Abstract
Background: Gaucher disease (GD) is a hereditary lysosomal storage disorder characterized by the accumulation of glucosylceramide, mainly in the cells of the reticuloendothelial system, due to a deficiency of the enzyme acid β-glucosidase (GBA). Diagnosis is usually based on measurement of GBA activity in peripheral leukocytes. The purpose of this study was to evaluate the ability of screening for GBA and chitotriosidase activity using dried blood spots on filter paper (DBS-FP) to identify individuals at high risk for GD in high-risk populations such as that of Tabuleiro do Norte, a small town in Northeastern Brazil.
Methods: Between 1 June 2007 and 31 May 2008, 740 consented residents and descendants of traditional families from Tabuleiro do Norte were submitted to screening with DBS-FP. Subjects with GBA activity < 2.19 nmol/h/mL were referred to the analysis of GBA and chitotriosidase activity in peripheral leukocytes and in plasma, respectively. Subjects at highest risk for GD (GBA activity in peripheral leukocytes < 5.6 nmol/h/mg protein) were referred to molecular analysis to confirm diagnosis.
Results: Screening with DBS-FP identified 135 subjects (18.2%) with GBA activity < 2.19 nmol/h/mL, 131 of whom remained in the study. In ten of these (7.6%), GBA activity in leukocytes was 2.6–5.5 nmol/h/mg protein. Subsequent molecular analysis confirmed six cases of heterozygosity and four normals for GD.
Conclusion: DBS-FP assay was shown to be an effective initial GD-screening strategy for high-prevalence populations in developing regions. Diagnosis could not be established from GBA activity in leukocytes alone, but required confirmation with molecular analysis.
Keywords: Acid β-glucosidase, Diagnosis, Dried blood spots, Gaucher disease, Screening
Introduction
Gaucher disease (GD), the most common hereditary lysosomal storage disorder in humans (Beutler and Grabowski 2001), is caused by a deficiency of the enzyme acid β-glucosidase (GBA) (EC 3.2.1.45) (Brady et al. 1966) which leads to the accumulation of glucosylceramide, mainly in the cells of the reticuloendothelial system (Amaral et al. 2000; Charrow et al. 2000; Beutler and Grabowski 2001). More than 200 mutations have already been identified in the GBA gene (Abrahamov et al. 1995), justifying the heterogeneity of GD phenotypes. The three main clinical presentations are: type I – non-neuropathic form, accounting for 94% of cases; type II – acute neuropathic form (<1% of cases); and type III – subacute neuropathic form (5% of cases) (Charrow et al. 2000; Grabowski 2004).
The most common clinical manifestations of GD include anemia, thrombocytopenia, hepatosplenomegaly, and skeletal complications (pain crisis, bone injury, cortical and medullar bone infarction, medullar expansion, osteopenia, osteonecrosis, and pathological fractures) (Kaplan et al. 2006; Kishnani et al. 2009). Unfortunately, due to the low incidence and the variability of clinical manifestations of GD, many patients are misdiagnosed or remain undiagnosed (Mistry and Germain 2007).
GD is pan-ethnic. The incidence in the USA is estimated to be 1:40,000, with a higher prevalence among Ashkenazi Jews (1:400 to 1:800) (Grabowski 2004). In February 2009, 5,356 cases had been identified throughout the world, 531 of which in Brazil (ICGG Gaucher Registry 2009). Twenty of the Brazilian cases come from the State of Ceará (population: ~8.2 million), and, of these, as many as seven come from a small town (Tabuleiro do Norte) of only approximately 28,000 inhabitants. The high estimated prevalence of GD in Tabuleiro do Norte (1:4,000), probably the highest in Brazil, is believed to be due to a low migration rate and high level of inbreeding over many generations and possibly to the existence of Jewish heredity in some of the local families (Vieira 2000). Further analyses of the mutations of patients in Tabuleiro do Norte will help confirm this hypothesis.
