Abstract
Objectives/Hypotheses
The objective was to assess the utility of selected “resonant voice” exercises for the reduction of acute vocal fold inflammation. The hypothesis was that relatively large-amplitude, low-impact exercises associated with resonant voice would reduce inflammation more than spontaneous speech and possibly more than voice rest.
Study Design
The study design was prospective, randomized, double-blind.
Methods
Nine vocally healthy adults underwent a 1-hr vocal loading procedure, followed by randomization to (a) a spontaneous speech condition, (b) a vocal rest condition, or (c) a resonant voice exercise condition. Treatments were monitored in clinic for 4 hr, and continued extra-clinically until the next morning. At baseline, immediately following loading, after the 4-hr in-clinic treatment, and 24 hr post baseline, secretions were suctioned from the vocal folds bilaterally and submitted to enzyme-linked immunosorbent assay (ELISA) to estimate concentrations of key markers of tissue injury and inflammation: IL-1β, IL-6, IL-8, TNF-α, MMP-8, and IL-10.
Results
Complete data sets were obtained for 3 markers -- IL-1β, IL-6, and MMP-8 -- for one subject in each treatment condition. For those markers, results were poorest at 24-hr follow-up in the spontaneous speech condition, sharply improved in the voice rest condition, and best in the resonant voice condition. Average results for all markers, for all responsive subjects with normal baseline mediator concentrations, revealed an almost identical pattern.
Conclusions
Some forms of tissue mobilization may be useful to attenuate acute vocal fold inflammation.
Keywords: vocal fold inflammation, wound healing, tissue mobilization, resonant voice, Agent-Based Modeling
INTRODUCTION
Traditional management of acute vocal fold injury emphasizes voice conservation. Classically, patients with acute injury are advised to restrict both amount and loudness of phonation to facilitate recovery (1, 2). The underlying physiological rationale for this approach is reasonable. Perpendicular impact stress to the vocal fold tissue is thought to be the most direct cause of phonotrauma (3–5). Therefore, restricting phonation post-traumatically should minimize aggravating stresses, presumably enhancing the inherent tissue healing phenotype and also minimizing the likelihood of new injury during the recovery period.
This approach is imminently sensible. However, emerging data from other domains suggest the counterintuitive notion that in some cases, tissue mobilization may be anabolic, optimizing the resolution of inflammation and also the long-term outcome of injury (e.g., ref 6). Although these principles have translated to clinical practice in other fields, they have not yet been systematically explored in the context of vocal fold injury. Recent data from our laboratory suggested that dynamic biomechanical strain limited the inflammatory phenotype in vocal fold fibroblasts in vitro (7), suggesting a putative anti-inflammatory role for some forms of vocal motion over voice rest. However, the clinical translation of these preliminary findings is tenuous. First, the forces placed upon the fibroblasts in our in vitro investigations only dimly mimic the in vivo phonatory environment. Second, the value of mobilization or exercise following vocal fold injury in humans has not yet been reported. As such, the current study sought to systematically investigate the potential for tissue mobilization or exercise in the form of “resonant voice exercises” as a means to improve outcomes in patients with acute vocal fold injury.
Relevant background is as follows. Given their anatomic position, the vocal folds are inherently susceptible to various sources of insult, ranging from chemical to surgical injury and mechanical trauma from phonation. Regardless of the source, in most cases, tissue injury initiates a cascade of biochemical events ideally leading to the reconstitution of functional tissue. The initial stage of the wound healing response is commonly referred to as the inflammatory phase. Events in this phase control the flow of blood into the injury site, recruit inflammatory cells, neutrophils and macrophages, to ensure a sanitary, viable wound environment, and perhaps most importantly, produce growth factors and cytokines that regulate subsequent events in wound healing. In fact, processes in the acute phase of wound healing may influence the quality of the ultimate outcome of healing. Specifically, consensus exists that limiting the magnitude of the inflammatory response generally leads to improved tissue architecture and function in the long term (8).
Most relevant to laryngology is the need to limit the development of benign vocal fold lesions such as scar. In fact, attempts are often made pharmacologically to inhibit vocal fold inflammation with steroids (systemic, per-oral or intra-muscular). This practice is particularly prevalent in the management of voice problems within the performing arts community (9), but has also shown promise for problems in other patients with benign vocal fold lesions (10) as well as Reinke’s edema (11, 12). Although clinical evidence suggests that steroids may be a satisfactory therapeutic option in the short term, the long-term negative consequences with prolonged steroid use often outweigh the therapeutic benefits.
Ideally, therapeutic intervention for vocal fold inflammation should be developed to attenuate the inflammatory response, but also circumvent the potential negative consequences of pharmacological treatments. Mechanical signaling paradigms appear to meet these criteria. Specifically, in vitro and in vivo data from other tissues suggest that some forms of tissue mobilization may be inherently anti-inflammatory. For example, low levels of mechanical signaling reduced gene expression for many pro-inflammatory mediators, including cyclooxygenase-2, in cells from a number of connective tissues in vitro (13–16). The anabolic effects of mechanical signaling are thought to be due to the inhibition of NF-κB translocation into the nucleus via the inhibition of I-κB degradation (17, 18). These processes have only recently been elucidated in the vocal folds (7).
In other domains, in vitro data have translated to clinical practice. For example, historically, the primary treatment for severe ankle inversion sprains was complete immobilization. In contrast, contemporary management approaches involve tissue mobilization in these patients yielding improved outcomes including decreased pain ratings and improved range of motion (19). Furthermore, tissue mobilization has been associated with decreased fibrosis in the surgically-injured patellar tendon (20). These emerging data provide the primary theoretical foundation for the systematic investigation of (a) vocal fold inflammation, and (b) the role of vocal fold mobilization tasks that may modulate post-injury vocal fold inflammation.
The fundamental challenge in this type of investigation is methodological. Until recently, no methods allowed for the quantitative characterization of vocal fold inflammation in humans. Our laboratory reported on the putative utility of assaying secretions collected from the vocal fold surfaces for biochemical mediators of wound healing. In our initial report, we described the collection of secretions from a single subject before and after one hour of high-intensity vocal loading. Secretions were then assayed for key pro-inflammatory mediators including interleukin (IL)-1β, tumor necrosis factor (TNF)-α, and several key matrix metalloproteinases (MMPs). Soluble inflammatory mediators were indeed captured in the assays. As important, increased mediator levels corresponded with the clinical appearance of vocal fold injury (21). Specifically, concentrations of IL-1β, TNF-α, and MMP-8 increased sharply following vocal loading. In contrast, levels of transforming growth factor (TGF-β) and prostaglandin (PGE2) remained constant suggesting some degree of selectivity of this assay, in that not every mediator elevated in the underlying tissue is detectable in airway secretions. Several subsequent reports have provided evidence regarding the validity of the approach. Findings in those reports have replicated expected results for inflammatory mediators in acute and chronic vocal fold injury in both animal models and human pathologies (22–24). Furthermore, the approach has been validated in the oral biology literature (for example, ref 25) and more recently, in the laryngology literature (26). Thus, there is peer-reviewed support for the general validity of the approach to provide insight into the inflammatory state of vocal fold tissue.
The present study employs this method to explore the potential utility of vocal fold tissue mobilization in the form of “resonant voice” exercises to limit inflammation subsequent to acute phonotrauma. Resonant voice has been defined perceptually as a voicing pattern associated with anterior oral vibratory sensations in the context of “easy” phonation (27, 28). This voicing pattern has been shown to be associated with barely ad- or abducted vocal folds engaged in relatively large-amplitude, low-impact vocal fold vibrations (27, 29–31). These biomechanical features of resonant voice make it an attractive rehabilitation approach for investigation into the potential therapeutic properties in acute phonotrauma. Specifically, the large-vibration feature of resonant voice may help to limit the influx of inflammatory mediators into the tissue, due to cell deformation associated with cyclic tensile strain, while at the same time increasing concentrations of anti-inflammatory mediators unleashed by tissue motion (32–34). In this sense, the relatively large vocal fold vibrations associated with resonant voice may function as something of a biological “healing” factor in acute injury. At the same time, the low-impact feature of resonant voice may function as a biological “prevention” mechanism by limiting new injury to the tissue during the recovery period. In addition to these biological considerations, an anecdotal case report provided clinical evidence that resonant voice may offer therapeutic benefits in patients with acute phonotrauma (27).
Together, these considerations prompted the generation of the primary experimental hypotheses for the present study: voice rest would generally enhance resolution of acute vocal fold inflammation compared to spontaneous speech post-traumatically, but inflammation would be most improved following resonant voice exercises, 4 hr after the initiation of treatment and 24 hr post baseline. Specific predictions were that concentrations of inflammatory mediators IL1-β, IL-6, IL-8, MMP-8, and TNF-α would be greatest 4 hr after treatment initiation and 24 hr post baseline in the spontaneous speech condition, smallest in the resonant voice condition, and intermediate in the voice rest condition. Conversely, concentrations of an anti-inflammatory mediator, IL-10, should be greatest at the same time points in the resonant voice condition, and smallest in the voice rest condition, due to influx of this mediator triggered by tissue mobilization. Evidence to this effect would be consistent with results from published in vitro studies suggesting that some forms of tissue mobilization may have value in limiting the inflammatory response in human vocal folds (7).
