 2010). However, toluene abuse also produces several severe adverse effects, including motor incoordination, hypothermia, and mental confusion, such as delusions and amnesia (Andersen et al., 1983; Meulenbelt et al., 1990; Saito and Wada, 1993; Chouaniere et al., 2002). Behavioral disturbances that result from toluene use, including motor incoordination, cognitive impairment (Lo et al., 2009), and hypothermia (Gordon et al., 2010), have been reported in rats. Additionally, the reward-enhancing effects of toluene have been studied using the intracranial self-stimulation (ICSS) procedure in rats. In geneal, a lowering of ICSS thresholds reflects increased brain reward sensitivity induced by the administration of drugs of abuse. However, toluene has been shown to increase the threshold current of self-stimulation in the study of Yavich and Zvartau (1994), using a rate-intensity protocol. On the other hand, auto-titration procedure that offers a rate-independent assessment of the ICSS thresholds demonstrated that toluene significantly reduced ICSS thresholds in rats (Bespalov et al., 2003).
An improved understanding of the mechanisms of action of toluene may aid in the identification of therapeutic approaches to counter the problem of toluene abuse. Mice may be an important tool in the investigation of the neurobiological effects of toluene with the recent advances that have been made in genetic engineering in this species. Several studies have used mice to study the acute effects of toluene and found that toluene exposure could result in changes in locomotor activity (Bushnell et al., 1985; Wood and Colotla, 1990; Bowen and Balster, 1998; Bowen et al., 2010; Conti et al., 2012), disturbances of gait, mobility, and righting reflex, impaired psychomotor coordination (Tegeris and Balster, 1994) and recognition memory (Win-Shwe and Fujimaki, 2012). However, the effects of toluene on rotarod test, rectal temperature, and brain stimulation reward in mice remain to be revealed. In the present study, the dose-dependent effects of toluene on ICSS thresholds in mice were determined by a discrete-trial current-intensity procedure which provides a current-intensity-threshold measure to avoid the problems inherent in rate-dependent studies. Additionally, the effects of toluene on rotarod test, rectal temperature, and novel object recognition test in mice were also assessed.
Toluene alters the function of a large number of receptors and ion channels (Lubman et al., 2008). However, the mechanisms that underlie toluene-induced behavioral and physiological responses remain unclear although the effects of toluene have been suggested to be at least partially mediated through the inhibition of N-methyl-d-aspartate (NMDA) receptor activity. Toluene blocks NMDA receptor-mediated currents in vitro (Cruz et al., 1998) and also induced partial substitution for the discriminative properties of the NMDA receptor antagonist phencyclidine (PCP) (Bowen et al., 1999). A recent study from our laboratory demonstrated that toluene-induced locomotor hyperactivity, motor incoordination, and memory impairment in rats was reversed by d-serine, a selective co-agonist for the glycine site of NMDA receptors (Lo et al., 2009). These findings suggest that the positive modulation of NMDA receptors may effectively block several behavioral disturbances induced by toluene. However, remaining unknown is whether the positive modulation of NMDA receptors blocks the stimulation reward-enhancing and hypothermic effects of toluene.
Sarcosine is an NMDA receptor co-agonist (Zhang et al., 2009), a competitive inhibitor of the type I glycine transporter (GlyT1) (Smith et al., 1992; Lopez-Corcuera et al., 1998; Eulenburg et al., 2005), and an important intermediate in one-carbon metabolism (Wittwer and Wagner, 1981; Chen et al., 2010). Sarcosine induced less NMDA receptor desensitization and larger increases in intracellular Ca2+ levels compared with glycine (Zhang et al., 2009). Additionally, sarcosine is more potent than d-serine in reducing the anti-seizure effect of MK-801 (Long et al., 2006) and as an add-on treatment for schizophrenia (Lane et al., 2010). Sarcosine appears to act as a potent positive allosteric NMDA receptor modulator in vitro and effectively exerts its effect in vivo. Thus, the present study assessed the effects of sarcosine on toluene-induced motor incoordination and recognition memory impairment to test the hypothesis that the positive modulation of NMDA receptors by sarcosine administration in mice suppresses acute behavioral responses elicited by toluene. Furthermore, the effects of sarcosine on toluene-induced stimulation reward enhancement and hypothermia were evaluated.
Materials and methods
Animals and drugs
Male NMRI mice (8–9 weeks, 33–40 g) were supplied by the Laboratory Animal Center of Tzu Chi University (Hualien, Taiwan) and housed in groups of four to five mice per cage on a 12 h/12 h light/dark cycle with ad libitum access to water and food. The experiments conducted at Tzu Chi University were performed in accordance with the Republic of China animal protection law (Chapter III: Scientific Application of Animals) and approved by the Review Committee of Tzu Chi University. The ICSS experiments were conducted at the University of California, San Diego, in accordance with the guidelines of the American Association for the Accreditation of Laboratory Animal Care, and the National Research Council’s Guide for Care and Use of Laboratory (NIH Publication No. 85-23, revised 1996) and approved by the University of California San Diego Institutional Animal Care and Use Committee. Male C57BL/6J mice were used for the ICSS experiments and purchased from Jackson Laboratories (Sacramento, CA, USA).
Toluene (high-performance liquid chromatography grade, 99.8%, Mallinckrodt Baker Inc., KY, USA) was diluted in corn oil (0, 250, 500, and 750 mg/kg) to achieve an injection volume of 10 ml/kg and administered intraperitoneally. Sarcosine (Sigma, St. Louis, MO, USA) was dissolved in saline and intraperitoneally injected in volumes of 10 ml/kg and administered 30 min prior to toluene treatment.