The diagnosis of GD is mainly based on morphological findings (detection of Gaucher cells in tissues), enzyme activity, and molecular analysis (Beutler and Grabowski 2001). The definitive diagnosis requires the determination of GBA activity in peripheral leukocytes (Beutler and Saven 1990; Charrow et al. 1998) or in cultured fibroblasts from skin biopsies (Charrow et al. 1998; Beutler and Grabowski 2001).
High-risk populations may be screened with GBA and chitotriosidase activity assays using dried blood spots on filter paper (DBS-FP), followed by confirmatory tests in suspected cases (Chamoles et al. 2002; Civallero et al. 2006). DBS-FP is simple and time-saving, and samples are easy to transport. When combined with GBA activity assay in peripheral leukocytes, DBS-FP is a reliable and cost-effective strategy for public health-sponsored screening of lysosomal storage diseases in newborns and/or high-risk populations (Li et al. 2004; Meikle et al. 2004; Evans et al. 2005; Gelb et al. 2006). Moreover, the existence of effective treatment providing good control of the disease and considerable improvement in quality of life (Damiano et al. 1998; Masek et al. 1999; Giraldo et al. 2005; Weinreb et al. 2007) makes screening of high-risk populations imperative to diagnose new cases and initiate treatment when indicated (Civallero et al. 2006).
Some GD mutations are more strongly associated with disease severity and prognosis than others. Consequently, molecular diagnosis of GD is a very important aid in genetic counseling and population-screening programs for GD in high-risk groups. Molecular analysis makes it possible to identify homozygous and heterozygous subjects and subsidizes efforts to prevent the emergence of new cases in the population (Mistry et al. 1992; Abrahamov et al. 1995).
The purpose of this study was to evaluate the ability of screening for GBA activity with DBS-FP to identify individuals at high risk for GD in high-risk populations such as that of Tabuleiro do Norte, a small town in Northeastern Brazil.
Methods
Study Design and Population
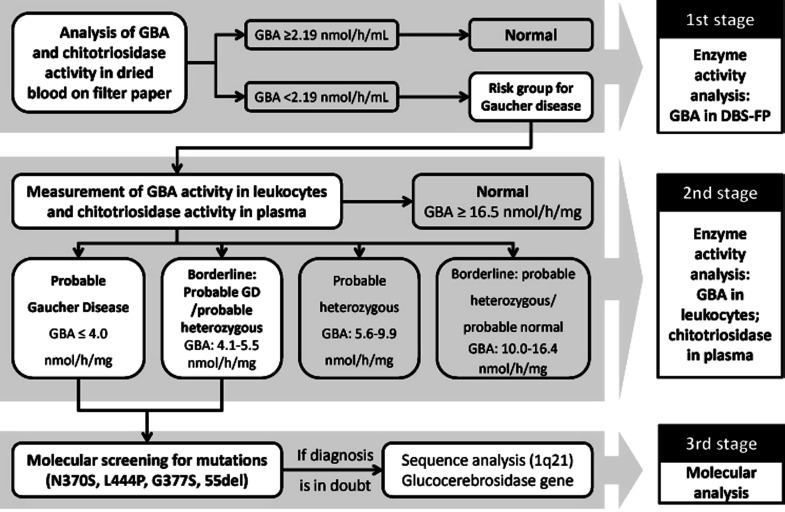
The study was conducted in three stages: (1) evaluation of GBA and chitotriosidase activity in blood spots on filter paper; (2) evaluation of GBA and chitotriosidase activity in leukocytes and plasma, respectively; and (3) molecular analysis of samples from subjects highly suspected of GD.
This cross-sectional study included 740 residents and descendants of traditional families from Tabuleiro do Norte. The sample size was calculated with the formula for infinite populations. The level of statistical significance and sampling error was set at 5% and 3.6%, respectively. Due to lack of prior data, the total sample size was calculated from the 50% proportion.
The inclusion criteria for the study were: (1) being a resident and descendant of traditional families from Tabuleiro do Norte; (2) participation in health education sessions for GD; and (3) signing an informed consent form. Parents, siblings, and children of the seven patients who had a previously confirmed diagnosis of GD were excluded from the study. The study protocol was approved by the research ethics committee of Hospital Geral César Cals (Fortaleza, Brazil).