A secondary hypothesis regarded the ability of non-invasive aerodynamic and perceptual measures to quantitatively capture the time-varying inflammatory status of the vocal folds. Specifically, we hypothesized that phonation threshold pressures (PTP) from high-pitched, quiet phonation and direct magnitude estimation of perceived phonatory effort (DME) would not covary tightly with inflammatory mediator concentrations, despite claims that these measures may be useful in the detection of vocal fold injury (35, 36). Our skepticism was related to the fact that both PTP and DME reflect multidimensional factors (e.g.(37); Colton, personal communication), only a limited number of which would be captured by the inflammatory status of the tissue. A tertiary hypothesis, incidental to the main focus of the study but nonetheless valuable to entertain, regarded the relationship between PTP and DME values. These physiological and psychological indices of phonatory effort have long been suggested to covary (38). However, careful scrutiny of the literature indicates that the covariance may be considerably weaker than often assumed (38).
MATERIALS AND METHODS
General Paradigm
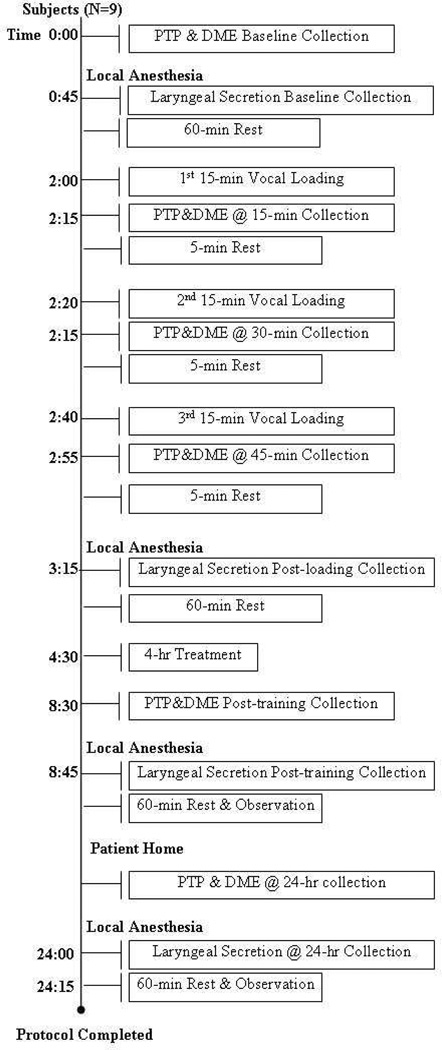
The general experimental paradigm is displayed in Figure 1. Nine vocally healthy subjects participated in a between-subjects design. All subjects first produced vocalization samples for PTP and DME measures, as described in detail shortly. Then, following the delivery of a local anesthetic to the larynx, laryngeal secretions were suctioned from the vocal fold surfaces bilaterally. Subjects then rested for 60 minutes to allow for dissipation of anesthetic effects, and subsequently underwent a 60-min vocal loading session. During loading, subjects alternated 15 minutes of loud phonation with five minutes of rest, for a total of three cycles over the 60-minute loading period. PTP and DME data collection occurred at the boundary between phonation and rest periods during loading, for a total of three PTP and DME collection time points during and immediately after the loading époque. Laryngeal secretions were again collected 20–30 minutes after the completion of loading. Subjects were then randomly assigned, stratifying by gender, to either (1) spontaneous speech, (2) voice rest, or (3) resonant voice exercise conditions, described shortly. Subjects underwent treatment conditions for four hours in the clinic, under the supervision of a voice trainer who, for the majority of subjects, was blinded to the experimental hypotheses. Secretion, PTP, and DME samples were collected again at the end of the 4-hr period, and subjects were sent home with instructions to persist with their respective treatments during waking hours, until their return to the clinic the next morning. Subjects were also instructed to avoid alcohol, smoke, late-night eating, and any voice use outside of the prescribed treatment for the evening. Subjects returned 24 hours after initial baseline for collection of final PTP, DME and laryngeal secretion samples. The 4-hr treatment period was motivated by parallel in vitro studies of vocal tissue inflammation in our laboratory (7). The 24-hr time point was motivated by an attempt to control for potential time-of-day effects in the baseline versus follow-up data.
Figure 1.
General experimental paradigm for between-subjects investigation. Within-subject effects were investigated in one subject who followed the same procedures, but participated in all three treatments on different days separated by 1–6 months.
Participants
The study was approved by the Institutional Review Board at the University of Pittsburgh. A total of nine subjects participated in the study: six females (21–46 years) and three males (21–29 years). Subjects were compensated for their participation. Both females and males were included in the study so that the population of individuals who may have acute phonotraumatic injury would be proportionately represented in terms of gender. The ratio of females to males in our study (2:1) was guided by conservative estimates in the literature regarding the frequency of voice problems in females over males in the population (e.g. (39, 40).
Specific inclusion criteria were: (1) males or female between the ages of 18 and 55 years, (2) in good overall health by self-report, (3) lack of extreme gag response to light base of tongue palpation based on clinical examination; (4) nasal patency sufficient to pass a flexible scope at least unilaterally; (5) normal hearing as tested bilaterally at 20dB to 8,000 Hz, (6) ability to produce “resonant voice” during initial training as determined by an examiner perceptually, despite no history of formal voice training of any type by subject report, and (7) ability to produce loud voice, between 85–95 dB measured 25 cm from the mouth. Exclusion criteria were: (1) self-report of a current voice problem or a voice problem more than once monthly during the preceding year; (2) previously diagnosed speech and /or language deficits in adulthood (childhood disorders were not exclusionary); (3) current use of any medications that might influence voice (e.g., diuretics, decongestants, antihistamines) or signs of currently active allergic process; (4) known or suspected allergy to any anesthetic, in particular lidocaine; (5) current smoker per subject report, and (6) report of pregnancy. Also, (7) females enrolled in the study were scheduled to participate at the mid-month mark in the menstrual cycle (i.e, non menstruating). Finally, (8) subjects with vocal fold lesions or risk of hemorrhage were excluded, based on laryngological examination.
Experimental design
The between-subjects study used a two-way, mixed model design with time (baseline, post loading, 4-hr post treatment start, and 24-hr post-baseline follow-up) as the within-subjects factor and treatment condition (spontaneous speech, voice rest, and resonant voice) as the between-subjects factor. Dependent variables for the primary research question were concentrations of inflammatory mediators IL1-β, IL-6, IL-8, MMP-8, TNF-α, and IL-10, normalized to baseline values and therefore unitless. Dependent variables pertinent to secondary and tertiary research questions were PTP and DME data (cm H20 and self-ratings, respectively). Subjects were blinded to experimental hypotheses and all data analyses were conducted by individuals blinded by subjects’ condition. Thus, the study was double-blinded.
Equipment
Phonation Threshold Pressure (PTP) data was obtained using the Aerophone II Phonatory Function Analyzer system with a Rothenberg (1973) circumferentially vented face mask (#5 Ambu). The Aerophone II was calibrated prior to each day of data collection. Target pitches for PTP and DME measures (C4, 262 Hz; males, and C5, 523 Hz; females) were provided in free field using a Dell RT 7D00 keyboard, and a target syllable production rate of 88 beats per minute for syllable utterance in the PTP task was guided by a Sabine MT-8000 metronome (41). Aerophone data were later transferred to a separate computer (Dell Pentium 4 Prescott DT, 3.6GHZ) with a custom software program for data analysis.
Rigid videolaryngostroboscopy was performed using the KayPentax Model 9016. Flexible endoscopy was performed using an Olympus 1300446 2 mm channel chip-tip flexible laryngoscope.
Vocal loading trials were recorded using a Panasonic SV 3900 professional digital audio tape deck and SX202 Dual Microphone Preamp Symetrix microphone converter box. The microphone was an AKG Acoustics C410 headset miniature condenser microphone with a behind-the-neck headband (fundamental frequency range 20–2,000 Hz; maximum sound pressure level 123 dB SLP). Microphone calibration was established individually for each subject at the onset of vocal loading. For calibration, a number 33–2050 Radio Shack Sound Level Meter and the microphone were positioned in parallel at a 45-degree angle three inches from the subject’s mouth. A Servox AG 51109 electrolarynx was used to generate a calibration tone delivered to the center of the subject’s lips. The intensity level was noted from the sound level meter, and was announced and recorded on the digital audio tape.
Procedures
Approximately 30 days prior to their participation in the experimental component of the protocol, subjects provided informed consent and were prescreened in the clinic for gag response and nasal patency. Individuals showing heightened gag in response to light base of tongue palpation and subjects with poor nasal patency were excluded from further participation. On the initial day of the experiment proper, subjects first received pre-training in Phonation Threshold Pressure (PTP) and Direct Magnitude Estimation (DME) data collection procedures. For PTP, subjects produced repeated sets of /pi pi pi pi pi/ utterances as quietly as possible at C4 (262 Hz; males) or C5 (523 Hz; females) indicated with a keyboard and a rate of 88 beats per minute indicated by a metronome. The target pitches were chosen based on empirical observations that high pitches are the most sensitive to various experimental manipulations (42, 43), and clinically, most subjects appear able to produce those particular non-ultra-high pitches even under conditions of vocal fold inflammation. The target rate of syllable production was based on reports that this general range of rates facilitates valid estimation of subglottic pressure from oral pressure data (Holmberg, Hillman, & Perkell, 1988). Pitches were verified perceptually by a trained examiner to an accuracy of about one quarter tone for each syllable. The examiner also monitored subjects’ loudness for PTP trials, empirically, continually encouraging them to phonate as quietly as possible. Training for the PTP task continued until the examiner and the subject considered that the subject could perform the task reliably according to criteria, typically five minutes or less. Subjects were then trained in Direct Magnitude Estimation (DME) procedures, which required subjects to rate their perceived phonatory effort for the preceding set of PTP trials on a scale on which “1” represented comfortable effort, “2” represented twice as much effort as comfortable, and so forth (44, 45). There was no upper (or lower) limit to the scale.
