Abstract
Quantification of mitochondrial DNA (mtDNA) content is an essential tool for the diagnosis of mtDNA depletion syndrome (MDS). Samples collected and processed for anatomopathology studies represent a unique source of archived biological material. Thus, the possibility to study mtDNA copy number in these specimens would be a useful way to screen for MDS. In this study, we designed and validated the methodology to determine mtDNA content by quantitative real-time polymerase chain reaction (qRT-PCR) in formalin-fixed paraffin-embedded (FFPE) muscle tissue. We studied 14 frozen muscle biopsies and compared the results with a portion of the same biopsy embedded in paraffin. Our results showed a similar variability among frozen and FFPE muscle biopsies. Patients with MDS detected in frozen muscle were also confirmed in their corresponding FFPE samples, which validate the usefulness of this approach. We conclude that the analysis of mtDNA copy number in FFPE muscle tissue by qRT-PCR is a useful method for the molecular screening of patients suspected to have MDS when frozen biopsies are not available. Analysis of these samples would facilitate retrospective studies and diagnostic procedures.
Introduction
Biogenesis and homeostasis of the mitochondria is tightly regulated, and requires the expression and coordination of both, nuclear and mitochondrial encoded proteins. Therefore, mitochondrial disorders are a group of complex dual genome diseases that can be caused by molecular defects in both nuclear or mitochondrial genomes including point mutations, deletions, duplications, and reduction in mitochondrial DNA (mtDNA) copy number, known as mtDNA depletion (Rötig and Poulton 2009).
mtDNA depletion syndromes (MDS) are a heterogeneous group of autosomal recessive disorders characterized by a reduction of the mtDNA content in a tissue-specific manner. They are caused by molecular defects in nuclear genes responsible for the biogenesis and maintenance of mtDNA integrity and usually affect different tissues and organs with high energetic demand, such as liver, skeletal muscle, and nervous system (Suomalainen and Isohanni 2010). Currently, MDS are divided into different syndromes caused by mutations in at least nine genes: myopathic form associated with mutations in TK2 (OMIM # 609560); encephalomyopathic with renal tubulopathy form associated with RRM2B (OMIM #612075); encephalomyopathic with methylmalonic aciduria associated with mutations in SUCLA2 and SUCLG1 (OMIM #612073 and 245400); hepatocerebral form associated with mutations in DGUOK, MPV17, and TWINKLE (OMIM # 251880, 256810 and 271245); MNGIE syndrome associated with mutations in TYMP and POLG (OMIM # 603041 and 613662). Mutations in POLG are also associated with Alpers Syndrome (OMIM #203700) (Dimmock et al. 2010).
Quantification of mtDNA content is an essential tool for the diagnosis of MDS and is based on Southern blot or quantitative real-time polymerase chain reaction (qRT-PCR) analysis (Suomalainen and Isohanni 2010). Although DNA from fresh frozen tissues is the suitable sample for the diagnosis of MDS, the most widely used method to collect samples is formalin fixation followed by paraffin block inclusion. The feasibility of performing mtDNA quantification on formalin-fixed paraffin-embedded (FFPE) tissues would facilitate diagnostic procedures and allow a large number of retrospective studies.
Material and Methods
Biological Samples
We studied 14 frozen skeletal muscle biopsies and compared them with a portion of the same biopsy embedded in paraffin. The age distribution and number of controls were: 0–1 year (n = 12); 6 years (n = 1), and 50 years (n = 1). To validate the methodology, we analyzed four patients with known reduction of mtDNA copy number. Briefly:
Patient1: She was admitted to the hospital at 7 days of life with bradypnea. She progressively developed hypothermia, mydriasis, and edema. She died few hours after admission. Biochemical analysis showed hyperammonemia, increased excretion of lactate, and hypertransaminasemia; 90% of mtDNA depletion in muscle biopsy by qRT-PCR was detected, while respiratory chain activities in the same muscle biopsy were normal. Mutations in DGUOK and MPV17 were excluded.
Patient 2: He was admitted to the hospital at 30 h of life with feeding refuse, irritability, dehydration, severe metabolic acidosis, hyperammonemia, hyperlactatemia, and altered hepatic enzymes. At 8 days of age, neurological deterioration, as well as renal and hepatic insufficiency, was evident. He presented two further episodes of metabolic acidosis and liver involvement. He died of hepatic failure at 2 months of age; mtDNA content in muscle biopsy, determined by southern blot, showed 89% mtDNA depletion. Mitochondrial respiratory chain activities (complex I–IV) were low. Genetic studies excluded mutations in DGUOK and RRM2B.