The population of Tabuleiro do Norte was informed of the study by the local media (newspapers and radio shows) and through educational lectures on GD. Thus, the recruitment of volunteers was nonrandom.
Laboratory Tests
Evaluation of GBA and Chitotriosidase Activity in Blood Spots on Filter Paper
All enzyme assays were performed at the Laboratory of Inborn Errors of Metabolism (Hospital das Clínicas, Porto Alegre, RS, Brazil). A 2-mL peripheral blood sample was collected from the forearm of each volunteer and placed in a heparin-coated test tube. Samples were homogenized, aspirated, separated into four parts, and dripped onto filter paper (903 Protein Saver Card, Whatman Inc., USA). Venipuncture was preferred over fingerstick to obtain a sample large enough for analysis. The filter paper was air-dried for 4 h, packed individually in a sealed plastic bag, and shipped at room temperature to the referral laboratory for measurement of GBA and chitotriosidase activity. Individual 3-mm disks containing approximately 3.6 μL of whole blood were incubated at 37°C with appropriate artificial substrates (4-methylumbelliferyl-β-d-glucoside and 4-methylumbelliferyl-N,N′,N″-triacetyl-β-chitotrioside) and dilution buffers. Samples were centrifuged and then submitted to fluorometric analysis (nanomoles of substrate hydrolyzed per hour per milliliter of blood) (Civallero et al. 2006).
Evaluation of GBA and Chitotriosidase Activity in Leukocytes and Plasma
New 10-mL blood samples were collected from subjects with GBA activity below 2.19 nmol/h/mL and stored in heparin-coated tubes. The samples were used to measure GBA activity in leukocytes (Peters et al. 1976) and chitotriosidase activity in plasma (Hollak et al. 1994). Leukocytes and plasma were separated immediately after collection, frozen, and shipped in dry ice to a referral laboratory 4,000 km away. The shipment was delivered within 48 h of collection.
The parameters for GBA activity in leukocytes used in the present study to classify the subjects in five groups (Table 1) were established by the referral laboratory based on enzyme analysis of samples from subjects previously diagnosed by molecular biology as homozygous, heterozygous, and normals for GD.
Table 1.
Classification adopted by the referral laboratory based on GBA activity in peripheral leukocytes
| Classification | GBA activity in leukocytes (nmol/h/mg protein) |
|---|---|
| Probable GD | ≤ 4.0 |
| Borderline between probable GD and probable heterozygous | 4.1–5.5 |
| Probable heterozygous | 5.6–9.9 |
| Borderline between probable heterozygous and probable normal | 10.0–16.4 |
| Normal | ≥ 16.5 |
Molecular Analysis of Mutations
Samples from the subjects most suspected of GD (GBA in leukocytes < 5.6 nmol/h/mg protein) were screened for the major Brazilian mutations (N370S, L444P, G377S, and 55del) at the Department of Genetics and Evolutionary Biology of the Institute of Bio-Sciences (University of São Paulo, SP, Brazil). DNA was extracted from oral mucosa by the method of Richards et al. (1993). The mutations N370S, L444P, G377S, and 55del were analyzed with restriction fragment length polymorphism. After endonuclease digestion, fragments were submitted to 12% polyacrylamide gel electrophoresis (N370S and G377S) or 2% agarose gel electrophoresis (L444P and 55del) (Rozenberg et al. 2006).
When diagnosis was in doubt, samples from subjects with probable GD (GBA activity ≤ 4.0 nmol/h/mg protein) were shipped to the Molecular Development Laboratory of the Department of Pediatrics, University of Washington, USA, for sequence analysis of the entire coding region of the glucocerebrosidase gene (1q21) using dried blood on FTA filter paper. The use of FTA filter paper facilitates access of samples to diagnostic centers and thus provides an effective means of performing population-based mutational analysis of Gaucher disease internationally (Devost and Choy 2000). Sequencing will identify approximately 95% of mutations. It will often not identify deletions or rearrangements interfering with primer-binding sites.