After subjects completed pre-training in PTP and DME procedures, the first set of formal samples of these measures was collected. For baseline as well as all subsequent PTP and DME data collection time points, three sets of /pi pi pi pi pi/ strings were collected using the foregoing criteria, and one DME value was extracted to reflect the subject’s perception of phonatory effort for the preceding set of PTP trials.
Following completion of initial PTP and DME data collection, a larygologist examined the subject’s oral cavity, oropharynx and nasal cavity and placed a cotton pledget soaked with lidocaine and decongestant into the subject’s most patent nasal cavity. The oropharynx was also anesthesized via topical, aerated 4% plain lidocaine. Rigid laryngoscopy with stroboscopy was performed to obtain baseline images of the larynx. Then, 4% lidocaine was dripped onto the endolarynx through the working channel of a flexible laryngoscope. After about five minutes, subsequent to verification of anesthesia of the vocal folds to light touch, a one-millimeter, plastic suction catheter was passed through the working channel of the scope and guided down to the free edge and superior surface of the vocal folds and gentle suction was applied. This procedure allowed for the collection of a small amount of vocal fold secretions, about 100 microliter (µl). Secretions were captured in a modified sinus trap and then transferred into a 0.2ml microfuge tube via a 1cc syringe. The tubes were labeled using codes that could not be traced to the subject or the subject’s condition, except by way of a master list retained by one investigator not involved with data analysis. Tubes were placed on dry ice and stored at −80°C until analysis.
Secretion collection was followed by a 60-min rest period to allow for dissipation of the anesthetic. During that period, subjects were monitored for their compliance with instructions to be completely silent and refrain from eating or drinking. Subjects then initiated participation in a 60-minute vocal loading session. For the loading session, subjects repeated three cycles of 15 min of loud voice production alternated with 5 min voice rest. Collection of PTP and DME data, which took less than 30 seconds, occurred at the boundary of the loading and resting phases. For vocal loading itself, subjects used theatre monologues, other written material, or simply engaged in conversation with an investigator.
Although acoustic analyses of vocal loading were not planned, loading trials were audio recorded (see Equipment) in case such analyses should become relevant. For loading, a sound level meter was positioned at a constant distance of 25 cm from the subject’s mouth so that an experimenter could monitor relative intensity levels during loading. The examiner monitored the meter nearly constantly and cued subjects to maintain a target intensity range of 75–90 dB during phonatory loading.
About 20–30 minutes after loading was completed, subjects received laryngeal anesthesia and underwent secretion collection as previously. Subjects then rested again for 60 minutes to allow for the dissipation of anesthetic effects. As previously, during that period subjects were silent and refrained from eating or drinking. Subsequently, subjects were randomized to one of three treatment conditions, described in detail shortly: (1) spontaneous speech; (2) voice rest; or (3) resonant voice. Randomization was constrained by stratification and counterbalancing by gender: two females and one male were assigned to each treatment condition, during each of three runs of the protocol on separate days. Subjects then underwent treatments for four hours in the clinic, as monitored by one of two voice experts. Six subjects (two subjects in each of three experimental conditions, including four females and two males distributed across the conditions) were monitored by a doctoral level singing voice specialist with approximately 20 years of experience, who was entirely naïve to the experimental hypotheses. Three subjects (one subject in each of three experimental conditions, including two females and one male) were monitored by a doctoral level speech-language pathologist and teacher of singing, who was informed about the experimental hypotheses. Although interventionist blinding was not central to the experimental hypotheses at this stage of inquiry, a combination of blinded as well as unblinded examiners was used so that later explorations of the data might provide some window on whether experimental biases might influence biological results. After the four-hr in-house treatments, subjects were dismissed from the clinic and were instructed to continue with the same general treatment procedures they had received extra-clinically for the remainder of the day (evening) and next morning until they returned to the clinic. Specifics are provided shortly.
Upon their arrival in the clinic the next morning, all subjects were silent except during elicitation of PTP data and in phonation during laryngeal examination and secretion collection procedures. Compliance with extra-clinical requirements was assessed with a checklist indicating subjects’ self-reported adherence to requirements and specific times that any exercises were completed. Based on their reports, all subjects were compliant with all requirements (data available upon request).
Treatments
Voice rest
For the voice rest condition, subjects were required to maintain absolute silence after vocal loading. No phonation or whispering was allowed. Subjects were encouraged to communicate with pen and paper as needed. After four hours of in-house monitoring, subjects were dismissed with the instruction to refrain from any voice use until they returned to clinic for repeated baseline data collection the following morning.
Spontaneous Speech
For the spontaneous speech treatment, during the 4-hr in-house monitoring period, subjects spoke with an investigator in what they considered a normal voice about topics that interested them for alternating intervals of 16 minutes followed by 4-minute periods of complete silence. All spontaneous speech trials were audio recorded as occurred for vocal loading. After four hours of in-house monitoring of this regimen, subjects were dismissed with the instruction to continue to use normal conversational speech until their return to the clinic the next morning for repeated baseline data collection.
Resonant Voice
Resonant voice exercises involved repeated prolongations of /m/, /n/, “ng” and /j/ prolongations, attending to anterior oral vibrations in the context of “easy voice” (27, 28). Prolongations were produced in a conversational pitch and loudness range that the clinician and subject agreed was comfortable for the subject, and also in pitch glides and scales that included notes as high as were comfortable for the subject. Following indications in the literature, the achievement of “resonant voice” was determined perceptually by the clinician and the subject together based on affirmative answers to the questions: “Do you feel vibrations in the front of your face?” “Is voice easy?” (e.g., Verdolini, 2000). Both investigators involved in subject monitoring had extensive clinical experience, 20 years or more, producing and training resonant voice. During the four-hour in-house treatment period, resonant voice exercises were produced for alternating cycles of 4 minutes followed by 16 min of voice rest. None of the subjects in the resonant voice group, or in the other two groups, had any prior experience with resonant voice.
All resonant voice trials were recorded for later post-hoc evaluations, should they become relevant. Throughout all phases of the treatment, resonant voice training and trouble-shooting by the clinician focused on the experiential dimension rather than biomechanical, verbal explanations (see for example, (46)). That is, instructions oriented towards the subject’s discovery of resonant voice rather than biomechanical prescriptions around its production. When the four-hour period of in-house treatment had been completed, the subject was sent home with instructions to maintain voice rest except for four minutes of resonant voice exercises every 30 minutes, during waking hours, until the return to the clinic the next morning for repeated baseline data collection.
The next morning, 24 hours following the acquisition of baseline data, subjects returned to the clinic for final PTP, DME, and laryngeal secretion collection procedures as previously. Subjects were then monitored in-house, while they refrained from eating or drinking for 60 minutes post secretion collection. Finally, subjects were debriefed regarding the experimental hypotheses and were dismissed.
Data reduction
Secretion analysis
All secretion analyses were carried out by an investigator who was blinded to subjects’ conditions (time point and treatment condition). For the analyses, a known volume of secretion was aliquoted for analysis and served as the dilution factor. The appropriate volume of sterile saline was added to the tube to bring the total volume up to 2.0 ml. Standard enzyme-linked immunoassays were performed for IL-1β, IL-6, IL-8, TNF-α, matrix metalloproteinase (MMP)-8, and IL-10 utilizing the manufacturer's recommended protocol (R&D Systems, Minneapolis, MN). These particular markers were selected based on previous work in our laboratory regarding marker levels in laryngeal secretions. In addition, IL-6 and IL-8 were included as they are ubiquitous mediators of inflammation. IL-10, an anti-inflammatory cytokine, was assayed to determine if anti-inflammatory cytokines are measurable in secretions and to determine if this cytokine may be a relevant indicator of tissue health. All samples were run in duplicate on the same kit to avoid inter-kit variability. Numeric results were generated based on the standard curve of each assay. Results were calculated as the amount of marker per milliliter of secretion; they were then normalized to the baseline levels for each individual subject and combined into groups for data analysis.
PTP and DME data extraction
Custom software was used to analyze PTP data. Analysis for each /pi pi pi pi pi/ production was derived from syllables 2–5 (Holmberg, Hillman & Perkell, 1988). Specifically, the software identified the temporal midpoint between adjacent oral pressure peaks for syllables 2–3, 3–4, and 4–5. Estimated subglottal pressures were interpolated for that time-point from oral pressures, using a straight line from peak pressure in the earlier syllable to the peak pressure of the latter syllable. For each 5-syllable /pi/ utterance, average values were calculated for PTP and included in the analyses. Ten percent of the PTP data were randomly selected and were re-analyzed by a second investigator to determine inter-investigator reliability, using a Pearson r correlation.
DME data were recorded straight from subjects’ responses at the time of data collection. Reliability checks for DME data were not possible because of the nature of the data collection. That is, we would have had to ask subjects to repeat, within a few moments, their DME estimates for an immediately preceding PTP trial, and they would clearly have remembered what they just said, or alternately we would have had to ask them to recall, at a later time, their sense of phonatory effort for earlier trials. Neither approach was appealing, and thus reliability for DME measures was not evaluated.
Statistical analyses
Inflammatory biomarkers
First, assumptions required for Analysis of Variance (ANOVA) in this two-factor mixed-model design were evaluated. Results revealed that assumptions of normality and homogeneity of variance were patently violated in these biological data. In fact, inspection of the full data set (Table 1) revealed that considerable variability was seen in the data across subjects.
Table 1.