Patient 3: She presented at 5 days of age with axial hypotonia, poor eye contact, and sweating. Biochemical studies revealed an increased excretion of lactate and methylmalonate. Mitochondrial respiratory chain activities (complex I–IV) showed a slight reduction of all the complexes; mtDNA content, determined by qRT-PCR, showed 87% mtDNA depletion in muscle biopsy. A new mutation in SUCLA2 was identified, which has been predicted to be disease causing according to Polyphen database (unpublished results and currently under study in our laboratory).
Patient 4: She is the third daughter of a family with two other affected siblings. Few hours after birth, she presented a severe hepatic insufficiency, metabolic acidosis, hypoglycemia, hyperammonaemia, and high lactate. Mitochondrial respiratory chain activities were normal (complex I–IV); mtDNA content, determined by qRT-PCR in muscle biopsy, showed 80% depletion. Genetic analysis of DGUOK identified the previously described p.H226R mutation (Dimmock et al. 2008) in homozygosity.
Informed consent has been obtained for all the samples used.
Experimental Design and Sample Preparation
To minimize the natural variability of mtDNA content within tissue samples, we prepared and processed separately two adjacent portions from each frozen tissue. DNA was directly extracted from one of the portions, while the other one was subjected to formalin fixation and paraffin block inclusion. Briefly, before DNA isolation, paraffin sections were dewaxed in xylene at room temperature and subjected to a series of alcohol washing steps, following standard procedures (Gilbert et al. 2007). Then, the samples were air-dried and DNA was isolated using Qiamp DNA mini kit (Qiagen, GmbH, Germany).
Quantification of mtDNA Content
Analysis of mtDNA copy number was performed by qRT-PCR using Taqman technology in a Step One plus real-time PCR system (Applied Biosystems, Foster City, CA, USA) (Table 1). This method is based on the amplification of the 12S rRNA (mtDNA target gene) and the RNaseP (endogenous nuclear control gene) to normalize the DNA content in each sample. PCRs were carried out in triplicate using 15 ng of DNA from both frozen and FFPE samples. Levels of mtDNA were relatively quantified by evaluating Ct values using the comparative Ct (ΔΔCt) method. Briefly, mtDNA content was calculated from the difference of the delta Ct of each sample (Ct12SrRNA−CtRNaseP) compared with the reference value obtained as the average of delta Ct from the controls studied. Final values were expressed in relative units and were obtained through the equation 2−ΔΔCt where: ΔΔCt = (Ct12SrRNA sample − CtRNaseP sample) – (average ΔCt controls).
Table 1.
Oligonucleotides and reagents used for qRT-PCR
| Gene | Primers | Probes | Fluorescent product |
|---|---|---|---|
| 12S rRNAa | CCACGGGAAACAGCAGTGAT | (6FAM)TGCCAGCCACCGCG(BHQ1) | 6FAM/BlackHole Quencher |
| CTATTGACTTGGGTTAATCGTGTGA | |||
| RNAsePb | Taqman RNase P Control Reagents Kit. Part Number 4316844 | VIC-TAMRA | |
aOligonucleotides are located at positions 158 and 280 according to 12SrRNA sequence NC_012920; PCR amplification yields a 122 bp product
bAccording to the manufacturer, Taqman technology uses amplicons around 100 bp in length
Statistical Analysis
Wilcoxon signed-rank (nonparametric two-related sample test) and Spearman correlation test were performed using SPSS16.0 statistical software.
Results and Discussion
FFPE specimens represent an important and unique source of archived biological material. According to the literature, molecular studies have been successfully performed in this type of samples (Gnanapragasam 2009; Yu et al. 2008). However, as far as we know, the reliability of qRT-PCR technology for mtDNA copy number determination in FFPE tissues remains still to be elucidated. Moreover, the heterogeneous clinical spectrum of MDS makes the mtDNA copy number study an essential tool before undertaking specific gene sequencing analysis (Bai et al. 2004; Dimmock et al. 2010). Thus, the possibility to detect mtDNA content in FFPE specimens would represent a useful way to screen for MDS. Our aim was to optimize the methodology to determine mtDNA content by qRT-PCR in FFPE muscle tissue.