Statistical Analysis
The collected data were submitted to descriptive analysis (frequency distribution and central tendency), followed by comparative analysis with the Mann–Whitney test and the chi-square test for categorical variables. Variables included gender, age, GBA activity in DBS-FP and in leukocytes, and chitotriosidase activity in DBS-FP and in plasma. The level of statistical significance was set at 5% (p < 0.05).
Subjects were initially distributed in two groups based on GBA activity in DBS-FP: (a) subjects at risk for GD and (b) normals (Fig. 1). The two groups were compared with regard to gender, age, and chitotriosidase activity. Samples from subjects at risk were subsequently submitted to analysis of GBA activity in leukocytes and classified in five categories according to criteria adopted by the referral laboratory (Table 1). Finally, these categories were compared with regard to chitotriosidase activity.
Fig. 1.
Study design of screening for Gaucher disease in Tabuleiro do Norte (Ceará, Brazil)
Results
From 1 June 2007 to 31 May 2008, 740 volunteers aged 31.4 ± 19.2 years (range: 1–85), 496 (67.0%) of whom were female, were enrolled in the study. None of the participants had symptoms compatible with GD or were first-degree relatives of the seven residents of Tabuleiro do Norte known to have GD.
The mean GBA and chitotriosidase activity measured in the DBS-FP assay was 3.4 ± 1.53 nmol/h/mL (range: 0.4–12.0) and 30.8 ± 25.8 nmol/h/mL (range: 0.0–242.0), respectively. GBA levels were below the adopted cutoff value for risk of GD (2.19 nmol/h/mL) in 135 (18.2%) participants. Subjects below and above cutoff did not differ significantly with respect to gender or age, but chitotriosidase activity in DBS-FP was significantly lower among the former (Table 2).
Table 2.
Comparison of gender, age, and chitotriosidase levels in DBS-FP between subjects at risk for GD and normals, according to the adopted cutoff value (<2.19 nmol/h/mL)
| Variable | At risk for GD (GBA <2.19 nmol/h/mL), n = 135 | Normal (GBA ≥ 2.19 nmol/h/mL), n = 605 | p value |
|---|---|---|---|
| Female gender | 87 (64.4%) | 409 (67.6%) | >0.05a |
| Age (years) | 32.0 ± 19.2 | 31.4 ± 19.3 | >0.05b |
| Chitotriosidase (nmol/h/mL) | 25.4 ± 21.9 | 32.0 ± 26.4 | 0.0014b |
aChi-square test
bMann–Whitney test
Four of the 135 subjects at risk for GD did not wish to participate in the second part of the study. New blood samples were collected from the remaining 131 subjects to determine GBA activity in peripheral leukocytes and chitotriosidase activity in plasma. Four subjects (3.1%) with GBA levels in the range 2.6–4.0 nmol/h/mg protein were classified as “probable GD,” 6 (4.6%) were “borderline between probable GD and probable heterozygous,” 63 (48.1%) were classified as “probable heterozygous,” and 55 (42.0%) were “borderline between probable heterozygous and probable normal.” Only three (2.3%) displayed normal GBA activity levels (Table 3). Subjects with probable GD did not differ significantly from subjects in the four other GBA ranges with regard to gender and age (p > 0.05) but displayed higher plasma chitotriosidase levels (82.75 ± 13.5 nmol/h/mL vs. 50.49 ± 54.80 nmol/h/mL, p = 0.023).
Table 3.