Complete Data Set. Raw Data Values for Marker Concentrations across Time Points, for All Subjects. (BL = Baseline; Post = 20–30 Minutes Post Loading; 4hr = 4 Hr after Initiation of Treatment; 24hr = 24 Hr Post Baseline). “Invalid Data” are Shown in Orange. “Pre-inflamed (Only) Data” are in Yellow. “Non-Responsive Data” are in Green. “Pre-Inflamed-Plus-Non-Responsive Data” are in Blue (see Text for Explanation).
| Time-point | Subject | Sex | IL-1β (pg/ml) |
IL-6 (pg/ml) |
IL-8 (pg/ml) |
TNF-α (pg/ml) |
MMP-8 (ng/ml) |
IL-10 (pg/ml) |
|
|---|---|---|---|---|---|---|---|---|---|
| Spontaneous Speech | BL | 3 | F | 83 | 20 | 1500 | 3 | 100 | 42 |
| Post | 103 | 27 | 7,200 | 4 | 303 | 83 | |||
| 4hr | 266 | 116 | 6,600 | 3 | 333 | 75 | |||
| 24hr | 1,066 | 1,333 | 23,000 | 18 | 1300 | 175 | |||
| BL | 6 | F | 128 | 632 | 2,151 | 33 | 178 | 187 | |
| Post | 28 | 314 | 300 | 36 | 30 | 204 | |||
| 4hr | 88 | 546 | 835 | 119 | 80 | 732 | |||
| 24hr | 466 | 98 | 1,443 | 32 | 276 | 202 | |||
| BL | 8 | M | 1,333 | 167 | 29,180 | 54 | 1,667 | 166 | |
| Post | 405 | 664 | 3,564 | 73 | 1,297 | 140 | |||
| 4hr | 15 | 220 | 420 | 52 | 37 | 265 | |||
| 24hr | 57 | 107 | 440 | 46 | 32 | 346 | |||
| Voice Rest | BL | 1 | M | 115 | 4 | 4,800 | 217 | 52 | 183 |
| Post | 222 | 43 | 4,100 | 7 | 188 | 30 | |||
| 4hr | 408 | 85 | 15,000 | 8 | 719 | 117 | |||
| 24hr | 215 | 37 | 7,900 | 13 | 104 | 120 | |||
| BL | 5 | F | 691 | 488 | 2,183 | 35 | 254 | 223 | |
| Post | 98 | 37 | 512 | 41 | 28 | 550 | |||
| 4hr | 79 | 198 | 733 | 43 | 45 | 120 | |||
| 24hr | 39 | 236 | 411 | 39 | 32 | 300 | |||
| BL | 7 | F | 770 | 26 | 3,187 | 77 | 82 | 151 | |
| Post | 45 | 24 | 1,889 | 35 | 36 | 143 | |||
| 4hr | 179 | 20 | 2,038 | 40 | 95 | 124 | |||
| 24hr | 30 | 15 | 744 | 49 | 28 | 120 | |||
| Resonant Voice | BL | 2 | F | 80 | 6 | 620 | 13 | 80 | 6 |
| Post | 121 | 60 | 3,900 | 8 | 121 | 60 | |||
| 4hr | 296 | 77 | 2,600 | 10 | 296 | 77 | |||
| 24hr | 36 | 0 | 1,300 | 1 | 36 | 0 | |||
| BL | 4 | M | 34 | 154 | 2,307 | 77 | 43 | 667 | |
| Post | 17 | 257 | 503 | 33 | 26 | 236 | |||
| 4hr | 17 | 500 | 252 | 35 | 16 | 2,635 | |||
| 24hr | 34 | 371 | 701 | 34 | 18 | 213 | |||
| BL | 9 | F | 18 | 75 | 8,494 | 48 | 28 | 256 | |
| Post | 710 | 536 | 33,333 | 60 | 1,188 | 423 | |||
| 4hr | 13 | 26 | 188 | 62 | 48 | 257 | |||
| 24hr | 1,333 | 412 | 22,206 | 55 | 1,667 | 374 | |||
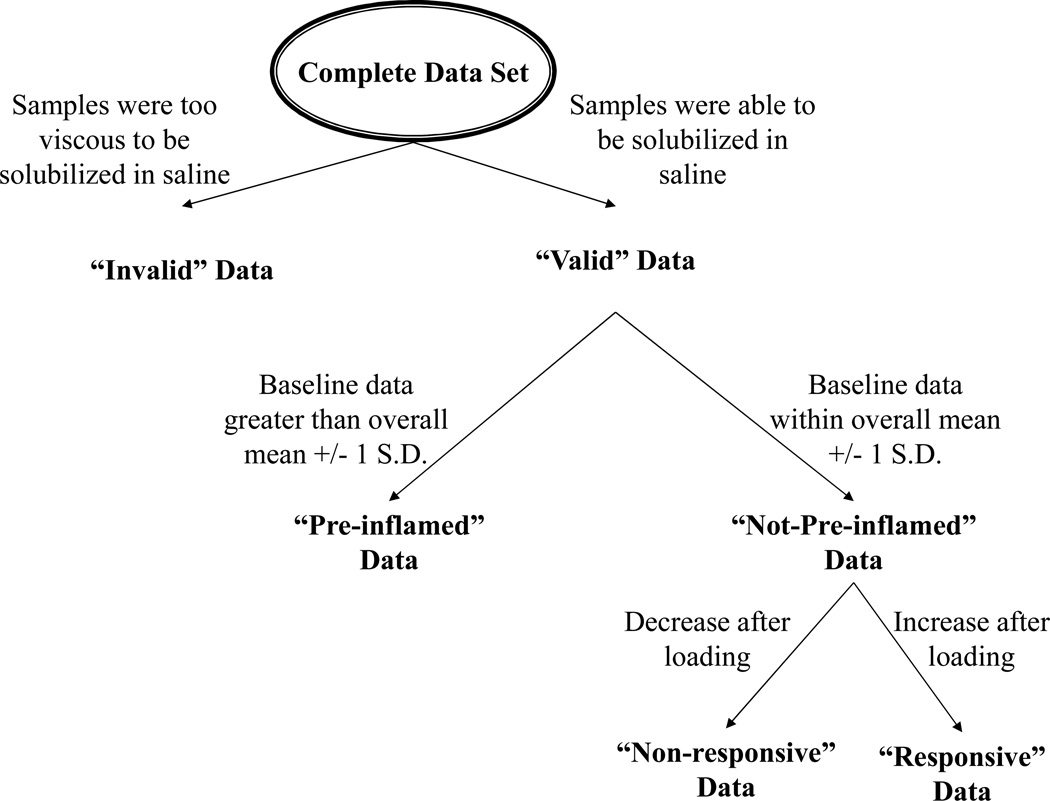
Inspection of the data further revealed that the data sorted into three main types across subjects and inflammatory markers (Figure 2): (1) data showing high baseline concentrations of pro-inflammatory markers (≥ 1 standard deviation in the total data set, designated as “pre-inflamed” data); (2) data showing normal baseline concentrations of markers (< 1 standard deviation in the data set), but paradoxically decreasing post loading (“non-responsive” data); and (3) data showing normal baseline concentrations of markers (< 1 standard deviation in the data set) and numeric increase after loading (“responsive”). Also, Table 1 shows that a limited number of data points were invalid due to thick secretions that precluded ELISA analysis. Data in each of these categories were relatively evenly distributed across subjects and treatment groups.
Figure 2.
Samples with high viscosity could not be solubilized in saline during the dilution procedure, and were thus considered “invalid.” Remaining data were then sorted into three main categories: “pre-inflamed data”, “non-responsive data” and “responsive data,” as described in the text.
In light of these findings, the most straightforward approach to our primary experimental question was to focus the main analyses on the “responsive” data set for subjects showing normal baseline mediator concentrations. Completely fortuitously, it turned out that one subject in each of the treatment conditions provided the optimal data set, all having valid, responsive data for IL1-β, IL-6, and MMP-8 (Subjects 1, 2, and 3, shown among unshadowed data, Table 1). Of note, those subjects were among those who received their respective treatments by a blinded clinician. We proceeded to normalize those subjects’ data to their own baseline values for the noted markers, and evaluated the normalized findings relative to the predicted pattern of results using non-parametric, binomial tests, one for the 4-hr and one for the 24-hr time point. For each test, the question was asked whether the data were positioned in the predicted position for the particular marker in question. Specifically, were normalized mediator concentrations for the inflammatory mediators (IL-1β, IL-6, IL-8, TNF-α, matrix metalloproteinase (MMP)-8) greatest for spontaneous speech, lower for voice rest, and lowest for resonant voice conditions? For the anti-inflammatory mediator, IL-10, which is reportedly triggered by tissue motion (32), were normalized concentrations lowest for voice rest (no mobilization), intermediate for spontaneous speech (some mobilization), and greatest for resonant voice, due to the relatively large-amplitude nature of this latter voicing modality? For both 4- and 24-hr data, each occurrence of a marker in the predicted position within the panel of markers was considered a “success.” Separate binomial tests were then used to evaluate the likelihood of the number of “successes” for the total number of “trials” (3 markers × 3 conditions = 9 “trials”), for each time point. To evaluate the fuller data set, averages for all valid, baseline-normal, responsive data from all subjects were then evaluated with similar binomial tests to assess results compared to protection. To control for alpha inflation, we applied a Bonferroni correction to an overall alpha level of .05, dividing by four tests, such that the alpha level for each test was .0125.
PTP and DME
The experimental questions surrounding PTP and DME regarded (1) the extent to which these measures might capture biological variations in the tissue as determined by correlation with inflammatory marker concentrations, and secondarily, (2) the extent to which PTP and DME might covary with each other. For both analyses, we used curve estimation to identify linear, quadratic, and curvilinear relations between PTP and normalized inflammatory concentrations, and between DME and normalized inflammatory concentrations, for the focus data set (Subjects 1, 2, and 3, for IL1-β, IL-6, and MMP-8). To address the relation between PTP and DME data, data were used from all subjects and all time points to identify linear, quadratic and curvilinear relations. Again, Bonferroni corrections were used in statistical testing (see Results).
