Abstract
Pompe disease is characterized by deficiency or absence of activity of the lysosomal enzyme acid alpha-glucosidase. As a result of ineffective metabolism, glycogen progressively accumulates in muscle tissues. Patients with an aggressive classic infantile-onset form generally rapidly die of cardiorespiratory failure. A cross-reactive immunological material (CRIM)-negative status is predictive of high anti-alglucosidase alfa antibody titers and usually a poor clinical outcome of enzyme replacement therapy (ERT). CRIM-positive patients can also develop robust antibody titers complicating therapeutic management.
We successfully used an immune modulation therapy (IMT) protocol in a CRIM-positive infantile-onset patient with Pompe disease in whom infusions had to be temporarily discontinued because of safety concerns despite administration of pre-infusion medication. Prior to discontinuation, she had shown signs of clinical deterioration and continuous ventilation support through a tracheostomy was required. She was found to be positive for anti-alglucosidase alfa antibodies (1:6,400). IMT (rituximab, methotrexate and intravenous gamma globulin) was started, ERT was safely reintroduced during the IMT induction phase and, subsequently, the enzyme dose was increased, all without any complications. Antibodies disappeared, IMT was tapered and discontinued, and cadiomyopathy steadily improved. During 1 year of follow-up, she remained ventilator dependent and no gains in motor skills were noticed; motor functions will be closely monitored during sustained ERT.
Although the reversal of clinical decline in our CRIM-positive and antibody-positive infant with Pompe disease cannot be solely attributed to IMT, our experiences with this protocol may be helpful to other physicians encountering comparable therapeutic dilemmas.
Introduction
Pompe disease (OMIM #232300), also known as glycogen storage disease type II, is a treatable lysosomal storage disorder caused by the presence of a mutation in the gene encoding acid alpha-glucosidase (GAA) (Hirschhorn and Reuser 2001). Affected individuals have deficient or no activity of lysosomal GAA and are unable to effectively metabolize glycogen. The pathological hallmark of Pompe disease is accumulation of glycogen in muscle tissues (Hirschhorn and Reuser 2001; van der Ploeg and Reuser 2008).
The spectrum of clinical presentations is continuous and wide. At the most severe end, patients have little, if any, residual GAA activity and usually present with cardiomyopathy, hypotonia and muscle weakness, respiratory distress, feeding difficulties, and failure to thrive during early infancy (Kishnani et al. 2006a). Death from cardiorespiratory failure generally occurs within the first year of life. Patients with a nonclassical infantile, juvenile or late-onset form generally have >1% of normal residual GAA activity and cardiomyopathy is more attenuated or absent. Although the disease course is less aggressive, progressive limb and respiratory muscle involvement can lead to wheelchair and/or ventilator dependency, and ultimately death (van der Ploeg and Reuser 2008). The clinical diversity in Pompe disease can largely be explained by the considerable genotypic variability; more than 350 mutations and sequence variants have been identified in the GAA gene (www.pompecenter.nl). The combined incidence of all forms of Pompe disease has been estimated at 1:40,000 (Ausems et al. 1999; Martiniuk et al. 1998).
Until 2006, when cause-specific enzyme replacement therapy (ERT) opened a new era in the treatment of Pompe disease, only supportive care to alleviate symptom could be offered. ERT with recombinant human GAA (rhGAA; alglucosidase alfa, Myozyme®34) has shown major beneficial effects in patients throughout the disease spectrum (Kishnani et al. 2006a, b; Nicolino et al. 2009; Kishnani et al. 2007; Amalfitano et al. 2001; van der Ploeg et al. 2010). These benefits included reduction of the risk of invasive ventilation, prolongation of survival, improvement in hypertrophic cardiomyopathy and, among a subset of infantile-onset patients, improvement in motor function, motor skills and functional dependence. It has become apparent that not all infantile-onset patients respond satisfactorily to ERT. A cross-reactive immunological material (CRIM)-negative status has been reported to predict poorer clinical outcome, particularly because of the presence of high titers of anti-alglucosidase alfa IgG antibodies (Kishnani et al. 2010). High antibody titers also increase the likelihood of infusion-associated reactions (IARs) that may complicate therapeutic management (Lipinski et al. 2009). Successful elimination of anti-alglucosidase alfa antibodies with immune modulation therapy (IMT) can play an important role in maximizing the benefits of ERT (Mendelsohn et al. 2009) and in the prevention of severe IARs. We report a case of Pompe disease in a female CRIM-positive and antibody-positive infant in whom ERT had to be interrupted because of safety concerns and could be successfully reintroduced after start of IMT.