Naive mice were used in each experiment, in which each mouse underwent one procedure only. To determine the effects of various doses of toluene on rotarod performance, novel object recognition memory, and rectal temperature, groups of six mice per dose of toluene (0, 250, 500, and 750 mg/kg) were used for each experiment. The dose range of toluene used was based on the study in rats (Lo et al., 2009). To test whether sarcosine pretreatment reverses the effects of toluene in these tests, 40 mice were divided into five groups (control, 300 mg/kg sarcosine + oil, saline + toluene, 100 mg/kg sarcosine + toluene, and 300 mg/kg sarcosine + toluene) in each experiment. The doses of sarcosine chosen were based on the previous study, which are effective to enhance the prepulse inhibition in mGluR5 KO mice (Chen et al., 2010). The ICSS experiments were conducted using a within-subjects Latin square design, such that each mouse received all of the treatments. Nine and eight mice were used to determine the dose-dependent effects of toluene and sarcosine pretreatment, respectively.
Rotarod motor coordination test
Motor coordination was assessed using an automated rotarod apparatus (TSE systems, Bad Homburg, Germany). A computer recorded the latency to fall in seconds. The mice were first trained on the rotarod at a constant speed of 20 rotations per minute (rpm) until all of the mice were able to spend at least 3 min on the rod. The mice were then treated with toluene (250, 500, or 750 mg/kg) or vehicle 30 min prior to testing since the brain toluene level reached the peak at 30 min after injection and returned to the basal level after 2h (Win-Shwe et al., 2007). The mice were tested at 20 rpm at 15 min intervals for 120 min. To test the effects of sarcosine pretreatment, sarcosine (0, 100, and 300 mg/kg) was administered 30 min prior to the toluene (750 mg/kg) or corn oil (vehicle) injection.
Novel object recognition test
The experimental apparatus consisted of a Plexiglas open field box (50 × 50 × 25 cm) located in a sound-attenuated room and illuminated with a 20-W light bulb. The novel object recognition procedure consisted of habituation, training, and retention sessions. A video camera recorded behavior during the training and retention phases. Habituation was conducted in three consecutive daily sessions, during which each mouse was allowed to individually explore the box without objects for 10 min. During the training session, each animal was placed in the box, and after 5 min, two identical objects (plastic items) were simultaneously introduced in two corners. Each animal was allowed to explore the objects for 5 min. An animal was considered to explore the object when its head was facing the object at a distance of approximately 1 cm or less between the head and object or when it was touching or sniffing the object. The time spent exploring each object was recorded using stopwatches by an experimenter blind to the treatment condition. After the training session, the mice were immediately returned to their home cages. The retention session was conducted 24 h after the training session. The animals were returned to the same box as during the training session, and one of the two objects of the training session was replaced with a novel object. The animals were allowed to explore the box freely for 5 min, and the time spent exploring each object was recorded as described above. The objects and chambers were cleaned with 70% ethanol after each use. The preference index in the retention session, defined as the ratio of the amount of time spent exploring the novel object and total time spent exploring both objects was used to evaluate recognition memory. In the training session, the preference index was defined as the ratio of the time spent exploring the object that replaced the original object in the retention session and the total exploration time. The mice received toluene (250, 500, and 750 mg/kg) or corn oil (vehicle) 30 min prior to initiating the training session. To test the effect of sarcosine pretreatment, sarcosine (0, 100, and 300 mg/kg) was administered 30 min prior to the toluene (750 mg/kg) or corn oil (vehicle) injection.
Body temperature
Rectal temperature was measured by a thermistor probe and digital thermometer (Singa Technology, Taipei, Taiwan). Baseline rectal temperatures were recorded before the mice were injected with toluene. After the injection of toluene (250, 500, and 750 mg/kg) or corn oil (vehicle), rectal temperatures were recorded at 15 min intervals for 120 min followed by a 30 min interval. Changes in toluene-induced body temperature were calculated as the difference in rectal temperature from the baseline value for each mouse. To test the effect of sarcosine, sarcosine (0, 100, 300 mg/kg) was administered 30 min prior to the toluene (750 mg/kg) or corn oil (vehicle) injection.
Intracranial self-stimulation: Surgery, apparatus, and procedure
For ICSS surgery, the mice were anesthetized by inhalation of 1–3% isoflurane in oxygen and positioned in a stereotaxic frame (Kopf Instruments, Tujunga, CA, USA). Stainless steel bipolar electrodes (0.2 mm diameter, 6 mm length; Plastics One, Roanoke, VA, USA) were implanted into the medial forebrain bundle at the level of the lateral hypothalamus using the following coordinates from flat skull as previously described (Stoker et al., 2008): anterior/posterior, 1.58 mm; medial/lateral, 1.0 mm; dorsal/ventral, 5.3 mm; (Paxinos and Franklin, 2001). Four stainless steel screws (3.2 mm length; Plastics One, Roanoke, VA, USA) were fixed to the skull to keep the electrode in place together with the application of a resin ionomer (Den-Mat, Santa Maria, CA, USA) and dental acrylic (Ortho-Jet, Lang Dental, Wheeling, IL, USA). The mice were allowed 7 post-surgery recovery days.
ICSS training and testing were conducted in eight Plexiglas operant chambers (30.5 × 24 × 27 cm; Med Associates, St. Albans, VT, USA). Each operant chamber was enclosed in a light- and sound-attenuated chamber (40 × 60 × 63.5 cm). Intracranial stimulation was delivered by constant-current stimulators (Stimtech model 1200c, San Diego Instruments, San Diego, CA, USA). The animals were connected to the stimulation circuit through flexible bipolar leads (Plastics One, Roanoke, VA, USA) attached to gold-contact swivel commutators (model SL2C, Plastics One, Roanoke, VA, USA) mounted above the operant chamber. The operant response required by the animals was a simple one-quarter turn of a wheel manipulandum (5.5 cm diameter, 4 cm width) that extended 1.5 cm from one wall of the operant chamber. The stimulation parameters, data collection, and all test session functions were controlled by a computer.