RESULTS
Inflammatory mediators
Full data set
Raw values for the complete data set are shown in Table 1. As noted, variability in the data was shown with respect to baseline and immediate post-loading time points, before subjects were assigned to experimental condition. Table 1 highlights markers showing “pre-inflamed,” “non-responsive,” “responsive,” and “invalid” states, as defined in the Methods section.
Focus data set
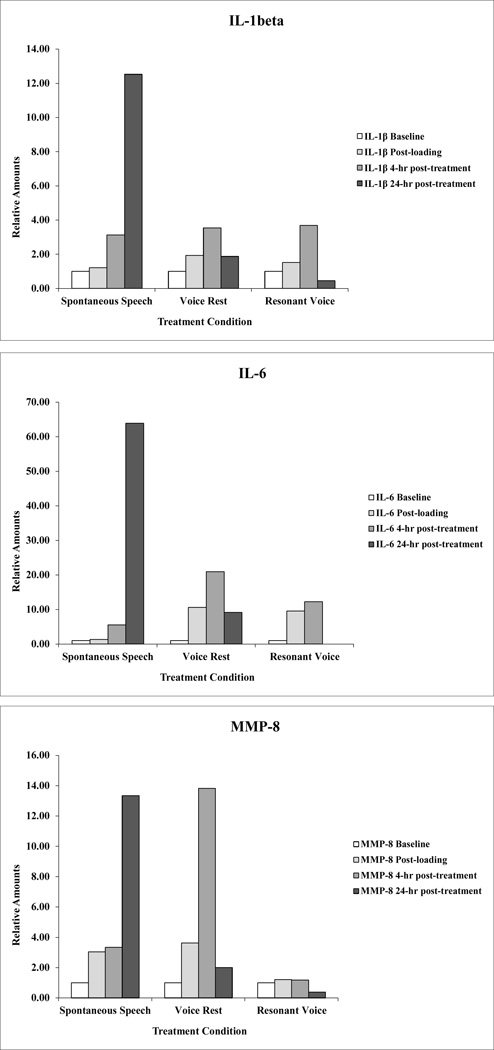
Results for the non-pre-inflamed, responsive data set – which we designate as the “focus data set” -- are summarized in Table 2 and Figure 3 (Subjects 3, 1, and 2). Those displays show normalized baseline concentrations of IL-1β, IL-6, and MMP-8 for the identified subjects as a function of treatment condition and time point. At the 4-hour time point, no clear pattern of results was observed. In contrast, the 24-hour data revealed that the normalized inflammatory concentrations for the identified markers were 100% aligned with predictions. Values for inflammatory mediators IL-1β, IL-6, and MMP-8 were greatest for spontaneous speech, lower for voice rest, and lowest for resonant voice. Values for the anti-inflammatory mediator IL-10 were greatest for resonant voice, intermediate for spontaneous speech, and lowest for voice rest. The probability of that result, which involved 9/9 prediction “successes” (3 markers × 3 subjects) was statistically significant at p = 0.004.
Table 2.
Normalized (Unitless Ratio) Data Values for Concentrations of IL-1β, IL-6, & MMP-8 across Time Points, for Subjects 1, 2, and 3 (see text; BL = Baseline; Post = 20–30 Minutes Post Loading; 4hr = 4 Hr after Initiation of Treatment; 24hr = 24 Hr Post Baseline).
| Time- point |
Subject | Sex | IL-1β | IL-6 | MMP-8 | |
|---|---|---|---|---|---|---|
| Spontaneous Speech | BL | 3 | F | 1.00 | 1.00 | 1.00 |
| Post | 1.21 | 1.32 | 3.04 | |||
| 4hr | 3.13 | 5.56 | 3.33 | |||
| 24hr | 12.52 | 63.86 | 13.34 | |||
| Voice Rest | BL | 1 | M | 1.00 | 1.00 | 1.00 |
| Post | 1.93 | 10.62 | 3.62 | |||
| 4hr | 3.54 | 20.94 | 13.82 | |||
| 24hr | 1.87 | 9.16 | 2.00 | |||
| Resonant Voice | BL | 2 | F | 1.00 | 1.00 | 1.00 |
| Post | 1.51 | 9.54 | 1.21 | |||
| 4hr | 3.68 | 12.25 | 1.18 | |||
| 24hr | 0.45 | 0.00 | 0.38 | |||
Figure 3.
Normalized (unitless ratio) values for three markers, IL1-β, IL-6, and MMP-8 for Subjects 3 (spontaneous speech), 1 (voice rest) and 2 (resonant voice) at baseline, immediate post loading, 4 hr post treatment onset, and 24 hr post baseline time points (see text).
Fuller data set
A binomial test using average data following data reduction, including all valid, non-pre-inflamed, responsive normalized data for all subjects similarly revealed a benefit of voice rest over spontaneous speech, and of resonant voice over voice rest, at the 24-(but not the 4-) hr time point. Table 3 shows the results. Specifically, again, for the 4-hr time point no discernible pattern was detected. However, at the 24-hr time point, normalized data for IL-1β, IL-6, and MMP-8 showed the identical pattern of results as for the focus data set. Moreover, including additional markers not available in the focus data set, a conceptually similar pattern was seen. Average concentrations for the inflammatory mediator IL-8 were worst (greatest) for spontaneous speech and best (lowest) for resonant voice at 24 hr (data were not available for this marker in the rest condition). For TNF-α, values were marginally greater for the resonant voice as compared to the voice rest condition, but as predicted, worst for spontaneous speech. Moreover, average concentrations of IL-10 – the anti-inflammatory mediator we evaluated – were best (greatest) for resonant voice, intermediate for spontaneous speech, and worst (lowest) for voice rest. The overall p value for this data set was statistically significant at 0.002, using a binomial test (15/17 mediators in predicted position).
Table 3.
Full Data Set. Individual Normalized (Unitless) Values (for Data from 1 Subject Only) and Means (for Data from > 1 Subject; Standard Error of Means in Parentheses) for Each Marker at Each Time Point after Data Reduction (Removal of Invalid, Pre-Inflamed and Unresponsive Markers) and Normalization to Baseline Values. SS=Spontaneous Speech; Rest = Voice Rest; RV=Resonant Voice.
| IL-1β | IL-6 | |||||
| SS | Rest | RV | SS | Rest | RV | |
| N | 1 | 1 | 1 | 2 | 1 | 2 |
| Post | 1.21 | 1.93 | 1.51 | 2.65 (1.33) | 10.62 | 8.31 (1.24) |
| 4hr post | 3.13 | 3.54 | 3.68 | 3.44 (2.12) | 20.94 | 6.30 (5.96) |
| 24hr post | 12.52 | 1.87 | 0.45 | 32.25 (31.61) | 9.16 | 2.72 (2.72) |
| IL-8 | TNF-α | |||||
| SS | Rest | RV | SS | Rest | RV | |
| N | 1 | 0 | 1 | 1 | 1 | 1 |
| Post | 4.57 | Nil | 6.22 | 1.25 | 1.18 | 1.26 |
| 4hr post | 4.18 | Nil | 4.23 | 0.96 | 1.22 | 1.30 |
| 24hr post | 14.81 | Nil | 2.08 | 4.69 | 1.11 | 1.14 |
| MMP-8 | IL-10 | |||||
| SS | Rest | RV | SS | Rest | RV | |
| N | 1 | 1 | 1 | 2 | 1 | 1 |
| Post | 3.04 | 3.62 | 1.21 | 1.53 (0.44) | 2. | 1.16 |
| 4hr post | 3.33 | 13.82 | 1.18 | 2.85 (1.07) | 0.56 | 1.59 |
| 24hr post | 13.34 | 2.00 | 0.38 | 2.62 (1.54) | 1.38 | 4.09 |
Relations across PTP, DME and inflammatory mediator data
Inter-investigator reliability for PTP was 0.997 (p < 0.05; Pearson’s correlation) for 10% of randomly selected utterances (reliability for DME values was not assessed; see Methods). Raw PTP and DME data are shown in Table 4. For the focus data set across all time points, all linear, quadratic and cubic slopes were about as close to 0.00 as they could get, ranging from −0.001 to −0.045 (Table 5). Oddly, all slopes were also numerically negative. R2s – which were necessarily positive due to squaring of values -- ranged between 0.127 and 0.652 (Table 5). Using Bonferroni protection for multiple comparisons (.05 alpha level/18 tests for 3 markers * 2 dependent variables * 3 levels of relation, p = .0028), none of the relations achieved statistical significance. Stated differently, there was no evidence that either PTP or DME might function as a non-invasive proxy for inflammatory mediator concentrations.
Table 4.