Case Report
Feeding difficulties, failure to thrive and muscle weakness were first noticed by the parents of the female infant at the age of 4 months. She had been born after an uneventful pregnancy and delivery, and birth weight (3,752 g), length (52 cm) and Apgar score (10) were normal. The family history was unremarkable and her 5-years-older half sister was healthy. Once admitted to our hospital, clinical examination revealed hypotonia, tachycardia, and macroglossia. Ultrasound examination showed cardiomyopathy (left ventricular mass index (LVMI) 174.4 g/m2) and hepatomegaly. The diagnosis infantile-onset Pompe disease was suspected and confirmed by demonstration of deficient GAA activity (3% of normal) in lymphocytes, and by genetic studies [Gly309Arg (925 G > A), Gln757X (2269 C > T)].
A month later, ERT was initiated at a dose of 20 mg/kg of Myozyme® administered once every other week. Soon after, she was discharged home and improvements in motor functions, with attainment of new motor milestones, were noticed over a 10-month period. LVMI reduced by 20%. Recurrent upper respiratory tract infections occurred, but the girl remained ventilator-free. At 10 months of ERT, IARs became more frequent despite pre-treatment with diphenhydramine and prednisone, and selection of a slower infusion rate. Infusions were complicated by episodes of tachycardia, hyper- or hypotension, irritability, pallor, tachypnea, wheezing, and oxygen desaturation. The reactions were managed by temporarily slowing or halting the infusion and administering symptomatic treatment.
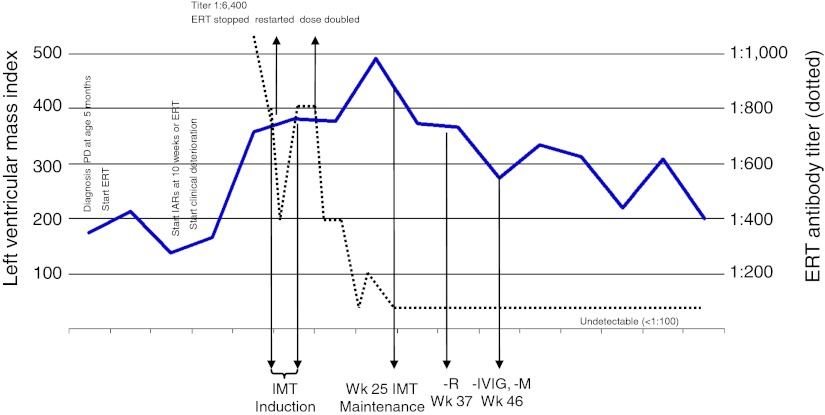
Her general condition gradually deteriorated with an increase in muscle weakness and hypotonia. Despite intensive conservative treatment, continuous ventilation support through a tracheostomy had to be initiated 1 year after start of ERT. To investigate possible causes of her clinical deterioration, an anti-alglucosidase alfa IgG antibody assay was ordered and a positive test result (antibody titer 1:6,400) was received after 6 weeks; determination of anti-alglucosidase-specific IgE antibodies could not be performed because of logistic reasons. LVMI had significantly increased to 379.8 g/m2 and IARs (wheezing, oxygen desaturation, bradycardia) had become more serious. Figure 1 depicts the patient’s clinical course including changes in LVMI and anti-alglucosidase antibody titers over time. After careful consideration, it was decided to temporarily discontinue ERT. Four weeks later, IMT was started, involving administration of rituximab, methotrexate, and intravenous gamma globulin (IVIG). The IMT protocol (Fig. 2) was approved by the University Hospital of Split Institutional Review Board, and written informed consent was obtained from the parents. At day 5 of IMT, ERT was restarted and diphenhydramine and prednisone were administered only prior to the first ERT infusion. No side effects occurred. Antibody titers ranged between 1:800 at start of IMT (result received 8 weeks after start of IMT).and 1:400 at 10 weeks of ERT. Although the LVMI tended to stabilize, the overall response to ERT was felt to be inadequate. After careful consideration and having sought expert advice, the dose of ERT was increased to 40 mg/kg every other week. Subsequently, antibody titers decreased and LVMI reduced from IMT week 19 onward. At week 25 of IMT (15 weeks after ERT dose increase), antibody titers had become undetectable. No adverse reactions associated with IMT or ERT had occurred. Twelve weeks thereafter, IMT was tapered. Rituximab was discontinued at week 37 of IMT, and the last doses of IVIG and methotrexate were administered in week 46 of the protocol. Currently, more than a year after the IgG antibodies had become undetectable, antibodies are still untraceable. No IARs to ERT have occurred. Although LVMI continues to decrease (a ~60% reduction as compared to the highest value measured), there were no other significant clinical improvements and the girl remained ventilator dependent.