Frequency distribution of GBA activity in peripheral leukocytes of 131 subjects with suspected GD, according to the classification adopted by the referral laboratory
| Classification | N (%) | CI 95% | Chitotriosidase (nmol/h/mL ± SD) |
|---|---|---|---|
| Probable GD | 4 (3.1%) | 0.8–7.6% | 82.75 ± 13.5 |
| Borderline between probable GD and probable heterozygous | 6 (4.6%) | 1.7–9.7% | 61.47 ± 72.53 |
| Probable heterozygous | 63 (48.1%) | 39.3–57.0% | 43.37 ± 36.0 |
| Borderline between probable heterozygous and probable normal | 55 (42.0%) | 33.4–50.9% | 57.89 ± 69.54 |
| Normal | 3 (2.3%) | 0.5–6.5% | 42.33 ± 41.54 |
The GBA and chitotriosidase activity assays were repeated for the ten subjects in the two lowest ranges of GBA activity, confirming the initial results. The same subjects were submitted to additional evaluations, including clinical examinations, laboratory tests (complete peripheral blood count and aminotransferase dosage), X-ray scanning of pelvis, femurs, lumbar spine and chest, abdominal ultrasonography, magnetic resonance imaging of femurs, and bone densitometry of the lumbar spine. To further confirm the diagnosis, samples of oral mucosa were submitted to molecular screening for the four major Brazilian GD mutations (N370S, L444P, G377S, and 55del). Four subjects presented none of the mutations, but six subjects were found to be heterozygous (G377S/-). Complete individual genetic counseling was given upon study completion.
The four subjects with GBA activity in leukocytes below 4.1 nmol/h/mg protein (two heterozygous male siblings, mutation G377S/-, 12 and 17 years of age with GBA activity of 4.0 and 2.6 nmol/h/mg protein, respectively, and two unrelated females aged 39 and 44 years with GBA activity of 4.0 nmol/h/mg protein) were asymptomatic and presented no significant clinical changes in laboratory or imaging tests. Thus, to test for mutations other than N370S, L444P, G377S, and 55del, dried blood samples on FTA filter paper were collected and submitted to sequence analysis of the entire coding region of the glucocerebrosidase gene (1q21). The DNA analysis confirmed the diagnosis of two heterozygous subjects (p.G377S/wt) and two normals (wt/wt).
Discussion
The results of our study show that GBA activity assay in DBS-FP is an efficient screening strategy for the identification of subjects at risk of GD in high-prevalence populations. It also draws attention to the risk of false-positive diagnosis of GD in asymptomatic subjects when the measurement of GBA activity in leukocytes is used as the sole criterion. Molecular analysis was indispensable in this study to clarify doubts and confirm diagnosis. Only one mutation (G377S) was detected among the ten subjects at highest risk for GD. The fact that G377S is the third-most common mutation in subjects with GD in Portugal and Spain (Amaral et al. 1996) and rare in Ashkenazi Jews suggests that the mutation observed in Tabuleiro do Norte may be due to Portuguese ancestry. Further studies are necessary to clarify this issue.
The initial GBA activity assay in DBS-FP identified 135 of 740 subjects to be at risk for GD. The adopted cutoff value (<2.19 nm/h/mL) was above the value recommended by Civallero et al. (2006) (1.78 nmol/h/mL) to reduce the number of false-negative results and ensure the identification of all potential GD patients and carriers.
Four of the 135 patients selected in the initial screening withdrew from the study. Of the remaining 131 subjects, 4 were classified as “probable GD” (0.54%; CI 95%; 0.2–1.5%) and 124 as “possible heterozygous” (16.8%; CI 95%; 14.2–19.7%). The level of GBA activity was normal for the remaining 608 subjects (605 in DBS-FP, 3 in leukocytes) (82.6%; CI 95%; 79.6–85.2%). Thus, to identify one case of probable GD, 185 apparently healthy subjects had to be screened, and to identify one case of probable heterozygosity, only six subjects needed to be examined. In a high-risk population such as that of Tabuleiro do Norte, correct identification of affected individuals and carriers, including molecular screening for mutations, is essential to clarify doubts about diagnosis and provide adequate genetic counseling and, consequently, to reduce the incidence and prevalence of GD.