Raw Data Values for Phonation Threshold Pressure (PTP, in cmH20) and Direct Magnitude Estimation of Phonatory Effort (DME; Unitless) across Time Points for Each Subject.
| Time-point | Subject | PTP (cm/H20) | DME | |
|---|---|---|---|---|
| Spontaneous Speech | BL | 3 | 5.36 | 3 |
| Post | 3.19 | 1 | ||
| 4hr | 2.28 | 2 | ||
| 24hr | 3.28 | 1 | ||
| BL | 6 | 11.34 | 2 | |
| Post | 5.28 | 2.5 | ||
| 4hr | 4.04 | 2 | ||
| 24hr | 5.18 | 2 | ||
| BL | 8 | 3.97 | 2 | |
| Post | 6.79 | 3 | ||
| 4hr | 6.91 | 3 | ||
| 24hr | 5.44 | 2 | ||
| Voice Rest | BL | 1 | 8.55 | 2 |
| Post | 5.63 | 2 | ||
| 4hr | 4.33 | 1 | ||
| 24hr | 7.08 | 1 | ||
| BL | 5 | 3.98 | 1 | |
| Post | 8.22 | 2 | ||
| 4hr | 6.51 | 1 | ||
| 24hr | 5.74 | 1 | ||
| BL | 7 | 4.45 | 1 | |
| Post | 7.51 | 3 | ||
| 4hr | 7.49 | 2 | ||
| 24hr | 6.73 | 1 | ||
| Resonant Voice | BL | 2 | 6.94 | 3 |
| Post | 4.45 | 3 | ||
| 4hr | 2.97 | 2 | ||
| 24hr | 6.84 | 3 | ||
| BL | 4 | 6.32 | 1 | |
| Post | 4.04 | 1.6 | ||
| 4hr | 3.23 | 1 | ||
| 24hr | 4.86 | 1.1 | ||
| BL | 9 | 7.65 | 5 | |
| Post | 6.33 | 2 | ||
| 4hr | 7.42 | 2 | ||
| 24hr | 6.01 | 3 | ||
Table 5.
Linear, Quadratic, and Cubic Slopes (b), R2, and p Values for Phonation Threshold Pressure (PTP, in cm H20) and Direct Magnitude Estimation of Phonatory Effort (DME, unitless) versus IL-1β, IL-6, and MMP-8. Referent Data are from Focus Data Set (Subjects 1, 2, and 3) Collapsed over 4 Time Points (Baseline, Post-loading, 4 hr Post Treatment Onset and 24 hr Post Baseline). (With Bonferroni Correction for Overall α Set at .05, for 18 Tests, Criterion for Each Test is .003. None of the Tests Achieved Statistical Significance.)
|
PTP- IL-1β |
PTP- IL-6 |
PTP- MMP-8 |
|
| Linear | |||
| b (slope) | −.003 | −.002 | −.003 |
| R2 | .193 | .127 | .228 |
| (p) | (.153) | (.255) | (.116) |
| Quadratic | |||
| b (slope) | −.012 | −.045 | −.010 |
| R2 | .293 | .652 | .370 |
| (p) | (.210) | (.009) | (.125) |
| Cubic | |||
| b (slope) | −.015 | −.045 | −.028 |
| R2 | .285 | .652 | .513 |
| (p) | (.399) | (.009) | (.108) |
|
DME- IL-10β |
DME- IL-6 |
DME- MMP-8 |
|
| Linear | |||
| b (slope) | −.002 | −.001 | −.001 |
| R2 | .317 | .158 | .374 |
| (p) | (.057) | (.200) | (.035) |
| Quadratic | |||
| b (slope) | −.006 | −.008 | −.004 |
| R2 | .473 | .240 | .495 |
| (p) | (.056) | (.290) | (.046) |
| Cubic | |||
| b (slope) | −.010 | −.008 | −.007 |
| R2 | .480 | .240 | .510 |
| (p) | (.137) | (.290) | (.110) |
Similarly, no clear evidence was seen of any linear, quadratic, or cubic relations between PTP and DME as sometimes suggested in the literature (38, 47–49) (slopes ranged from −0.216 to 0.537; p-values were 0.10–0.25).
DISCUSSION
In extra-laryngeal tissues, mobilization has been shown to be anabolic and to facilitate a regenerative tissue healing response. To date, these phenomena have not been formally reported in the context of vocal fold injury. In fact, traditional practice in rehabilitation of acutely injured vocal folds mandates limited voice use and limited vocal intensity, and even voice rest. Preliminary in vitro data from our laboratory suggest that the cellular response to mechanical signaling in the vocal folds may, in fact, mimic other connective tissues, pointing to the possibility that voice rest may not be the ideal approach to optimize healing outcomes in all patients with acute injury (7). One potential limitation to this type of investigation in humans is methodological: traditional methods to investigate the inflammatory phase of healing are not feasible in the human vocal folds, in vivo. As such, our laboratory described the immunoassay of soluble mediators of wound healing present in secretions collected from the surface of the vocal folds as a means to quantify these wound healing events (21).
Utilizing this approach, we sought to determine the effects of tissue mobilization in the resolution of vocal fold inflammation following acute phonotrauma induced in the laboratory. Biological data from our study suggest that voice rest and resonant voice exercises yielded improved post-traumatic inflammatory profiles in subjects with interpretable data at 24 hr post baseline, compared to spontaneous speech, for which post-traumatic profiles were generally worse than baseline. Specifically, although no discernible pattern of results was evident after 4 hr of in-clinic interventions, for subjects with interpretable data in both focus and fuller data sets, with one minor exception normalized concentrations of inflammatory mediators IL-1β, IL-6, IL-8, TNF-α, and MMP-8 were greatest at 24 hr post baseline for the spontaneous speech condition, improved following voice rest, and lowest following resonant voice exercises (the exception was that average concentration of TNF-α was about the same for voice rest and resonant voice at 24 hr). Moreover, again for both focus and fuller data sets, average normalized concentrations of the anti-inflammatory mediator IL-10 was greatest at 24 hr following resonant voice exercises, somewhat lower for spontaneous speech, and lowest following absolute voice rest, the only condition involving no vocal fold vibrations whatsoever post-traumatically.
If tissue mobilization improves the acute inflammatory profile in vocal fold tissue compared to rest, the question can be asked why resonant voice appeared to reduce inflammation but spontaneous speech actually increased it. Potentially, as previously discussed, the combination of relatively large-amplitude, low-impact vibrations associated with resonant voice is critical for its benefit. However, it is likely that dose-dependency is also in play. The spontaneous speech condition involved 16 minutes of phonation followed by 4 min of voice rest during the in-clinic treatment, as compared to the reverse pattern that was used for resonant voice. It is reasonable to posit that there is an ideal phonation dose to improve acute inflammation, as shown in our in vitro studies (7, 50). We are currently working with computational modeling ultimately to address dose-dependency in inflammation (51, 52). However, in the meantime, the present data clearly show that a limitation of phonation time is not the only factor in inflammation control. If it were, results would have been best for voice rest – and they were not.
One issue that is critical to address is the variability in the biological data reported here. In recruiting vocally healthy individuals, as clinically assessed, we assumed participants would have low concentrations of inflammatory markers at baseline, and that concentrations would increase post loading, consistent with findings from our previous work (21). Although all subjects had normal values for at least one inflammatory mediator at baseline, 7/ 9 subjects also had abnormally high baseline values for one or more mediators. Several factors may have been contributory in baseline data. For example, asymptomatic episodic laryngopharyngeal reflux or casual environmental exposures to pollutants could have elevated certain inflammatory concentrations at baseline for some subjects. Moreover, perhaps clinical examination and self-report are insufficient to detect vocal fold inflammation, which instead requires more sophisticated technology such as the technology used here. A similar question can be posed regarding some subjects’ apparent non-response to the vocal loading protocol, based on inflammatory mediatory concentrations. Again, although all subjects showed responsiveness in at least one mediator post loading, 6/9 subjects did not show any increase in one or more mediators post load. A plausible speculation is that the loading protocol did not exceed the threshold required for injury for those subjects, for those particular markers. In fact, it is clear that the wound healing response is complex, involving many cell types and soluble mediators (53–57), for which patterns may vary across individuals. One component in biological responses may be a genetically-predetermined balance between inflammatory and anti-inflammatory mediators in wound healing (58–60). Moreover, in our study we did not control for voicing modality during loading. A fundamental premise in voice therapy is that some modalities – for example pressed voice, associated with high impact stresses – are more damaging to the tissue than others (61). We therefore expect variability in individuals’ response to vocal loading and injury based on the interaction of genetic and phonatory variables, at minimum.
Having said as much, one potentially disconcerting aspect of the data is seen in PTP measures, for which values actually decreased within the immediate post-loading window in 6/9 subjects. This finding contrasts with general clinical expectations that PTP should increase with laryngeal injury (62). Stated differently, although biological data indicated vocal loading was inflammatory to some degree for all subjects, the PTP data pose the question of whether the loading protocol constituted actual loading for fully two-thirds of subjects. This possibility is interesting to entertain. Visual observations of physical changes to the larynx provide no help to address this issue, as such observations were not made systematically and no spontaneous observations emerged along these lines. However, recent data suggest PTP fluctuates with training alone (63). Thus, perhaps some of the fluctuations seen in PTP were due to learning rather than biological factors. Moreover, as for biological data, several factors regulate PTP, one of them being glottal gap between the vocal folds width, which is directly related to threshold pressure (64, 65). As laryngeal tissue becomes engorged with the initial results of inflammation, perhaps the glottal gap is decreased thus paradoxically reducing PTP. In sum, comparing results for the biological mediators in the present study versus PTP, we may need to entertain the notion that perhaps clinical concepts around PTP as an indicator of laryngeal injury may be misguided, at least where acute injury is concerned.
Although we are cautiously optimistic about the interpretable results that emerged in the data above the noise, we would be remiss to exclude discussion regarding the validity of the secretion analysis technique. We acknowledge the limitations of the assays, as seen in some of the noted challenges encountered in data collection. However, independent data do indicate there is co-variation between mediator concentrations in secretions and underlying tissue. Using an animal model of controlled subglottic mucosal injury, Hebda’s laboratory demonstrated that IL-1β and other soluble inflammatory mediators became elevated in secretions within 24 hours post injury, the response reflected degree of injury, and a positive correlation was shown between mediator increase in the secretions and localized up-regulation of expression of the mediator in the subglottic tissue (66–69). Although we recognize that the specific cell-source of these markers is not known, Hebda’s findings appear to support the utilization of select inflammatory mediators in secretions as surrogate markers for phonotrauma. Outstanding questions invite further investigation.