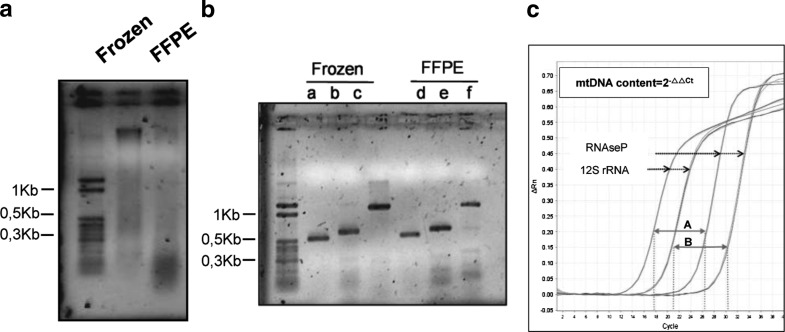
Limitations of molecular biology studies in FFPE tissues have been widely reported to be due to the high fragmentation of nucleic acids. We visualized the integrity of the DNA from frozen and FFPE samples in agarose gels and results showed that the DNA was highly fragmented in comparison with that extracted from frozen samples (Fig. 1a). Despite the concentration of DNA is lower in FFPE than in frozen muscle samples, the purity of both is almost identical and suitable for molecular studies (data not shown). According to the literature, the median DNA fragment length in FFPE samples is around 400 bp and mainly depends on the tissue type and the methodology of fixation (Gilbert et al. 2007; Lehmann and Kreipe 2001). To determine the extent of the degradation of the DNA in FFPE samples, we performed conventional PCR. Two nuclear and one mtDNA fragments of around 1,200, 700 and 500 bp, respectively, were successfully amplified (Fig. 1b). The lower intensity of the 1,200 bp fragment in the FFPE samples is probably due to DNA degradation during formalin fixation and sample processing. Because the qRT-PCR amplicons had around 100 bp, we considered that the DNA obtained from FFPE samples was suitable for mtDNA determination. However, FFPE samples showed higher Ct values in comparison with their corresponding frozen tissue (Fig.1c). This phenomenon could be explained by the fact that the DNA degradation in FFPE samples might reduce the number of intact amplicons, although the starting DNA amounts in the qRT-PCR were the same in both FFPE and frozen muscle. Because the delta Ct values obtained in both kind of samples were slightly different (mean; −10.54 and −11.72, respectively) the two groups were analyzed separately.
Fig. 1.
Integrity of the DNA obtained from frozen and FFPE muscle biopsy. (a) Total genomic DNA was visualized in 1% agarose gel. MW, molecular weight marker (b) DNA products from frozen and FFPE muscle biopsy obtained by PCR amplification. MW, molecular weight marker; lanes b, c, e, and f correspond to nuclear DNA fragments; lanes a and d correspond to a mtDNA fragment. (c) qRT-PCR amplification plot in frozen (A) and FFPE (B) samples
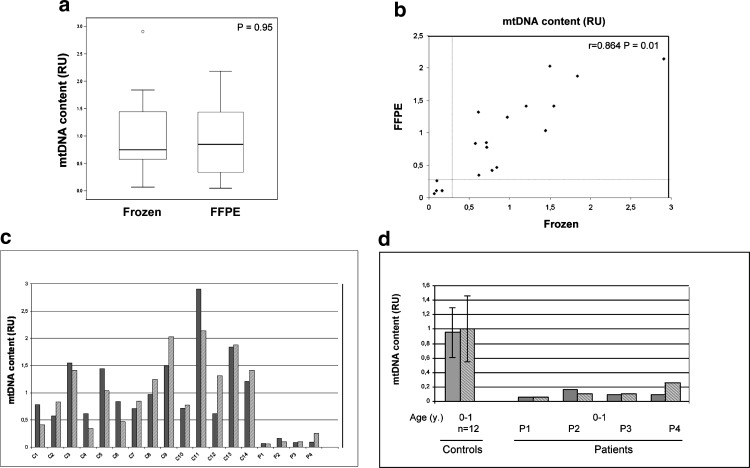
A similar variability in the mtDNA content between both groups has been observed, reference range: 0.57–2.90 and 0.41–2.02, in frozen and FFPE samples, respectively (Fig. 2a). Wilcoxon signed-rank test showed no statistically significant differences (p-value = 0.95). To assess the level of agreement of paired samples, the Spearman’s correlation test was applied and showed a good correlation within specimens (r = 0.864; p < 0.01). Altogether these data demonstrate that the mtDNA content in frozen and FFPE muscle samples was comparable. Despite the variability observed within these two groups, the comparative study showed similar behavior for each sample (Fig. 2b–c). Nevertheless, as has been reported previously (Bai et al. 2004; Morten et al. 2007; Dimmock et al. 2010), the observed variability could be explained by the differences in the age of control individuals. Interestingly, one of the control samples (C11), showing the highest mtDNA content, belongs to an adult while the remaining samples are from pediatric controls (Fig. 2c).
Fig. 2.
