Abstract
There are many registries in Latin America as dialysis and kidney transplantation, breast cancer, primary immunodeficiency, acute coronary syndromes, but the focus here are the registries of lysosomal storage diseases (LSD) because is our experience. Registry of Gaucher disease, Fabry disease, Pompe disease, and mucopolysaccharidosis type I are comprehensive observational voluntary programs that aim to collect clinical and laboratory data of initiation, progression, and evolution of those diseases, with and without treatment, using questionnaires of quality of life and/or skills and functions. There are two more programs of LSD: Hunter outcome survey and Fabry outcome survey. The registries are a kind of phase IV clinical trials, postmarketing studies delineate additional information including the drug’s risks, benefits, and optimal use, and in addition we have data from natural history. The demographics of the Gaucher, Fabry, MPS I, and Pompe Registries show that a total of patients, being 16%, 8%, 15%, and 7%, respectively, of this population, and 19%, 19%, 18%, and 13%, respectively, of all physicians participating in the program are from Latin America. In the Gaucher Registry, we can observe that the percentage of children in Latin America (29%) is bigger than the rest of the world (20%), what can mean more severe disease in this population. These diseases are rare, and a database of clinical data from a larger number of patients gives us the opportunity to know about the natural history of these diseases, their phenotypic variability, and the response to specific enzyme replacement therapy in our population.
Keywords: Disease registries, Fabry registry, Gaucher registry, Latin America diseases registries, Mucoolisaccharidosis I registry, Pompe registry
Introduction
Lysosomal storage diseases (LSD) including in Latin America, we have registries of Gaucher disease, Fabry disease, Pompe disease, mucopolysaccharidosis type I, Hunter outcome survey (HOS), and Fabry outcome survey (FOS). The infrastructure of the Gaucher Registry, Fabry Registry, Pompe Registry, and mucopolysaccharidosis type I (MPS I Registry) is sponsored by Genzyme Corporation (Cambridge, MA, USA), and they gave us permission to publish the registries data from 2009. All these registries are comprehensive observational voluntary programs that aim to collect clinical and laboratory data of initiation, progression, and evolution of those diseases, with and without treatment, using questionnaires of quality of life and/or skills and functions. The privacy of doctors and patients is protected, and they are identified only by the initials of their names.
The Advisory Boards are formed by a body of independent physicians specialized in each disease, and they coordinate the regular meetings of those programs to assess the possibilities for scientific research; evaluate the quality and specificity of the collected data, making changes in these data whenever it is necessary; analyze the request of the registries data for publications by the participants; schedule the publications; create working groups among the participating physicians; and evaluate consultations on the registries.
The above-mentioned diseases are rare, and a database of clinical data from a larger number of patients gives us the opportunity to know more about the natural history of these diseases, their phenotypic variability, and the response to enzyme replacement therapy. Often patients present clinical manifestations that were not previously described in the literature. The physician participating in the program can query the registry database of that particular disease, questioning whether the observed event is recorded and, according to the response, the doctor may use this information for a publication or presentation at medical meetings, reverting the findings into a better knowledge about the disease.
The coordinators of the registries of LSD in Latin America have an educational role with physicians and patients, clarifying questions about the rare disease in question, its development, monitoring, complications, recommended follow-up examinations, and case discussions during the visit of the coordinator and/or at a distance. As the coordinators travel great distances for visits, often there is a request of lectures to physicians and patients from that region so that a larger number of people can solve their questions.
The 2009 Annual Report of the Fabry Registry (Sims et al. 2009) pointed out the importance of the characteristics of voluntary collection of observational data; patients are not randomized into groups “with treatment” and “no treatment,” and in general the treated patients constitute more severe cases, what generates inherent biases. This report recommends that these and other factors must be considered when the registration data are evaluated.
The registry programs have developed guidelines for patients, periodical clinical and laboratory evaluation, but because registration is voluntary, the doctor accompanying the patient is who settles the kind of assessments and their frequency for each patient. This reflects the quality of data, especially in the long term.
Databases that combine features of protocol-oriented and practice-oriented information have as a goal to create a large and diverse source of prospective longitudinal patient data (Thadani 2006). The registries cited here are examples of such a database.
Data from the registries of the diseases herein described were supplied by the databases with the consent of the Advisory Boards, and the data were collected until October 2009.
Data Presentation and Discussion
The first registry of LSD was the Gaucher Registry, initiated in 1991, to fulfill a requirement of the FDA to monitor a larger number of patients with and without treatment.