Turning to results for PTP and DME, as we hypothesized, there was no evidence of any links between these variables and mediator concentrations. Slopes for linear, quadratic, and cubic relations were essentially nil, failing to approach statistical significance. As such, results from our study do not provide optimism that either PTP or DME may be reasonable surrogates for more invasive estimates of acute vocal fold injury.
A further question regards the relation between PTP and DME. Logically, it would seem these physiological and perceptual estimates of phonation effort should covary. Our data showed no indication of any such relation. In fact, other recent data show these variables may not be as tightly related as sometimes assumed (38, 47–49). One possibility is that PTP and DME may capture different biological processes in vocal fatigue or injury, as PTP has been reported to return to baseline within about an hour of a vocal loading task in comparison to DME, which according to one report requires a full day (47). In that case, the measures would not be expected to covary tightly.
In summary, despite its limitations, the current study is the first to address systematically biological mechanisms in behavioral voice therapy for patients with acute vocal fold injury. Our data suggest that some forms of tissue mobilization may represent a rational treatment approach for acute mucosal injury, in some individuals. Although the direct translation to clinical practice is not yet straightforward, the current study certainly suggests some clinical value may be found in controlled, vocal exercise in the context of acute phonotrauma.
ACKNOWLEDGEMENTS
The study was supported by R01 DC5643 from the National Institute on Deafness and Other Communication Disorders. The authors acknowledge the substantial contributions of Dr. Priya Krishna and Maria Dietrich in data collection and management as well as Dr. Elaine Rubenstein for statistical consulting and Mr. Neil Szuminsky for the development of software for data analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Preliminary data were presented at the 34th Symposium: Care of the Professional Voice, Philadelphia, Pennsylvania, June 2005.
REFERENCES
- 1.Colton R, Casper JK. Understanding Voice Problems: A Physiological Perspective for Diagnosis and Treatment. 2nd ed. Baltimore: Williams & Wilkins; 1996. [Google Scholar]
- 2.Boone DR, McFarlane SC. The Voice and Voice Therapy. 5th ed. Englewood Cliffs: Prentice-Hall, Inc.; 1994. [Google Scholar]
- 3.Gray SD. Benign Pathologic Responses of the Larynx. NCVS Status and Progress Report. 1997;11:135–148. [Google Scholar]
- 4.Gunter HE. Modeling mechanical stresses as a factor in the etiology of benign vocal fold lesions. Journal of Biomechanics. 2004;37:1119–1124. doi: 10.1016/j.jbiomech.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Titze IR. Mechanical stress in phonation. J Voice. 1994;8(2):99–105. doi: 10.1016/s0892-1997(05)80302-9. [DOI] [PubMed] [Google Scholar]
- 6.Ferretti M, Gassner R, Wang Z, et al. Biomechanical signals suppress proinflammatory responses in cartilage: early events in experimental antigen-induced arthritis. Journal of immunology. 2006;177(12):8757–8766. doi: 10.4049/jimmunol.177.12.8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Branski RC, Perera P, Verdolini K, et al. Dynamic biomechanical strain inhibits IL-1beta-induced inflammation in vocal fold fibroblasts. J Voice. 2007;21(6):651–660. doi: 10.1016/j.jvoice.2006.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dang C, Ting K, Soo C, et al. Fetal wound healing current perspectives. Clin Plast Surg. 2003;30(1):13–23. doi: 10.1016/s0094-1298(02)00067-6. [DOI] [PubMed] [Google Scholar]
- 9.Mishra S, Rosen CA, Murry T. Acute management of the performing voice. Otolaryngol Clin North Am. 2000;33(5):957–966. doi: 10.1016/s0030-6665(05)70257-7. [DOI] [PubMed] [Google Scholar]
- 10.Mortensen M, Woo P. Office steroid injections of the larynx. The Laryngoscope. 2006;116(10):1735–1739. doi: 10.1097/01.mlg.0000231455.19183.8c. [DOI] [PubMed] [Google Scholar]
- 11.Tateya I, Omori K, Kojima H, et al. Steroid injection for Reinke's edema using fiberoptic laryngeal surger. Acta oto-laryngologica. 2003;123:417–420. doi: 10.1080/00016480310001321. [DOI] [PubMed] [Google Scholar]
- 12.Tateya I, Omori K, Kojima H, et al. Steroid injection to vocal nodules using fiberoptic laryngeal surgery under topical anesthesia. Eur Arch Otorhinolaryngol. 2004;261(9):489–492. doi: 10.1007/s00405-003-0720-x. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal S. Low magnitude of tensile strain inhibits IL-1beta dependent induction of pro-inflammatory cytokines and induces synthesis of IL-10 in human periodontal ligament cells in vitro. Journal of Dental Research. 2001;80(5):1416–1420. doi: 10.1177/00220345010800050601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agarwal S, Long P, Gassner R, et al. Cyclic tensile strain suppresses catabolic effects of interleukin-1beta in fibrochondrocytes from the temporomandibular joint. Arthritis & Rheumatism. 2001;44(3):608–617. doi: 10.1002/1529-0131(200103)44:3<608::AID-ANR109>3.0.CO;2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Long P, Buckley MJ, Liu F, et al. Signalling by mechanical strain involves transcriptional regulation of proinflammatory genes in human periodontal ligament cells in vitro. Bone. 2002;30:547–552. doi: 10.1016/s8756-3282(02)00673-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Z, Buckley MJ, Evans CH, Agarwal S. Cyclic tensile strain acts as an antagonist of IL-1B actions in chrondrocytes. The Journal of Immunology. 2000;165:453–460. doi: 10.4049/jimmunol.165.1.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agarwal S, Deschner J, Long P, et al. Role of NF-kappaB transcription factors in antiinflammatory and proinflammatory actions of mechanical signals. Arthritis and rheumatism. 2004;50(11):3541–3548. doi: 10.1002/art.20601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deschner J, Hofman CR, Piesco NP, Agarwal S. Signal transduction by mechanical strain in chondrocytes. Current opinion in clinical nutrition and metabolic care. 2003;6(3):289–293. doi: 10.1097/01.mco.0000068964.34812.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green T, Refshauge K, Crosbie J, Adams R. A randomized controlled trial of a passive accessory joint mobilization on acute ankle inversion sprains. Physical Therapy. 2001;81(4):984–994. [PubMed] [Google Scholar]
- 20.Kamps BS, Linder LH, DeCamp CE, Haut RC. The influence of immobilization versus exercise on scar formation in the rabbit patellar tendoon after excision of the central third. Am J Sports Med. 1994;22(6):803–811. doi: 10.1177/036354659402200612. [DOI] [PubMed] [Google Scholar]
- 21.Verdolini K, Rosen CA, Branski RC, Hebda PA. Shifts in biochemical markers associated with wound healing in laryngeal secretions following phonotrauma: A preliminary study. Annals of Otology, Rhinology, & Laryngology. 2003;112(12):1021–1025. doi: 10.1177/000348940311201205. [DOI] [PubMed] [Google Scholar]
- 22.Branski RC, Verdolini K, Rosen CA, Hebda PA. Markers of wound healing in vocal fold secretions from patients with laryngeal pathology. Annals of Otology, Rhinology, and Laryngology. 2004;113(1):23–29. doi: 10.1177/000348940411300105. [DOI] [PubMed] [Google Scholar]
- 23.Branski RC, Hebda PA, Hake H, et al. Mucosal wound healing in a rabbit model of subglottic stenosis: biochemical analysis of secretions. Archives of Otolaryngology-Head & Neck Surgery. 2005;131:153–157. doi: 10.1001/archotol.131.2.153. [DOI] [PubMed] [Google Scholar]
- 24.Branski RC, Rosen CA, Hebda PA, Verdolini K. Cytokine analysis of acute wound healing in the larynx: A rabbit model. Journal of Voice. 2005;19(2):283–289. doi: 10.1016/j.jvoice.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Sakai A, Ohshima M, Sugano N, et al. Profiling the Cytokines in Gingival Crevicular Fluid Using a Cytokine Antibody Array. J Periodontol. 2006;77(5):856–864. doi: 10.1902/jop.2006.050340. [DOI] [PubMed] [Google Scholar]
- 26.Nakashima T, Tomita H, Chitose S, et al. Local immune status and tumour marker expression in the human larynx. J Laryngol Otol Suppl. 2009;(31):1–4. doi: 10.1017/S0022215109005003. [DOI] [PubMed] [Google Scholar]
- 27.Verdolini K. Resonant Voice Therapy. In: Stemple JC, editor. Voice Therapy: Clinical Studies. San Deigo: Singular Publishing Group; 2000. pp. 46–62. [Google Scholar]
- 28.Verdolini-Marston K, Burke MK, Lessac A, et al. Preliminary study of two methods of treatment for laryngeal nodules. Journal of Voice. 1995;9(1):74–85. doi: 10.1016/s0892-1997(05)80225-5. [DOI] [PubMed] [Google Scholar]