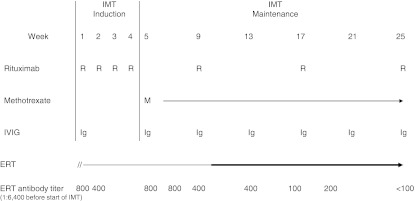
Fig. 1.
Immunomodulation therapy (IMT) protocol and anti-alglucosidase alfa antibody titers. Rituximab (R) 375 mg/m2/dose IV; after Wk 25, the patient received one additional administration in Wk 37. Methotrexate (M) 0.5 mg/kg weekly (orally or G-tube) to maintain absolute neutrophil count <500/μL and platelet count <50,000/μL. Discontinued in Wk 46. Intravenous immunoglobulin (IVIG) 500 mg/kg IV every 4 weeks. Monthly administrations continued till Wk 46. Alglucosidase alfa (ERT) restarted at 5 days of IMT at 20 mg/kg every other week (EOW). Dose increased to 40 mg/kg EOW in Wk 10 of IMT (continuing). After Wk 25, anti-alglucosidase alfa (ERT) antibody titers remained undetectable (<1:100)
Fig. 2.
Clinical course of the patient, including changes in left ventricular mass index and ERT antibodies over time. IMT Immunomodulation therapy, – R Rituximab stopped, – IVIG immunoglobulins stopped, – M Methotrexate stopped, PD Pompe disease, IAR Infusion-associated reaction, ERT Enzyme replacement therapy with alglucosidase alfa
Discussion
ERT with alglucosidase alfa is an essential component of the lifelong clinical care of patients with Pompe disease. However, treatment remains challenging, particularly in patients who have profound deficiency of GAA activity. Early start of ERT has proven to be critical and a better outcome can be anticipated in infants who are CRIM-positive and have remained free of ventilator support (Kishnani et al. 2010; Chien et al. 2008). Our CRIM-positive patient was immediately started on ERT after the diagnosis had been made at the age of 5 months. At 10 months of ERT, severe IARs started to occur and she deteriorated clinically (Fig. 1). The safety and efficacy concerns, and detection of anti-alglucosidase antibodies, forced us to consider other therapeutic approaches. ERT was temporarily discontinued, IMT was started, then ERT was reintroduced, and subsequently the enzyme dose was increased. All these therapeutic changes were well tolerated. In retrospect, it remains unsure to what extent each of the therapeutic measures has contributed to the disappearance of antibodies and reduction in LVMI.
Approximately 90% of ERT-treated CRIM-positive infants with Pompe disease develop IgG antibodies during the first months of treatment, but their functional consequences are still poorly understood (Kishnani et al. 2010). These infants have some endogenous GAA protein which may prevent development of robust antibody titers in most patients (Kishnani et al. 2010). A recent study in a limited number of ERT-treated CRIM-positive infants found that antibodies had no in vivo inhibitory effects on enzyme uptake or activity, also not in patients with the highest titer (1:51,200). Median LVMI decreased to near-normal levels. In contrast, CRIM-negative infants developed a strong immune response with much higher titers (up to 1:1,638,400), but not all had inhibitory activity. If compared to these data, the titer in our patient was only modestly elevated (highest titer 1:6,400). Because samples were not tested for inhibitory antibody activity, a role of immunogenic mechanisms in the patient’s clinical decline cannot be rigorously excluded. Until the interruption of ERT, she had received only single doses of prednisone prior to the infusions and no maintenance treatment. It remains unsure whether these biweekly administrations can have reduced the antibody titers.
Clinical studies found that the majority of infantile-onset infants experienced IARs on ERT. However, correlations between IARs and antibody titers are not consistent (Nicolino et al. 2009; Kishnani et al. 2007). IARs are observed in both patients with low and absent antibody titers, as well as in patients with high antibody titers. IARs rarely sustain and generally subside gradually. In most patients, IARs can be prevented by administering premedication and, if they occur, can be controlled by temporarily slowing or halting the infusion and administering symptomatic care (Kishnani et al. 2006a, b; Nicolino et al. 2009; Kishnani et al. 2007; Amalfitano et al. 2001; van der Ploeg et al. 2010). This usually allows continuation of ERT with appropriate pre-infusion medication. In our patient, the serious IARs were uncontrollable despite pre-treatment with diphenhydramine and prednisone and selection of a slower infusion rate. Because of logistic reasons, she could not be tested for anti-alglucosidase-specific IgE antibodies which have been demonstrated in a limited number of ERT-treated patients developing significant allergic reactions. Successful rechallenge with modified infusion regimens has been reported (Lipinski et al. 2009), and there is one case report describing immune modulation with monoclonal anti-IgE antibody (omalizumab) (Rohrbach et al. 2010).