The fact that chitotriosidase activity in plasma was significantly higher in subjects with GBA activity in leukocytes below 4.1 nmol/h/mg protein than in subjects classified as “probable heterozygous” or “normal” (p < 0.05) matches the results published by other researchers (Hollak et al. 1994; Aerts et al. 2003; Cabrera-Salazar et al. 2004; Schoonhoven et al. 2007). Surprisingly, chitotriosidase activity in DBS-FP was lower for subjects with GBA activity <2.19 nmol/h/mL in DBS-FP than for normal subjects (p < 0.05), suggesting that, at least in this study, chitotriosidase activity was not a useful marker of GD. The discrepancy between the measures of chitotriosidase activity in DBS-FP and in plasma is difficult to explain, but the results of one or both of the chitotriosidase assays may have been affected by unfavorable sample shipping conditions.
The recruitment process was the most important limitation of this study. Since the selection of participants was nonrandom and because relatives of previously diagnosed patients were likely to be particularly interested in the study, the prevalence of homozygotes and heterozygotes in the study population may not have been representative of the general population. Unfortunately, it was not possible to completely avoid recruitment bias in the study.
Screening with enzyme assays instead of molecular analysis may at first sight appear a limitation. However, molecular analysis was not an option for initial screening due to lack of previous knowledge of the genetic profile of the population and due to questions of cost-effectiveness and infrastructure (the study was conducted in a socioeconomically challenged region). Despite its apparent limitations, we believe that triage with DBS-FP may even be used to improve the cost-effectiveness of screening with molecular analysis.
To our knowledge, this study represents the largest population screening for GD in Brazil using DBS-FP. Initial screening with DBS-FP, followed by a confirmatory GBA activity assay in peripheral leukocytes and molecular analysis, was shown to be an effective GD-screening strategy for high-prevalence populations in developing regions. Further studies are needed to determine the cost-effectiveness of different methods, alone and in combination, used to screen for GD in other high-prevalence populations.
Acknowledgments
The authors would like to thank the team at Genzyme do Brasil, sponsor of the study. Also many thanks to Dr. Elisa Sobreira for scientific support, Prof. Antonio Toledo Jr. for assistance with the manuscript, and Dr. Erlane Marques Ribeiro for help with the study design. We are also in debt to João Márcio da Silva, head of the Municipal Department of Health, and to the staff of the Family Health Program in Tabuleiro do Norte for their cooperation. Last but not least, we are appreciative of the valuable support received from ACDG (Associação Cearense de Profissionais atuantes em Doenças Genéticas, Pacientes, Familiares e Voluntários).
Synopsis
Enzyme assay with DBS-FP was shown to be an effective initial GD-screening strategy for high-prevalence populations in developing regions when followed by measurement of GBA activity in leukocytes, but molecular analysis was necessary to confirm diagnosis (homozygous, heterozygous, and normals for GD).
Conflicts of Interest
Rigoberto Chaves and Tibelle Maurício received educational grants from Genzyme do Brasil to help develop the study. Genzyme do Brasil covered the travel expenses and training of Rômulo Maurício and sponsored the laboratory tests performed by Janice Coelho and Kristiane Michelin-Tirelli. No other conflicts of interest are reported.
Contributor Information
Rigoberto Gadelha Chaves, Email: rigobertogadelha@hotmail.com.