- 29.Berry DA, Verdolini K, Montequin DW, et al. A quantitative output-cost ration in voice production. J Speech Lang Hear Res. 2001;44(1):29–37. doi: 10.1044/1092-4388(2001/003). [DOI] [PubMed] [Google Scholar]
- 30.Peterson KL, Verdolini-Marston K, Barkmeir JM, Hoffman HT. Comparison of aerodynamic and electroglottographic parameters in evaluating clinically relevant voicing patterns. The Annals of otology, rhinology, and laryngology. 1994;103(5 Pt 1):335–346. doi: 10.1177/000348949410300501. [DOI] [PubMed] [Google Scholar]
- 31.Verdolini K, Druker DG, Palmer PM, Samawi H. Laryngeal adduction in resonant voice. J Voice. 1998;12(3):315–327. doi: 10.1016/s0892-1997(98)80021-0. [DOI] [PubMed] [Google Scholar]
- 32.Helmark IC, Mikkelsen UR, Borglum J, et al. Exercise increases interleukin-10 levels both intraarticularly and peri-synovially in patients with knee osteoarthritis: a randomized controlled trial. Arthritis research & therapy. 2010;12:R126. doi: 10.1186/ar3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keylock KT, Vieira VJ, Wallig MA, et al. Exercise accelerates cutaneous wound healing and decreases wound inflammation in aged mice. American journal of physiology. 2008;294(1):R179–R184. doi: 10.1152/ajpregu.00177.2007. [DOI] [PubMed] [Google Scholar]
- 34.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98(4):1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 35.Bastian RW, Keidar A, Verdolini-Marston K. Simple vocal tasks for detecting vocal fold swelling. Journal of Voice. 1990;4(2):172–183. [Google Scholar]
- 36.Eckel RC, Boone DR. The s/z ratio as an indicator of laryngeal pathology. J Speech Hear Dis. 1981;46:147–149. doi: 10.1044/jshd.4602.147. [DOI] [PubMed] [Google Scholar]
- 37.Titze IR. The physics of small-amplitude oscillation of the vocal folds. The Journal of the Acoustical Society of America. 1988;83(4):1536–1552. doi: 10.1121/1.395910. [DOI] [PubMed] [Google Scholar]
- 38.Verdolini K, Titze IR, Fennell A. Dependence of phonatory effort on hydration level. Journal of speech and hearing research. 1994;37(5):1001–1007. doi: 10.1044/jshr.3705.1001. [DOI] [PubMed] [Google Scholar]
- 39.Roy N, Merrill RM, Thibeault SL, et al. Prevalence of voice disorders in teachers and the general population. Journal of Speech, Language, and Hearing Research. 2004;47(281–293) doi: 10.1044/1092-4388(2004/023). [DOI] [PubMed] [Google Scholar]
- 40.Coyle SM, Weinrich BD, Stemple JC. Shifts in relative prevalence of laryngeal pathology in a treatment-seeking population. Journal of Voice. 2001;15(3):424–440. doi: 10.1016/S0892-1997(01)00043-1. [DOI] [PubMed] [Google Scholar]
- 41.Holmberg EB, Hillman RE, Perkell JS. Glottal airflow and transglottal air pressure measurements for male and female speakers in soft, normal, and loud voice. The Journal of the Acoustical Society of America. 1988;84(2):511–529. doi: 10.1121/1.396829. [DOI] [PubMed] [Google Scholar]
- 42.Verdolini K, Titze IR, Druker DG. Changes in Phonation Threshold Pressure with Induced Conditions of Hydration. Journal of Voice. 1990;4(2):142–151. [Google Scholar]
- 43.Finkelhor BK, Titze IR, Durham PL. The effect of viscosity changes in the vocal folds on the range of oscillation. Journal of Voice. 1988;1:320–325. [Google Scholar]
- 44.Wright HN, Colton RH. Some Parameters of Vocal Effort. The Journal of the Acoustical Society of America. 1972;51(1A):141. [Google Scholar]
- 45.Colton RH, Brown J. Some Relationships between Vocal Effort and Intraoral Air Pressure. The Journal of the Acoustical Society of America. 1973;53(1):296. [Google Scholar]
- 46.Wulf G, Prinz W. Directing attention to movement effects enhances learning: a review. Psychonomic bulletin & review. 2001;8(4):648–660. doi: 10.3758/bf03196201. [DOI] [PubMed] [Google Scholar]
- 47.Chang A, Karnell MP. Perceived phonatory effort and phonation threshold pressure across a prolonged voice loading task: a study of vocal fatigue. J Voice. 2004;18(4):454–466. doi: 10.1016/j.jvoice.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 48.Plexico LW, Sandage MJ, Faver KY. Assessment of phonation threshold pressure: a critical review and clinical implications. American journal of speech-language pathology / American Speech-Language-Hearing Association. 2011;20(4):348–366. doi: 10.1044/1058-0360(2011/10-0066). [DOI] [PubMed] [Google Scholar]
- 49.Sivasankar M, Fisher KV. Oral breathing increases Pth and vocal effort by superficial drying of vocal fold mucosa. Journal of voice : official journal of the Voice Foundation. 2002;16(2):172–181. doi: 10.1016/s0892-1997(02)00087-5. [DOI] [PubMed] [Google Scholar]
- 50.Branski RC. Vocal fold fibroblast response to mechanical stress [doctoral dissertation] Pittsburgh: University of Pittsburgh; 2005. [Google Scholar]
- 51.Li NYK, Verdolini K, Clermont G, et al. A patient-specific in silico model of inflammation and healing tested in acute vocal fold injury. PLoS One. 2008;3(7):e2789. doi: 10.1371/journal.pone.0002789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li NYK, Vodovotz Y, Kim KH, et al. Biosimulation of acute phonotrauma: An extended model. The Laryngoscope. 2011;121(11):2418–2428. doi: 10.1002/lary.22226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Broughton G, 2nd, Janis JE, Attinger CE. The basic science of wound healing. Plast Reconstr Surg. 2006;117(7 Suppl):12S–34S. doi: 10.1097/01.prs.0000225430.42531.c2. [DOI] [PubMed] [Google Scholar]
- 54.Gillitzer R, Goebeler M. Chemokines in cutaneous wound healing. Journal of leukocyte biology. 2001;69(4):513–521. [PubMed] [Google Scholar]
- 55.Henry G, Garner WL. Inflammatory mediators in wound healing. The Surgical clinics of North America. 2003;83(3):483–507. doi: 10.1016/S0039-6109(02)00200-1. [DOI] [PubMed] [Google Scholar]
- 56.Kirsner RS, Eaglstien WH. The Wound Healing Process. Dermatologic Clinics. 1993;11(4):629–640. [PubMed] [Google Scholar]
- 57.Moulin V. Growth factors in skin wound healing. European journal of cell biology. 1995;68(1):1–7. [PubMed] [Google Scholar]
- 58.Arcaroli J, Fessler MB, Abraham E. Genetic polymorphisms and sepsis. Shock. 2005;24(4):300–312. doi: 10.1097/01.shk.0000180621.52058.e1. [DOI] [PubMed] [Google Scholar]
- 59.Erbek SS, Yurtcu E, Erbek S, et al. Proinflammatory cytokine single nucleotide polymorphisms in nasal polyposis. Archives of otolaryngology--head & neck surgery. 2007;133(7):705–709. doi: 10.1001/archotol.133.7.705. [DOI] [PubMed] [Google Scholar]
- 60.Smith AJ, Humphries SE. Cytokine and cytokine receptor gene polymorphisms and their functionality. Cytokine Growth Factor Rev. 2009;20(1):43–59. doi: 10.1016/j.cytogfr.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 61.Berry DA, Verdolini K, Montequin D, et al. A quantitative output-cost ratio in voice production. Journal of Speech, Language, and Hearing Research. 2001;44(1):29–37. doi: 10.1044/1092-4388(2001/003). [DOI] [PubMed] [Google Scholar]
- 62.Jiang J, O'Mara T, Conley D, Hanson D. Phonation threshold pressure measurements during phonation by airflow interruption. The Laryngoscope. 1999;109(3):425–432. doi: 10.1097/00005537-199903000-00016. [DOI] [PubMed] [Google Scholar]
- 63.Dastolfo C. Effects of Repetition on Phonation Threshold Pressure Task Performance. Pittsburgh: University of Pittsburgh; 2011. [Google Scholar]
- 64.Titze IR. Phonation threshold pressure: a missing link in glottal aerodynamics. The Journal of the Acoustical Society of America. 1992;91(5):2926–2935. doi: 10.1121/1.402928. [DOI] [PubMed] [Google Scholar]
- 65.Titze IR, Schmidt SS, Titze MR. Phonation threshold pressure in a physical model of the vocal fold mucosa. The Journal of the Acoustical Society of America. 1995;97(5 Pt 1):3080–3084. doi: 10.1121/1.411870. [DOI] [PubMed] [Google Scholar]
- 66.Branski RC, Sandulache VC, Dohar JE, Hebda PA. Mucosal wound healing in a rabbit model of subglottic stenosis: biochemical analysis of secretions. Archives of otolaryngology--head & neck surgery. 2005;131(2):153–157. doi: 10.1001/archotol.131.2.153. [DOI] [PubMed] [Google Scholar]
- 67.Sandulache VC, Chafin JB, Li-Korotky HS, et al. Elucidating the role of interleukin 1beta and prostaglandin E2 in upper airway mucosal wound healing. Archives of otolaryngology--head & neck surgery. 2007;133(4):365–374. doi: 10.1001/archotol.133.4.365. [DOI] [PubMed] [Google Scholar]
- 68.Sandulache VC, Singh T, Li-Korotky HS, et al. Prostaglandin E2 is activated by airway injury and regulates fibroblast cytoskeletal dynamics. The Laryngoscope. 2009;119(7):1365–1373. doi: 10.1002/lary.20173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Singh T, Barsic M, Dohar JE, Hebda PA. Interleukin-1beta as a marker of mucosal injury and inflammation. Wound Repair Regen. 2008;16:A49. [Google Scholar]