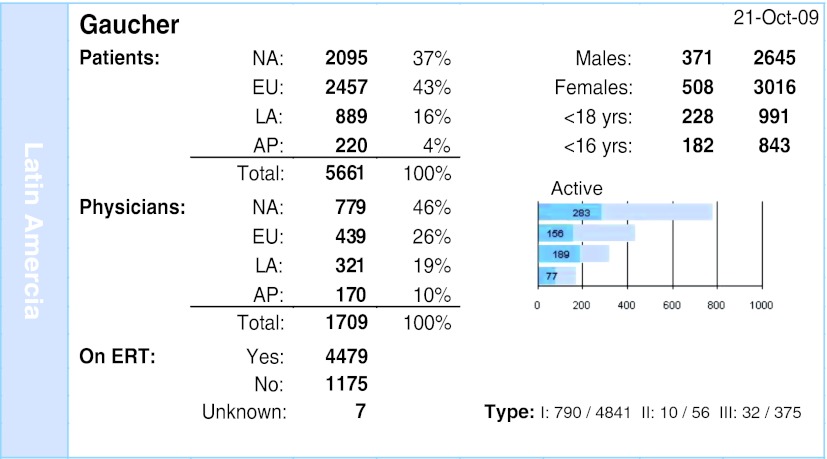
The demographics data of the Gaucher Registry (Fig. 1) show a total of 5,661 patients, being 16% of this population and 19% of all physicians participating in the program from Latin America. It is observed that the majority of patients have the type 1, the non-neuropathic form (92%). The largest proportion of patients receiving treatment live in Latin America (76%) in comparison with the rest of the world can probably be because most patients being diagnosed in Latin America are serious cases and not the oligosymptomatic. In the clinical practice, this information is important to give us the children’s treatment because if we diagnosed early is because they are more severe.
Fig. 1.
Gaucher Registry overview
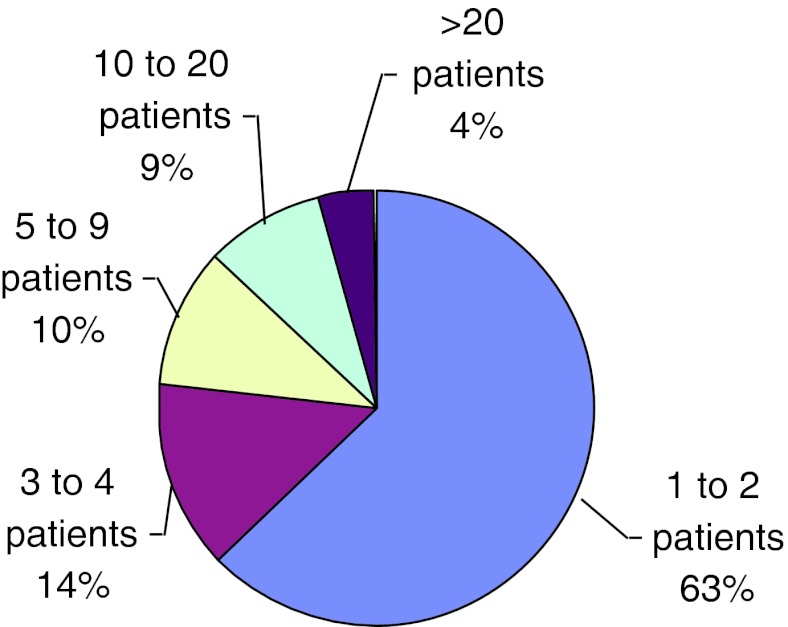
In the Latin America 2009 Report of the International Collaborative Group of Gaucher Disease (ICGG) (unpublished data), it is observed that the majority of physicians participating in the Gaucher Registry in Latin America have 1–2 patients; about 63% and 14% have 3–4, just like in the USA and Europe (Fig. 2). In countries with large territories such as Brazil, this means a lot of work, as coordinators have to travel large distances to collect the data. Sometimes they are assisted by a trained person who enters information in the online programs.
Fig. 2.