The safety and efficacy concerns made us decide to interrupt ERT and explore alternative therapeutic approaches. We were unaware that the antibody titer had come down spontaneously to 1:800 after the 4-week interruption of ERT but, based on the previously detected titer of 1:6,400, the option of IMT to suppress antibody formation and, thereby, secure safe administration of ERT, was considered. Moreover, IMT could eliminate a theoretical inhibitory effect of antibodies. There is only scarce published experience with tolerance induction protocols in Pompe disease. Methotrexate has been used in the CRIM-negative murine model (Joseph et al. 2008) and Mendelsohn et al. have successfully applied an IMT protocol in conjunction with ERT in a CRIM-negative infant; antibodies disappeared, cardiomyopathy normalized, but the boy became ventilator dependent (Mendelsohn et al. 2009). This protocol involves administration of rituximab (elective depletion of mature CD20+ B-cells), methotrexate (inactivation of rapidly multiplying B- and T-cells) and intravenous gamma globulin (to compensate for B-cell suppression induced by rituximab) (Fig. 2). After having obtained expert advice, it was decided to use this protocol in our patient. The restart of ERT 5 days after initiation of the IMT induction phase was well tolerated. At 10 weeks of IMT, the enzyme dose was increased (as Mendelsohn et al. have done) in an attempt to achieve optimum treatment effect. After having peaked at week 19, LVMI steadily decreased in the absence of antibodies and untoward reactions. Although cardiomyopathy majorly improved, gains in motor development were not observed. This may relate to observations showing that ERT effectively clears lysosomal glycogen from the heart but is less effective in clearing skeletal muscle, and that remodeling of muscle fibers and restoration of muscle function are time-consuming processes (van der Ploeg and Reuser 2008).
In summary, ERT had to be interrupted in our CRIM-positive and antibody-positive infantile patient with Pompe disease because of safety concerns. Given the observed clinical decline, IMT was introduced, ERT was safely restarted, and subsequently the enzyme dose was increased. Anti-alglucosidase antibodies disappeared and cardiomyopathy gradually improved. Although these observations cannot be solely attributed to IMT, our experiences with this protocol may be helpful to other physicians encountering comparable therapeutic dilemmas. It is advised to first explore all less-invasive options. The knowledge on clinical decline in a subset of CRIM-positive patients needs to be expanded. Encouraging physicians to periodically collect antibody samples in all ERT-treated patients with Pompe disease and to report clinical data will be critical in this endeavor.
Acknowledgment
The authors thank Nancy Mendelsohn, M.D., and Yoav Messinger, M.D., from Children’s Hospitals and Clinics of Minnesota for developing the IMT regimen and providing it to us.
Furthermore, the authors acknowledge the editorial support of Hans Ebels, M.D., from Genzyme Europe. The authors maintained full and independent responsibility for the content of this manuscript.
Abbreviations
- CRIM
Cross-reactive immunological material
- ERT
Enzyme replacement therapy
- GAA
Acid alpha-glucosidase
- IAR
Infusion-associated reaction
- IMT
Immune modulation therapy
- LVMI
Left ventricular mass index
Synopsis
Immune tolerance induction is a viable therapeutic option in poorly responding infantile, antibody-positive patients with Pompe disease.
Footnotes
Summary of Product Characteristics Myozyme®, http://www.ema.europa.eu/.
Competing interests: None declared.