The ICSS procedure used here has been described previously (Stoker et al., 2008). Initially, the mice were trained to turn the wheel manipulandum on a fixed-ratio 1 schedule of reinforcement with a 180 µA current. After the successful acquisition of this schedule (i.e., two sessions in which the mouse received 200 reinforcers in less than 10 min), the mice were trained in the discrete-trial current-threshold procedure. Each trial began with the delivery of a noncontingent electrical stimulus followed by a 7.5 s response window within which the subject could make a response to receive a second contingent stimulus identical in all parameters to the initial noncontingent stimulus. A response during this time window was labeled a positive response, and the lack of a response was labeled a negative response. During a 2 s period immediately after a positive response, additional responses had no consequences. The intertrial interval that followed either a positive response or the end of the response window (in the case of a negative response) had an average duration of 10 s (ranging from 7.5 to 12.5 s). Responses that occurred during the intertrial interval were labeled timeout responses and resulted in a further 12.5 s delay of the onset of the next trial. During training on the discrete-trial procedure, the duration of the intertrial interval and delay periods induced by timeout responses were gradually increased until both reached a duration of 10 s (ranging from 1 to 10 s during training). The animals were subsequently tested in the current-threshold procedure, in which stimulation current intensities were varied according to the classical psychophysical method of limits. A test session consisted of four alternating series of descending and ascending current intensities, starting with a descending series. Blocks of three trials were presented to the subject at a given stimulation intensity, and the intensity changed by 5 µA steps between blocks of trials. The initial stimulus intensity was set at approximately 30–40 µA above the baseline current threshold for each animal. Each test session typically lasted 30–40 min and provided two dependent variables for behavioral assessment: threshold and response latency. The threshold value of each series was defined as the midpoint in microamperes between the current intensity level at which the animal made two or more positive responses out of the three stimulus presentations and the level at which the animal made less than two positive responses. The animal’s estimated current threshold for each test session was the mean of the four series’ thresholds. The response latency was defined as the average time in seconds that elapsed between the delivery of the electrical stimulus and the turning of the wheel manipulandum for all of the trials that led to a positive response.
Two ICSS sessions were conducted daily, separated by 2 h. On drug injection days, toluene or vehicle was injected 15 min before the start of Session 2. The initial stimulus intensity was set at approximately 30–40 µA above the baseline current threshold for each animal, thus it took about 10–15 min to reach the threshold. Therefore, toluene was administrated 15 min prior to the start of Session 2. The effects of toluene on the threshold would be recorded approximately between 30–60 min after toluene injection. ICSS thresholds obtained during Session 2 are expressed as a percentage of the baseline values obtained during Session 1. Test days were separated by 48 h. On days between test days, the animals were similarly tested twice daily, and vehicle was injected 15 min prior to the start of Session 2. To test whether sarcosine could reverse the effect of toluene on ICSS thresholds, sarcosine (0, 300, or 600 mg/kg) was administered 30 min prior to toluene (500 mg/kg) or corn oil administration and 45 min prior to the start of Session 2.
Statistical analyses
All of the data are expressed as mean ± SEM. The data from the rotarod test and rectal temperature assessment were analyzed by two-way mixed-design analyses of variance (ANOVAs), with Time as the within-subjects factor. The data from the novel object recognition test were analyzed by one-way ANOVA. The effects of toluene on ICSS thresholds and response latencies were analyzed by one-way repeated-measures ANOVA. A two-way repeated measures ANOVA was used to analyze the effects of sarcosine and toluene on ICSS thresholds and response latencies. The Student-Newman-Keuls test was used for post hoc comparisons. Values of p < 0.05 were considered statistically significant.
Results
Dose-dependent effects of toluene on motor coordination, rectal temperature, novel object recognition, and ICSS thresholds
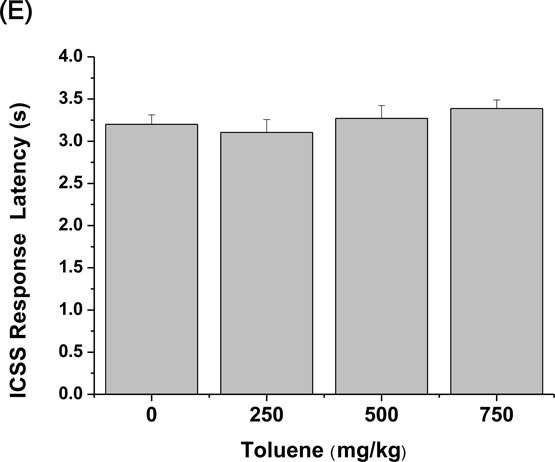
Rotarod performance, rectal temperature, novel object recognition, and ICSS thresholds were assessed after toluene treatment (0, 250, 500, and 750 mg/kg). Fig. 1A shows a dose-dependent impairment of motor coordination induced by toluene in the rotarod test. A mixed-design ANOVA revealed significant main effects of Toluene (F3,120 = 10.93, p < 0.001) and Time (F6,120 = 8.16, p < 0.001) and a significant Toluene × Time interaction (F18,120 = 3.09, p < 0.001). The Student-Newman-Keuls post hoc test revealed that 750 mg/kg toluene produced significant motor incoordination (p < 0.001). Fig. 1B shows the effects of toluene on novel object recognition. Toluene dose-dependently reduced the recognition index during the memory retention test session (F3,28 = 20.36, p < 0.001). Toluene impaired recognition memory at doses of 250 mg/kg (p < 0.01), 500 mg/kg (p < 0.01), and 750 mg/kg (p < 0.001), assessed by the Student-Newman-Keuls post hoc test. The effects of toluene on rectal temperature are shown in Fig. 1C. A mixed-design ANOVA revealed significant main effects of Toluene (F3,140 = 4.21, p < 0.001) and Time (F7,140 = 8.58, p < 0.001) and a significant Toluene × Time interaction (F21,140 = 1.808, p < 0.05). The Student-Newman-Keuls post hoc test revealed that 750 mg/kg toluene produced a significant hypothermic effect (p < 0.001). Fig. 1D shows the effects of toluene on ICSS thresholds. Baseline ICSS thresholds were 68.78 ± 21.17. A one-way repeated-measures ANOVA demonstrated that toluene dose-dependently lowered ICSS thresholds (F3,24 = 13.882, p < 0.001). The Student-Newman-Keuls post hoc test revealed that toluene at doses of 500 and 750 mg/kg but not 250 mg/kg significantly lowered ICSS thresholds (p < 0.001). Toluene did not affect the response latency in the ICSS test at any of the doses tested (Fig.1E). In summary, toluene dose-dependently resulted in impairment in the rotarod and novel object recognition tests and lowered rectal temperature and ICSS thresholds.