Distribution of all registry patients per physician among patients with all disease types in Latin America
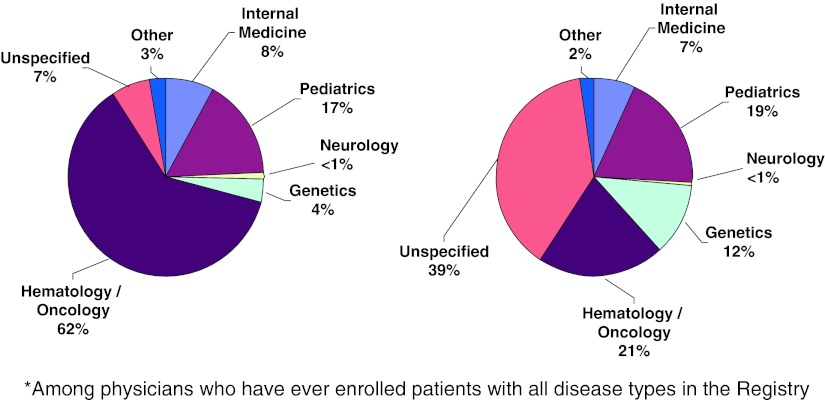
In the Latin American Annual Report (ICGG Gaucher Registry 2009), the number of hematologists involved in the treatment of patients with Gaucher disease in Latin America is almost three times higher compared to the rest of the world (unpublished data), and interestingly, the number of participating doctors without expertise specified is much greater in the rest of the world than in Latin America. We realized that in Latin America, Gaucher disease is yet best known among hematologists (Fig. 3).
Fig. 3.
Distribution of physician specialties (among physicians who have ever enrolled patients with all disease types in the registry) in Latin America and in the rest of the world
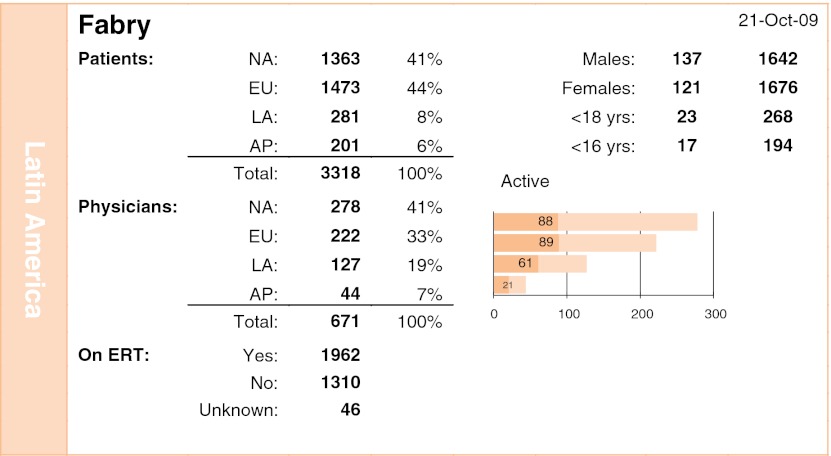
The Fabry Registry began in 2001 and has now 3,318 patients; 20% of doctors and 281 patients are from Latin America (Fig. 4). Approximately 40% of patients in Latin America are being treated with β-agalsidase, while in the rest of the world, the percentage is 59%, probably due to the difficulty in reimbursement for treatment by the governments of Latin America, and perhaps associated with the fact that the disease has a phenotypic impact smaller than other lysosomal diseases that use the enzyme replacement therapy as treatment, in addition to affecting more adults than children, which can also reinforce the poor appeal for inclusion in a high-cost treatment program.
Fig. 4.
Fabry Registry overview
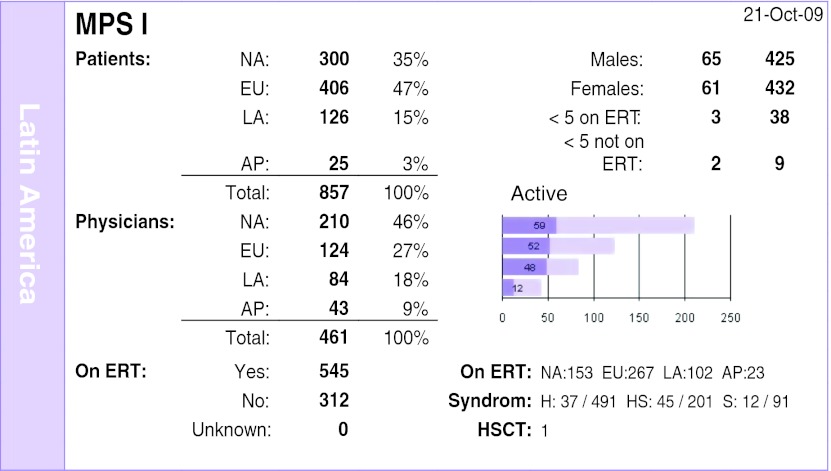
The total number of patients registered with MPS I is 857, being 126 in Latin America and accounting for 15% from the rest of the world. The percentage of doctors in Latin America is 18%. The percentage of patients receiving treatment is 80%, being the largest one, compared with 54% in North America and 66% in Europe (Fig. 5). It is hypothesized that most patients diagnosed in Latin America consist in the most severe cases, and therefore the rates of treatment are higher; the fact that the disease affects the children more than the adults also have a role in these results.