Geraldo Barroso Cavalcanti, Jr., Email: gbcjunior@hotmail.com
References
- Abrahamov A, Elstein D, et al. Gaucher's disease variant characterised by progressive calcification of heart valves and unique genotype. Lancet. 1995;346(8981):1000–1003. doi: 10.1016/S0140-6736(95)91688-1. [DOI] [PubMed] [Google Scholar]
- Aerts JM, Hollak C, et al. Biochemistry of glycosphingolipid storage disorders: implications for therapeutic intervention. Philos Trans R Soc Lond B Biol Sci. 2003;358(1433):905–914. doi: 10.1098/rstb.2003.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral O, Pinto E, Fortuna M, Lacerda L. Sa Miranda MC. Type 1 Gaucher disease: identification of N396T and prevalence of glucocerebrosidase mutations in the Portuguese. Hum Mutat. 1996;8:280–281. doi: 10.1002/(SICI)1098-1004(1996)8:3<280::AID-HUMU15>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Amaral O, Marcao A, et al. Gaucher disease: expression and characterization of mild and severe acid beta-glucosidase mutations in Portuguese type 1 patients. Eur J Hum Genet. 2000;8(2):95–102. doi: 10.1038/sj.ejhg.5200422. [DOI] [PubMed] [Google Scholar]
- Beutler E, Grabowski G. Gaucher disease. In: Scriver C, Beaudet A, Sly W, Valle D, editors. The metabolic and molecular bases of inheredited disease. New York: McGraw-Hill; 2001. pp. 3635–3668. [Google Scholar]
- Beutler E, Saven A. Misuse of marrow examination in the diagnosis of Gaucher disease. Blood. 1990;76(3):646–648. [PubMed] [Google Scholar]
- Brady RO, Kanfer JN, et al. Demonstration of a deficiency of glucocerebroside-cleaving enzyme in Gaucher's disease. J Clin Invest. 1966;45(7):1112–1115. doi: 10.1172/JCI105417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera-Salazar MA, O'Rourke E, et al. Correlation of surrogate markers of Gaucher disease. Implications for long-term follow up of enzyme replacement therapy. Clin Chim Acta. 2004;344(1–2):101–107. doi: 10.1016/j.cccn.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Chamoles NA, Blanco M, et al. Gaucher and Niemann-Pick diseases–enzymatic diagnosis in dried blood spots on filter paper: retrospective diagnoses in newborn-screening cards. Clin Chim Acta. 2002;317(1–2):191–197. doi: 10.1016/S0009-8981(01)00798-7. [DOI] [PubMed] [Google Scholar]
- Charrow J, Esplin JA, et al. Gaucher disease: recommendations on diagnosis, evaluation, and monitoring. Arch Intern Med. 1998;158(16):1754–1760. doi: 10.1001/archinte.158.16.1754. [DOI] [PubMed] [Google Scholar]
- Charrow J, Andersson HC, et al. The Gaucher registry: demographics and disease characteristics of 1698 patients with Gaucher disease. Arch Intern Med. 2000;160(18):2835–2843. doi: 10.1001/archinte.160.18.2835. [DOI] [PubMed] [Google Scholar]
- Civallero G, Michelin K, et al. Twelve different enzyme assays on dried-blood filter paper samples for detection of patients with selected inherited lysosomal storage diseases. Clin Chim Acta. 2006;372(1–2):98–102. doi: 10.1016/j.cca.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Damiano AM, Pastores GM, et al. The health-related quality of life of adults with Gaucher's disease receiving enzyme replacement therapy: results from a retrospective study. Qual Life Res. 1998;7(5):373–386. doi: 10.1023/A:1008814105603. [DOI] [PubMed] [Google Scholar]
- Devost NC, Choy FY. Mutation analysis of Gaucher disease using dot-blood samples on FTA filter paper. Am J Med Genet. 2000;94(5):417–420. doi: 10.1002/1096-8628(20001023)94:5<417::AID-AJMG14>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Evans MI, Galen RS, et al. Principles of screening. Semin Perinatol. 2005;29(6):364–366. doi: 10.1053/j.semperi.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Gelb MH, Turecek F, et al. Direct multiplex assay of enzymes in dried blood spots by tandem mass spectrometry for the newborn screening of lysosomal storage disorders. J Inherit Metab Dis. 2006;29(2–3):397–404. doi: 10.1007/s10545-006-0265-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldo P, Solano V, et al. Quality of life related to type 1 Gaucher disease: Spanish experience. Qual Life Res. 2005;14(2):453–462. doi: 10.1007/s11136-004-0794-y. [DOI] [PubMed] [Google Scholar]