Figure 1.
Effects of toluene on motor coordination, recognition memory and body temperature, and brain stimulation reward. (A) The latency to fall from the rotarod (n = 6), (B) the recognition index in the novel object recognition test (n = 6), (C) rectal temperature (n = 6), (D) ICSS thresholds (n = 9), and (E) ICSS response latency were determined after an injection of corn oil or toluene (250, 500, and 750 mg/kg). The data are expressed as mean ± SEM. Asterisks indicate a significant difference between toluene- and vehicle-treated mice (**p < 0.01, ***p < 0.001).
Effects of sarcosine on toluene-induced motor incoordination, recognition memory impairment, and the lowering of rectal temperature and ICSS thresholds
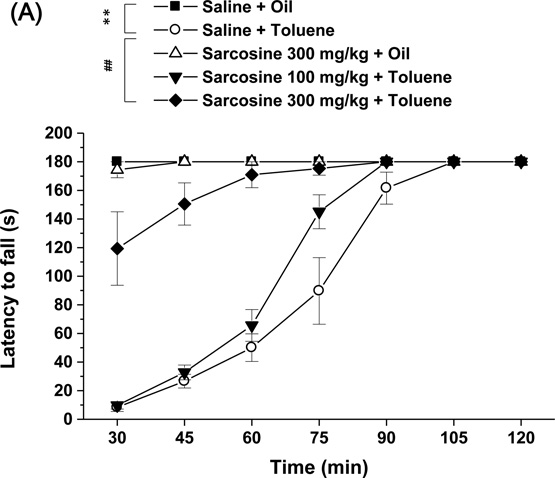
A mixed-design ANOVA revealed significant main effects of Treatment (F4,157 = 38.773, p < 0.001) and Time (F6,157 = 80.622, p < 0.001) on rotarod performance and a significant Treatment × Time interaction (F24,157 = 20.904, p < 0.001). The post hoc tests revealed that toluene significantly decreased the latency to stay on the rotarod, and 300 mg/kg but not 100 mg/kg sarcosine significantly reduced toluene-induced motor incoordination (Fig. 2A).
Figure 2.
Effects of sarcosine on toluene-induced motor incoordination, memory impairment, hypothermia, and enhancement of brain stimulation reward. Sarcosine (0, 100, and 300 mg/kg) was administered 30 min prior to vehicle or toluene (750 mg/kg) administration, and the mice were assessed in the (A) rotarod test (n = 7–9), (B) novel object recognition test (n = 8), and (C) rectal temperature test (n = 7–9). A Latin square design was used for the ICSS experiment (n = 8). (D) ICSS thresholds and (E) ICSS response latencies were measured. Sarcosine (0, 300, and 600 mg/kg) was administered 30 min prior to corn oil or toluene (500 mg/kg). The data are expressed as mean ± SEM. Asterisks indicate a significant difference between Saline + Oil mice and mice treated with toluene or sarcosine (**p < 0.01, ***p < 0.001). Pound signs indicate a significant difference between Saline + Toluene mice and Sarcosine + Toluene mice (##p < 0.01, ###p < 0.001).
The effects of sarcosine (100 and 300 mg/kg) on toluene (750 mg/kg)-induced impairment in the novel object recognition test is shown in Fig. 2B. A one-way ANOVA demonstrated a significant difference between groups in the retention session (F4,35 = 31.73, p < 0.001) but not in the training session. The Student-Newman-Keuls post hoc test revealed that sarcosine pretreatment (100 and 300 mg/kg) significantly attenuated the impairing effects of toluene on recognition memory (p < 0.01).
The effects of sarcosine (100 and 300 mg/kg) on the decrease in rectal temperature induced by toluene (750 mg/kg) are shown in Fig. 2C. A mixed-design ANOVA revealed a significant Treatment × Time interaction (F28,245 = 4.084, p < 0.001) and main effects of Treatment (F4,245 = 4.701, p < 0.01) and Time (F7,245 = 6.893, p < 0.001). The Student-Newman-Keuls post hoc test revealed that sarcosine (100 and 300 mg/kg) significantly prevented the toluene-induced hypothermic effect (p < 0.001).
A Latin square design was used to determine the effects of sarcosine pretreatment on the enhancement of brain stimulation reward induced by toluene in the ICSS procedure. As shown in Fig. 2D, sarcosine (300 and 600 mg/kg) did not affect toluene (500 mg/kg)-induced lowering of ICSS thresholds. The response latencies were not affected by sarcosine and toluene treatment (Fig. 2E).