Fig. 5.
MPS I Registry overview
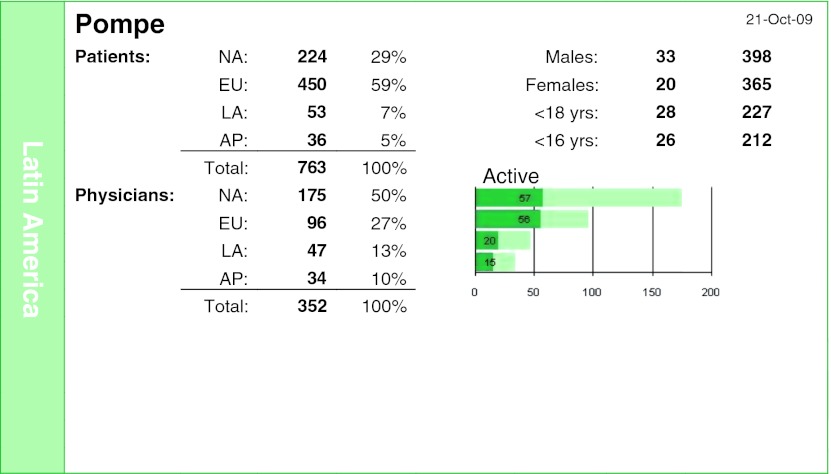
The Pompe Registry has 763 patients, 53 from Latin America representing 7% of the total, with 47 participating physicians, representing 13% (Fig. 6). About half of patients in Latin America are under 18 years of age, and in the rest of the world this percentage is 33%; so Latin America is diagnosing more children than adults, perhaps due to the more exuberant clinical characteristics and to the more severe clinical manifestations.
Fig. 6.
Pompe Registry overview
The scientific production generated by the registries so far is 22 publications, 18 of the Gaucher Registry – with only one publication of the Gaucher Registry focusing on Latin America, seven of the Fabry Registry, two of the MPS I Registry, but with scarce publications focusing on the patient population of Latin America. The only publication (Sobreira et al. 2007) that focuses on the Latin American population, in this case specifically the Brazilian patients in the Gaucher Registry, aimed at the phenotypic and genotypic heterogeneity in type I Gaucher disease. When Brazil was compared with the rest-of-the-world patients, Sobreira and colleagues concluded that Brazilian patients may have a more aggressive clinical form of the disease than the rest of the world, what emphasizes the need for caution in making generalizations about Gaucher disease across demographic groups.
Final Considerations
Databases are very useful for doctors interested in the enzyme replacement therapy available for lysosomal diseases, and also for governments to anticipate the costs associated with the treatment and follow-up. In the long term, we can calculate the impact of therapeutic measures applied on the quality of life and survival of the patients. The disadvantage of these existing programs is its high cost, they are voluntary-based and observational, and it is difficult to achieve clinical and laboratory data at the appropriate frequency and quality to better understand the disease progression and its response to treatment. In Latin America is important to have information about our population, in comparison with USA and Europe as severity of the disease, response to ERT, and other characteristics of these rare diseases to indivudualize their treatments.
Acknowledgments
The authors would like to thank Genzyme Corporation, Genzyme do Brasil, ICGG Gaucher Registry, Fabry Registry, MPS I Registry, Pompe Registry, to their support and the physicians and patients to their participation in the registries.
References
- Sims K, Lee P, Wilcox W, Wanner C, Martins AM (2009) Fabry Registry Annual Report 2009. Published by Genzyme Corporation. http://www.fabry.org/fsig.psf/PDFs/PDFsR/$File/2009_RADAR.pdf
- Sobreira E, Pires RF, Cizmarik M, Grabowski GA. Phenotypic and genotypic heterogeneity in Gaucher disease type 1: a comparison between Brazil and the rest-of-the-world. Mol Genet Metab. 2007;90:81–86. doi: 10.1016/j.ymgme.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Thadani R (2006) Formal trials versus observational studies. In: Metha A, Beck M, Sunder-Plassmann G (eds) Fabry disease, perspectives from 5 years of FOS. Oxford PharmaGenesis, Oxford. http://www.ncbi.nlm.nih.gov/bookshelf/ [PubMed]