References
- Amalfitano A, Bengur AR, Morse RP, Majure JM, Case LE, Veerling DL, et al. Recombinant human acid alpha-glucosidase enzyme therapy for infantile glycogen storage disease type II: results of a phase I/II clinical trial. Genet Med. 2001;3:132–138. doi: 10.109700125817-200103000-00007. [DOI] [PubMed] [Google Scholar]
- Ausems MG, Verbiest J, Hermans MP, Kroos MA, Beemer FA, Wokke JH, et al. Frequency of glycogen storage disease type II in The Netherlands: implications for diagnosis and genetic counselling. Eur J Hum Genet. 1999;7:713–716. doi: 10.1038/sj.ejhg.5200367. [DOI] [PubMed] [Google Scholar]
- Chien YH, Chiang SC, Zhang XK, Keutzer J, Lee NC, Huang AC, et al. Early detection of Pompe disease by newborn screening is feasible: results from the Taiwan screening program. Pediatrics. 2008;122:e39–45. doi: 10.1542/peds.2007-2222. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R, Reuser A. Glycogen storage disease type II: acid alpha-glucosidase (acid maltase) deficiency. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited disease. New York: McGraw-Hill; 2001. pp. 3389–3420. [Google Scholar]
- Joseph A, Munroe K, Housman M, Garman R, Richards S. Immune tolerance induction to enzyme-replacement therapy by co-administration of short-term, low-dose methotrexate in a murine Pompe disease model. Clin Exp Immunol. 2008;152:138–146. doi: 10.1111/j.1365-2249.2008.03602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishnani PS, Hwu WL, Mandel H, Nicolino M, Yong F, Corzo D. A retrospective, multinational, multicenter study on the natural history of infantile-onset Pompe disease. J Pediatr. 2006;148:671–676. doi: 10.1016/j.jpeds.2005.11.033. [DOI] [PubMed] [Google Scholar]
- Kishnani PS, Nicolino M, Voit T, Rogers RC, Tsai AC, Waterson J, et al. Chinese hamster ovary cell-derived recombinant human acid alpha-glucosidase in infantile-onset Pompe disease. J Pediatr. 2006;149:89–97. doi: 10.1016/j.jpeds.2006.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishnani PS, Corzo D, Nicolino M, Byrne B, Mandel H, Hwu WL, et al. Recombinant human acid [alpha]-glucosidase: major clinical benefits in infantile-onset Pompe disease. Neurology. 2007;68:99–109. doi: 10.1212/01.wnl.0000251268.41188.04. [DOI] [PubMed] [Google Scholar]
- Kishnani PS, Goldenberg PC, DeArmey SL, Heller J, Benjamin D, Young S, et al. Cross-reactive immunologic material status affects treatment outcomes in Pompe disease infants. Mol Genet Metab. 2010;99:26–33. doi: 10.1016/j.ymgme.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski SE, Lipinski MJ, Burnette A, Platts-Mills TA, Wilson WG. Desensitization of an adult patient with Pompe disease and a history of anaphylaxis to alglucosidase alfa. Mol Genet Metab. 2009;98:319–321. doi: 10.1016/j.ymgme.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Martiniuk F, Chen A, Mack A, Arvanitopoulos E, Chen Y, Rom WN, et al. Carrier frequency for glycogen storage disease type II in New York and estimates of affected individuals born with the disease. Am J Med Genet. 1998;79:69–72. doi: 10.1002/(SICI)1096-8628(19980827)79:1<69::AID-AJMG16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Mendelsohn NJ, Messinger YH, Rosenberg AS, Kishnani PS. Elimination of antibodies to recombinant enzyme in Pompe’s disease. N Engl J Med. 2009;360:194–195. doi: 10.1056/NEJMc0806809. [DOI] [PubMed] [Google Scholar]
- Nicolino M, Byrne B, Wraith JE, Leslie N, Mandel H, Freyer DR, et al. Clinical outcomes after long-term treatment with alglucosidase alfa in infants and children with advanced Pompe disease. Genet Med. 2009;11:210–219. doi: 10.1097/GIM.0b013e31819d0996. [DOI] [PubMed] [Google Scholar]
- Rohrbach M, Klein A, Köhli-Wiesner A, Veraguth D, Scheer I, Balmer C, et al. CRIM-negative infantile Pompe disease: 42-month treatment outcome. J Inherit Metab Dis. 2010;33:751–757. doi: 10.1007/s10545-010-9209-0. [DOI] [PubMed] [Google Scholar]
- van der Ploeg AT, Reuser AJ. Pompe’s disease. Lancet. 2008;372:1342–1353. doi: 10.1016/S0140-6736(08)61555-X. [DOI] [PubMed] [Google Scholar]
- van der Ploeg AT, Clemens PR, Corzo D, Escolar DM, Florence J, Groeneveld GJ, et al. A randomized study of alglucosidase alfa in late-onset Pompe’s disease. N Engl J Med. 2010;362:1396–1406. doi: 10.1056/NEJMoa0909859. [DOI] [PubMed] [Google Scholar]