Discussion
Toluene exposure dose-dependently disturbed motor coordination in the rotarod test, impaired recognition memory in the novel object recognition test, produced hypothermia, and lowered ICSS thresholds in mice. These findings are consistent with previous studies that assessed the behavioral and physiological responses to toluene exposure in rats. Specifically, studies in rats demonstrated that toluene dose-dependently produced a hypothermic response (Gordon et al., 2010) and impaired motor coordination and passive avoidance learning (Lo et al., 2009). Toluene has been shown to increase the threshold current of self-stimulation by a rate-intensity protocol (Yavich and Zvartau, 1994) which is a rate-dependent assessment of ICSS thresholds. Usually, the motor impairment produced by higher concentration or dose of toluene resulted in confounding impact on the rate-intensity protocol. In fact, toluene significantly reduced ICSS thresholds using another procedure (auto-titration) that offers a rate-independent assessment of the self-stimulation thresholds in rats (Bespalov et al., 2003), similar to our results in mice. The discrete-trial current-intensity procedure used in the present study provides a current-intensity-threshold measure without confounding related to rate changes. Therefore, two rate-independent procedures most commonly used to assess ICSS threshold revealed the ICSS threshold-lowering effects of toluene in rats and mice. The impaired effects of toluene on novel object recognition test has been demonstrated in mice (Win-Shwe and Fujimaki, 2012). The present study further revealed that toluene could elicit hypothermia, motor incoordination in rotarod test, and lowered ICSS thresholds in mice. Taken together, toluene appears to act on the central nervous system in rats and mice in a similar manner. Future studies in genetically engineered mice may promote our understanding of the molecular mechanisms that mediate the distinct behavioral and physiological responses to toluene.
Human toluene abusers typically sniff toluene and prefer to titer their dose to desired effect by repeatedly “huffing” very high concentrations for seconds to minutes at a time (Flanagan and Ives, 1994). This pattern of intentional toluene vapor abuse is difficult to mimic in experimental animals. Intraperitoneal injections are routinely used in animals to study the effects of drugs that are commonly abused by inhalation (e.g., cannabinoids and nicotine). In addition, intraperitoneal injections of toluene fully substitute for inhaled toluene in drug discrimination studies in mice (Shelton, 2007; Shelton and Slavova-Hernandez, 2009). Therefore, toluene was administered intraperitoneally in the present study. The results demonstrated that intraperitoneal toluene injection dose-dependently changed motor coordination, recognition memory, and body temperature in NMRI mice. Additionally, toluene dose-dependently lowered ICSS thresholds in C57BL/6J mice.
The results of the present study further demonstrated that sarcosine, a co-agonist of NMDA receptors at the glycine binding site and a GlyT1 inhibitor, significantly reduced toluene-induced motor incoordination in the rotarod test, cognitive impairment in the novel object recognition test, and hypothermia in NMRI mice. However, sarcosine did not modulate the lowering of ICSS thresholds induced by toluene in C57BL6/J mice. Overall, these findings suggest that NMDA receptor blockade induced by toluene may be associated with motor incoordination, recognition memory impairment, and hypothermia in response to toluene exposure, whereas the reward-enhancing effect of toluene may be independent of NMDA receptor inhibition. This conclusion is also supported by one of our previous studies that demonstrated that d-serine, also a selective co-agonist at the glycine binding site of NMDA receptors, reversed locomotor hyperactivity, motor incoordination, and learning impairment induced by toluene administration in rats (Lo et al., 2009). Indeed, the neurobehavioral profiles of toluene including biphasic effect on locomotor activity (Riegel and French, 1999; Riegel et al., 2003; Chan et al., 2004; Dell et al., 2011), ataxia (Lo et al., 2009), and learning impairments (Lo et al., 2009; Win-Shwe et al., 2010; Huerta-Rivas et al., 2012) overlap those of NMDA receptor antagonists, such as phencyclidine (PCP) and ketamine. Sarcosine and another GlyT1 inhibitor, N-(3-[4’-fluorophenyl]-3-[4’-phenylphenoxy]propyl)sarcosine (NFPS), have been reported to antagonize the effects of NMDA receptor channel blockers. Specifically, NFPS prevented MK-801-induced long-term potentiation (Manahan-Vaughan et al., 2008) and cognitive impairment in the novel object recognition test (Karasawa et al., 2008), whereas sarcosine reduced PPI deficits and hyperlocomotion induced by ketamine (Yang et al., 2010b). In conclusion, the results of the present study suggest that increased NMDA receptor activity that results from the activation of the glycine binding site could effectively attenuate several effects of toluene and NMDA receptor channel blockers. These findings further support the hypothesis that toluene and noncompetitive NMDA receptor antagonists may exert psychotomimetic effects through similar mechanisms.
Interestingly, however, sarcosine did not suppress the toluene-induced reductions in ICSS thresholds. These results suggest that the reward-enhancing effects of toluene may be mediated through a different neurobiological mechanism than the effects of toluene on rotarod performance, novel object recognition, and rectal temperature. Notably, the effects of sarcosine on the toluene-induced lowering of ICSS thresholds were assessed in C57BL/6J mice, whereas all of the other experiments were performed in NMRI mice. Although the results of dose-dependent effects of toluene showed that both stains of mice are sensitive to toluene, it is possible that NMRI and C57BL/6J mice have different sensitivity to sarcosine. However, sarcosine could diminish the expression of cocaine-induced conditioned place preference in C57BL/6J mice at the same doses used in the present study (Yang et al., 2010a). In addition, sarcosine (300 mg/kg) could reverse the toluene-induced hypothermia in C57BL/6J mice (Supplement Fig. 1). C57BL/6J mice appear to be sensitive to sarcosine. Therefore, the reward-enhancing effects of toluene, reflected by the lowering of ICSS thresholds, may be mediated through neurobiological mechanisms that are different from the mechanisms of toluene-induced motor incoordination, recognition memory impairment, and hypothermia, which appear to be mediated through NMDA receptor blockade.