- Grabowski GA. Gaucher disease: lessons from a decade of therapy. J Pediatr. 2004;144(5 Suppl):S15–S19. doi: 10.1016/j.jpeds.2004.01.050. [DOI] [PubMed] [Google Scholar]
- Hollak CE, van Weely S, et al. Marked elevation of plasma chitotriosidase activity. A novel hallmark of Gaucher disease. J Clin Invest. 1994;93(3):1288–1292. doi: 10.1172/JCI117084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICGG Gaucher Registry (2009) Relatório do Brasil: Brasil comparado ao resto do mundo. Relatório Anual de 2009
- Kaplan P, Andersson HC, et al. The clinical and demographic characteristics of nonneuronopathic Gaucher disease in 887 children at diagnosis. Arch Pediatr Adolesc Med. 2006;160(6):603–608. doi: 10.1001/archpedi.160.6.603. [DOI] [PubMed] [Google Scholar]
- Kishnani PS, DiRocco M, et al. A randomized trial comparing the efficacy and safety of imiglucerase (Cerezyme) infusions every 4 weeks versus every 2 weeks in the maintenance therapy of adult patients with Gaucher disease type 1. Mol Genet Metab. 2009;96(4):164–170. doi: 10.1016/j.ymgme.2008.12.015. [DOI] [PubMed] [Google Scholar]
- Li Y, Scott CR, et al. Direct multiplex assay of lysosomal enzymes in dried blood spots for newborn screening. Clin Chem. 2004;50(10):1785–1796. doi: 10.1373/clinchem.2004.035907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masek BJ, Sims KB, et al. Quality of life assessment in adults with type 1 Gaucher disease. Qual Life Res. 1999;8(3):263–268. doi: 10.1023/A:1008859420641. [DOI] [PubMed] [Google Scholar]
- Meikle PJ, Ranieri E, et al. Newborn screening for lysosomal storage disorders: clinical evaluation of a two-tier strategy. Pediatrics. 2004;114(4):909–916. doi: 10.1542/peds.2004-0583. [DOI] [PubMed] [Google Scholar]
- Mistry P, Germain DP. Therapeutic objectives in Gaucher disease. Rev Méd Interne. 2007;28(Suppl 2):S171–S175. doi: 10.1016/S0248-8663(07)78876-8. [DOI] [PubMed] [Google Scholar]
- Mistry PK, Smith SJ et al (1992) Genetic diagnosis of Gaucher's disease. Lancet 339(8798):889–892 [DOI] [PubMed]
- Peters S, Coyle P, et al. Differantiation of beta glucocerebrosidase form beta glucosidase in human tissues using sodium taurocholate. Arch Biochem Biophys. 1976;175:562–569. doi: 10.1016/0003-9861(76)90547-6. [DOI] [PubMed] [Google Scholar]
- Richards B, Skoletsky J, Shuber AP, Balfour R, Stern RC, Dorkin HL, Parad RB, Witt D, Klinger KW. Multiplex PCR amplification from the CFTR gene using DNA prepared from buccal brushes/swabs. Hum Mol Genet. 1993;2:159–163. doi: 10.1093/hmg/2.2.159. [DOI] [PubMed] [Google Scholar]
- Rozenberg R, Araujo FT, et al. High frequency of mutation G377S in Brazilian type 3 Gaucher disease patients. Braz J Med Biol Res. 2006;39(9):1171–1179. doi: 10.1590/S0100-879X2006000900004. [DOI] [PubMed] [Google Scholar]
- Schoonhoven A, Rudensky B, et al. Monitoring of Gaucher patients with a novel chitotriosidase assay. Clin Chim Acta. 2007;381(2):136–139. doi: 10.1016/j.cca.2007.02.042. [DOI] [PubMed] [Google Scholar]
- Vieira V (2000) Os Gadelhas no Mundo. http://www.gentree.org.br/artigos/gadelha.htm. Accessed 13 May 2009
- Weinreb N, Barranger J, et al. Imiglucerase (Cerezyme) improves quality of life in patients with skeletal manifestations of Gaucher disease. Clin Genet. 2007;71(6):576–588. doi: 10.1111/j.1399-0004.2007.00811.x. [DOI] [PubMed] [Google Scholar]