Comparative analysis of mtDNA content in frozen and FFPE muscle biopsies. (a) Similar levels of mtDNA were found in frozen and FFPE samples as determined by the Wilcoxon ranked test. (b) Spearman test showed statistically significant correlation between FFPE and frozen paired samples. Dotted line separates tissues with confirmed mtDNA depletion and delimits the threshold for mtDNA depletion (30% of mtDNA content in control samples). (c) mtDNA content in frozen and FFPE paired samples. C1–C14 correspond to control samples and P1–P4 to patients with previously confirmed mtDNA depletion. Results are shown in relative units (RU). Gray bars indicate frozen tissue, striped bars indicate FFPE samples. (d) Comparison of the mtDNA content of the four patients (P1–P4) with age-matched controls. Results are shown in relative units (RU). Gray bars indicate frozen tissue, striped bars indicate FFPE samples. Error lines represent the mean ± standard deviation
The four patients (P1–P4) with MDS detected in frozen muscle were clearly confirmed in their corresponding FFPE samples when compared with age-matched controls (Fig. 2d), which validates the usefulness of this material. Thus, despite the degradation of the DNA, mtDNA depletion can be detected in FFPE specimens in a similar manner to that performed in frozen tissues. However, the relatively small number of specimens analyzed in this study should be supported by further studies in larger series of cases to fully confirm our observations.
It has also been reported that many variables can influence the validity and reliability of molecular biology studies in FFPE tissues, such as tissue amount, length, storage, and fixation conditions (Gnanapragasam 2009; Gilbert et al. 2007). Therefore, the above-mentioned aspects should also be considered when analyzing mtDNA content in FFPE tissues.
In conclusion, analysis of mtDNA copy number in FFPE by qRT-PCR is a useful method for molecular screening of patients suspected to have MDS when frozen biopsies are not available. Analysis of these samples, which are a rich source of biological material, would facilitate retrospective studies and diagnostic procedures.
Acknowledgements
This research was supported in part by the Spanish Ministerio de Sanidad Grant PI08/90348. CIBERER, is an initiative of Instituto de Salud Carlos III.
References
- Bai RK, Perng CL, Hsu CH, Wong LJ. Quantitative PCR analysis of mitochondrial DNA content in patients with mitochondrial disease. Ann NY Acad Sci. 2004;1011:304–309. doi: 10.1196/annals.1293.029. [DOI] [PubMed] [Google Scholar]
- Dimmock D, Zhang Q, Dionisi-Vici C et al (2008) Clinical and molecular features of mitochondrial DNA depletion due to mutations in deoxyguanosine kinase. Hum Mutat 29(2):330–331 [DOI] [PubMed]
- Dimmock D, Tang L, Schmitt E, Wong L. Quantitative evaluation of the mitochondrial DNA depletion syndrome. Clin Chem. 2010;56:1119–1127. doi: 10.1373/clinchem.2009.141549. [DOI] [PubMed] [Google Scholar]
- Gilbert M, Haselkorn T, Bunce M, et al. The isolation of nucleic acids from fixed, paraffin-embedded tissues-which methods are useful and when? PLoS ONE. 2007;20(2):e537. doi: 10.1371/journal.pone.0000537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnanapragasam VJ. Unlocking the molecular archive: the emerging use of formalin-fixed paraffin-embedded tissue for biomarker research in urological cancer. BJU Int. 2009;105:274–278. doi: 10.1111/j.1464-410X.2009.08665.x. [DOI] [PubMed] [Google Scholar]
- Lehmann U, Kreipe H. Real-Time PCR analysis of DNA and RNA extracted from formalin-fixed and paraffin-embedded biopsies. Methods. 2001;25:409–418. doi: 10.1006/meth.2001.1263. [DOI] [PubMed] [Google Scholar]
- Morten KJ, Ashley N, Wijburg F, et al. Liver mtDNA content increases during development: a comparison of methods and the importance of age- and tissue-specific controls for the diagnosis of mtDNA depletion. Mitochondrion. 2007;7(6):386–395. doi: 10.1016/j.mito.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Rötig A, Poulton J. Genetic causes of mitochondrial DNA depletion in humans. Biochim Biophys Acta. 2009;1792:1103–1108. doi: 10.1016/j.bbadis.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Suomalainen A, Isohanni P. Mitochondrial DNA depletion syndromes-many genes, common mechanisms. Neuromuscul Disord. 2010;20:429–437. doi: 10.1016/j.nmd.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Yu J, Miller R, Zhang W, et al. Copy-number analysis of topoisomerase and thymidylate synthase genes in frozen and FFPE DNAs of colorectal cancers. Pharmacogenomics. 2008;9:1459–1466. doi: 10.2217/14622416.9.10.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]