Toluene, PCP, ketamine, and MK-801 facilitate brain stimulation reward, reflected by a lowering of ICSS thresholds (Bespalov et al., 2003) (Herberg and Rose, 1989; Carlezon and Wise, 1993), but unknown is whether the effects of these compounds on brain reward function are mediated by NMDA receptor blockade. In fact, competitive NMDA receptor antagonists, such as LY235959, that act at glutamate binding sites of the NMDA receptor did not lower ICSS thresholds in rats (Kenny et al., 2009). Thus, NMDA receptor channel blockers but not competitive antagonists may selectively target populations of NMDA receptors, perhaps voltage-dependently, that exert a tonic inhibitory influence on brain reward systems (Kenny et al., 2009). However, ketamine-induced increases in nucleus accumbens dopamine efflux, which play an important role in the reinforcing effects of drugs of abuse, may not result from NMDA receptor blockade but rather from the mobilization of dopamine storage pools to releasable sites (Hancock and Stamford, 1999). Therefore, although the NMDA receptor is the primary site of action of toluene, ketamine, and PCP, the reward-enhancing effects of these drugs may not be mediated through NMDA receptors. Determining whether sarcosine affects the reward-enhancing effects of PCP or ketamine would be interesting.
In conclusion, the present study demonstrated that acute toluene exposure dose-dependently induced motor incoordination, hypothermia, and recognition memory impairment in NMRI mice and facilitated brain stimulation reward in C57BL6/J mice. Sarcosine, an endogenous amino acid that is an NMDA receptor co-agonist, a competitive inhibitor of GlyT1, and an important intermediate in one-carbon metabolism, reversed toluene-induced motor impairment, hypothermia, and memory deficits in NMRI mice but not the toluene-induced facilitation of brain stimulation reward in C57BL/6J mice. Distinct neural pathways and neurotransmitter systems may contribute to different behavioral responses to toluene, and the present findings suggest that NMDA receptors may mediate the effects of toluene on motor incoordination, hypothermia, and memory impairment, but not brain stimulation reward. Sarcosine may therefore have potential to prevent acute toluene intoxications by occupational or intentional exposure.
Supplementary Material
Highlights.
-
●
Toluene induces impairments in Rotarod test and novel object recognition test.
-
●
Toluene lowers rectal temperature and ICSS thresholds in mice.
-
●
Sarcosine reverses toluene-induced changes in motor, memory and body temperature.
-
●
Sarcosine pretreatment does not affect toluene-induced reward enhancement.
Acknowledgements
This work was supported by grant NSC 95-2314-B-320-004 from the National Scientific Council of Taiwan to H-HC, grant R01DA023209 from the U.S. National Institute on Drug Abuse to AM, and a National Institute on Drug Abuse Distinguished International Scientist Collaborative Award to H-HC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors declare that there are no conflicts of interest.
References
- Andersen I, Lundqvist GR, Molhave L, Pedersen OF, Proctor DF, Vaeth M, Wyon DP. Human response to controlled levels of toluene in six-hour exposures. Scand J Work Environ Health. 1983;9:405–418. doi: 10.5271/sjweh.2393. [DOI] [PubMed] [Google Scholar]
- Anderson CE, Loomis GA. Recognition and prevention of inhalant abuse. Am Fam Physician. 2003;68:869–874. [PubMed] [Google Scholar]
- Bespalov A, Sukhotina I, Medvedev I, Malyshkin A, Belozertseva I, Balster R, Zvartau E. Facilitation of electrical brain self-stimulation behavior by abused solvents. Pharmacol Biochem Behav. 2003;75:199–208. doi: 10.1016/s0091-3057(03)00071-6. [DOI] [PubMed] [Google Scholar]
- Bowen SE, Balster RL. A direct comparison of inhalant effects on locomotor activity and schedule-controlled behavior in mice. Exp Clin Psychopharmacol. 1998;6:235–247. doi: 10.1037//1064-1297.6.3.235. [DOI] [PubMed] [Google Scholar]
- Bowen SE, Kimar S, Irtenkauf S. Comparison of toluene-induced locomotor activity in four mouse strains. Pharmacol Biochem Behav. 2010;95:249–257. doi: 10.1016/j.pbb.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen SE, Wiley JL, Jones HE, Balster RL. Phencyclidine- and diazepam-like discriminative stimulus effects of inhalants in mice. Exp Clin Psychopharmacol. 1999;7:28–37. doi: 10.1037//1064-1297.7.1.28. [DOI] [PubMed] [Google Scholar]
- Bushnell PJ, Evans HL, Palmes ED. Effects of toluene inhalation on carbon dioxide production and locomotor activity in mice. Fundam Appl Toxicol. 1985;5:971–977. doi: 10.1016/0272-0590(85)90178-2. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Wise RA. Phencyclidine-induced potentiation of brain stimulation reward: acute effects are not altered by repeated administration. Psychopharmacology. 1993;111:402–408. doi: 10.1007/BF02253528. [DOI] [PubMed] [Google Scholar]
- Chan MH, Chien TH, Lee PY, Chen HH. Involvement of NO/cGMP pathway in toluene-induced locomotor hyperactivity in female rats. Psychopharmacology. 2004;176:435–439. doi: 10.1007/s00213-004-1900-0. [DOI] [PubMed] [Google Scholar]
- Chen HH, Stoker A, Markou A. The glutamatergic compounds sarcosine and N-acetylcysteine ameliorate prepulse inhibition deficits in metabotropic glutamate 5 receptor knockout mice. Psychopharmacology. 2010;209:343–350. doi: 10.1007/s00213-010-1802-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouaniere D, Wild P, Fontana JM, Hery M, Fournier M, Baudin V, Subra I, Rousselle D, Toamain JP, Saurin S, Ardiot MR. Neurobehavioral disturbances arising from occupational toluene exposure. Am J Ind Med. 2002;41:77–88. doi: 10.1002/ajim.10030. [DOI] [PubMed] [Google Scholar]
- Conti AC, Lowing JL, Susick LL, Bowen SE. Investigation of calcium-stimulated adenylyl cyclases 1 and 8 on toluene and ethanol neurobehavioral actions. Neurotoxicol Teratol. 2012;34:481–488. doi: 10.1016/j.ntt.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Cruz SL, Mirshahi T, Thomas B, Balster RL, Woodward JJ. Effects of the abused solvent toluene on recombinant N-methyl-D-aspartate and non-N-methyl-D-aspartate receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1998;286:334–340. [PubMed] [Google Scholar]
- Dell CA, Gust SW, MacLean S. Global issues in volatile substance misuse. Substance use & misuse. 2011;46(Suppl 1):1–7. doi: 10.3109/10826084.2011.580169. [DOI] [PubMed] [Google Scholar]
- Eulenburg V, Armsen W, Betz H, Gomeza J. Glycine transporters: essential regulators of neurotransmission. Trends Biochem Sci. 2005;30:325–333. doi: 10.1016/j.tibs.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Flanagan RJ, Fisher DS. Volatile substance abuse and crime: data from U.K. press cuttings 1996–2007. Med Sci Law. 2008;48:295–306. doi: 10.1258/rsmmsl.48.4.295. [DOI] [PubMed] [Google Scholar]
- Flanagan RJ, Ives RJ. Volatile substance abuse. Bull Narc. 1994;46:49–78. [PubMed] [Google Scholar]
- Garland EL, Howard MO. Phenomenology of adolescent inhalant intoxication. Exp Clin Psychopharmacol. 2010;18:498–509. doi: 10.1037/a0021737. [DOI] [PubMed] [Google Scholar]
- Gordon CJ, Gottipolu RR, Kenyon EM, Thomas R, Schladweiler MC, Mack CM, Shannahan JH, Wallenborn JG, Nyska A, MacPhail RC, Richards JE, Devito M, Kodavanti UP. Aging and susceptibility to toluene in rats: a pharmacokinetic, biomarker, and physiological approach. J Toxicol Environ Health A. 2010;73:301–318. doi: 10.1080/15287390903421144. [DOI] [PubMed] [Google Scholar]
- Hancock PJ, Stamford JA. Stereospecific effects of ketamine on dopamine efflux and uptake in the rat nucleus accumbens. Br J Anaesth. 1999;82:603–608. doi: 10.1093/bja/82.4.603. [DOI] [PubMed] [Google Scholar]
- Herberg LJ, Rose IC. The effect of MK-801 and other antagonists of NMDA-type glutamate receptors on brain-stimulation reward. Psychopharmacology. 1989;99:87–90. doi: 10.1007/BF00634458. [DOI] [PubMed] [Google Scholar]
- Howard MO, Bowen SE, Garland EL, Perron BE, Vaughn MG. Inhalant use and inhalant use disorders in the United States. Addiction science & clinical practice. 2011;6:18–31. [PMC free article] [PubMed] [Google Scholar]
- Huerta-Rivas A, Lopez-Rubalcava C, Sanchez-Serrano SL, Valdez-Tapia M, Lamas M, Cruz SL. Toluene impairs learning and memory, has antinociceptive effects, and modifies histone acetylation in the dentate gyrus of adolescent and adult rats. Pharmacol Biochem Behav. 2012;102:48–57. doi: 10.1016/j.pbb.2012.03.018. [DOI] [PubMed] [Google Scholar]
- Karasawa J, Hashimoto K, Chaki S. D-Serine and a glycine transporter inhibitor improve MK-801-induced cognitive deficits in a novel object recognition test in rats. Behav Brain Res. 2008;186:78–83. doi: 10.1016/j.bbr.2007.07.033. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Chartoff E, Roberto M, Carlezon WA, Jr, Markou A. NMDA receptors regulate nicotine-enhanced brain reward function and intravenous nicotine self-administration: role of the ventral tegmental area and central nucleus of the amygdala. Neuropsychopharmacology. 2009;34:266–281. doi: 10.1038/npp.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane HY, Lin CH, Huang YJ, Liao CH, Chang YC, Tsai GE. A randomized, double-blind, placebo-controlled comparison study of sarcosine (N-methylglycine) and D-serine add-on treatment for schizophrenia. Int J Neuropsychopharmacol. 2010;13:451–460. doi: 10.1017/S1461145709990939. [DOI] [PubMed] [Google Scholar]
- Lo PS, Wu CY, Sue HZ, Chen HH. Acute neurobehavioral effects of toluene: involvement of dopamine and NMDA receptors. Toxicology. 2009;265:34–40. doi: 10.1016/j.tox.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Long KD, Mastropaolo J, Rosse RB, Manaye KF, Deutsch SI. Modulatory effects of d-serine and sarcosine on NMDA receptor-mediated neurotransmission are apparent after stress in the genetically inbred BALB/c mouse strain. Brain Res Bull. 2006;69:626–630. doi: 10.1016/j.brainresbull.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Lopez-Corcuera B, Martinez-Maza R, Nunez E, Roux M, Supplisson S, Aragon C. Differential properties of two stably expressed brain-specific glycine transporters. J Neurochem. 1998;71:2211–2219. doi: 10.1046/j.1471-4159.1998.71052211.x. [DOI] [PubMed] [Google Scholar]
- Lubman DI, Yucel M, Lawrence AJ. Inhalant abuse among adolescents: neurobiological considerations. Br J Pharmacol. 2008;154:316–326. doi: 10.1038/bjp.2008.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Wildforster V, Thomsen C. Rescue of hippocampal LTP and learning deficits in a rat model of psychosis by inhibition of glycine transporter-1 (GlyT1) Eur J Neurosci. 2008;28:1342–1350. doi: 10.1111/j.1460-9568.2008.06433.x. [DOI] [PubMed] [Google Scholar]
- Meulenbelt J, de Groot G, Savelkoul TJ. Two cases of acute toluene intoxication. Br J Ind Med. 1990;47:417–420. doi: 10.1136/oem.47.6.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. The mouse brain in stereotaxic coordinates. 2nd Ed. San Diego, CA: Academic Press; 2001. [Google Scholar]
- Riegel AC, Ali SF, French ED. Toluene-induced locomotor activity is blocked by 6-hydroxydopamine lesions of the nucleus accumbens and the mGluR2/3 agonist LY379268. Neuropsychopharmacology. 2003;28:1440–1447. doi: 10.1038/sj.npp.1300193. [DOI] [PubMed] [Google Scholar]
- Riegel AC, French ED. Acute toluene induces biphasic changes in rat spontaneous locomotor activity which are blocked by remoxipride. Pharmacol Biochem Behav. 1999;62:399–402. doi: 10.1016/s0091-3057(98)00062-8. [DOI] [PubMed] [Google Scholar]
- Saito K, Wada H. Behavioral approaches to toluene intoxication. Environ Res. 1993;62:53–62. doi: 10.1006/enrs.1993.1088. [DOI] [PubMed] [Google Scholar]
- Shelton KL. Inhaled toluene vapor as a discriminative stimulus. Behav Pharmacol. 2007;18:219–229. doi: 10.1097/FBP.0b013e328157f460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton KL, Slavova-Hernandez G. Characterization of an inhaled toluene drug discrimination in mice: Effect of exposure conditions and route of administration. Pharmacol Biochem Behav. 2009;92:614–620. doi: 10.1016/j.pbb.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KE, Borden LA, Hartig PR, Branchek T, Weinshank RL. Cloning and expression of a glycine transporter reveal colocalization with NMDA receptors. Neuron. 1992;8:927–935. doi: 10.1016/0896-6273(92)90207-t. [DOI] [PubMed] [Google Scholar]
- Stoker AK, Semenova S, Markou A. Affective and somatic aspects of spontaneous and precipitated nicotine withdrawal in C57BL/6J and BALB/cByJ mice. Neuropharmacology. 2008;54:1223–1232. doi: 10.1016/j.neuropharm.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegeris JS, Balster RL. A comparison of the acute behavioral effects of alkylbenzenes using a functional observational battery in mice. Fundam Appl Toxicol. 1994;22:240–250. doi: 10.1006/faat.1994.1028. [DOI] [PubMed] [Google Scholar]
- Win-Shwe TT, Fujimaki H. Acute administration of toluene affects memory retention in novel object recognition test and memory function-related gene expression in mice. J Appl Toxicol. 2012;32:300–304. doi: 10.1002/jat.1693. [DOI] [PubMed] [Google Scholar]
- Win-Shwe TT, Kageyama S, Tsukahara S, Nakajima D, Fujimaki H. Effect of D-cycloserine on spatial learning performance and memory function-related gene expression in mice following toluene exposure. J UOEH. 2010;32:127–140. doi: 10.7888/juoeh.32.127. [DOI] [PubMed] [Google Scholar]
- Win-Shwe TT, Mitsushima D, Nakajima D, Ahmed S, Yamamoto S, Tsukahara S, Kakeyama M, Goto S, Fujimaki H. Toluene induces rapid and reversible rise of hippocampal glutamate and taurine neurotransmitter levels in mice. Toxicol Lett. 2007;168:75–82. doi: 10.1016/j.toxlet.2006.10.017. [DOI] [PubMed] [Google Scholar]
- Wittwer AJ, Wagner C. Identification of the folate-binding proteins of rat liver mitochondria as dimethylglycine dehydrogenase and sarcosine dehydrogenase. Flavoprotein nature and enzymatic properties of the purified proteins. J Biol Chem. 1981;256:4109–4115. [PubMed] [Google Scholar]
- Wood RW, Colotla VA. Biphasic changes in mouse motor activity during exposure to toluene. Fundam Appl Toxicol. 1990;14:6–14. doi: 10.1016/0272-0590(90)90226-a. [DOI] [PubMed] [Google Scholar]
- Yang FY, Lee YS, Cherng CG, Cheng LY, Chang WT, Chuang JY, Kao GS, Yu L. D-cycloserine, sarcosine and D-serine diminish the expression of cocaine-induced conditioned place preference. J Psychopharmacol. 2010a doi: 10.1177/0269881110388333. published online 24 November 2010 as. [DOI] [PubMed] [Google Scholar]
- Yang SY, Hong CJ, Huang YH, Tsai SJ. The effects of glycine transporter I inhibitor, N-methylglycine (sarcosine), on ketamine-induced alterations in sensorimotor gating and regional brain c-Fos expression in rats. Neurosci Lett. 2010b;469:127–130. doi: 10.1016/j.neulet.2009.11.058. [DOI] [PubMed] [Google Scholar]
- Yavich L, Zvartau E. A comparison of the effects of individual organic solvents and their mixture on brain stimulation reward. Pharmacol Biochem Behav. 1994;48:661–664. doi: 10.1016/0091-3057(94)90328-x. [DOI] [PubMed] [Google Scholar]
- Zhang HX, Hyrc K, Thio LL. The glycine transport inhibitor sarcosine is an NMDA receptor co-agonist that differs from glycine. J Physiol. 2009;587:3207–3220. doi: 10.1113/jphysiol.2009.168757. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.