Abstract
Crigler–Najjar syndrome type I (CN-I, MIM #218800) is a rare and severe autosomal disorder. It is caused by deficiency of the liver enzyme responsible for bilirubin elimination, the uridine diphosphate glucuronosyltransferase 1A1 (UGT1A1; EC 2.4.1.17). Biologically, the disease manifests itself with severe and persistent unconjugated hyperbilirubinemia. Kernicterus is a well-known complication of severe unconjugated hyperbilirubinemia in infants and young children, especially in patients with CN-I.
Few articles have shown the efficiency of plasmapheresis for extreme hyperbilirubinemia.
In this report, we describe the efficiency of plasmapheresis for a rapid control of acute and severe unconjugated hyperbilirubinemia in a 6-year-old CN-I patient who had previously developed kernicterus in the neonatal period. In spite of intensification of phototherapy, the patient developed severe hyperbilirubinemia (up to 830 μmol/l, with bilirubin/albumin ratio at 1.2). With two plasmapheresis procedures, bilirubin serum concentration decreased to 420 μmol/ and bilirubin/albumin ratio to 0.55. Following the acute episode of very severe unconjugated hyperbilirubinemia, the child recovered and neurological examination was unchanged, thus suggesting that plasmapheresis possibly prevented further worsening of kernicterus.
Introduction
Crigler–Najjar syndrome type I (CN-I, MIM #218800) is a rare and severe autosomal disorder. It is caused by deficiency of the liver enzyme responsible for bilirubin elimination, the uridine diphosphate glucuronosyltransferase 1A1 (UGT1A1; EC 2.4.1.17). Biologically, the disease manifests with severe and persistent unconjugated hyperbilirubinemia. CN-I patients require lifelong phototherapy until liver transplantation or, in the near future, gene therapy. These patients are at permanent risk for bilirubin encephalopathy (kernicterus). The latter is due to the deposition of unconjugated bilirubin in the subcortical nuclei (globus pallidus, subthalamic nuclei and brainstem cranial nerve nuclei). Its manifestations include extra-pyramidal hypertonia, deafness, mental retardation.
Controlling unconjugated hyperbilirubinemia in CN-I patients relies on daily phototherapy. Bilirubin/albumin ratio is a useful tool in such patients; it has been reported that the high risk values are above 0.7 and that all CN-I patients who developed kernicterus had had values above 1.0 (Strauss et al. 2006). However, acute episodes of severe unconjugated hyperbilirubinemia may occur during infections and surgical procedures. In such situations few drugs have been reported to be useful. Synthetic heme analogues that are competitive inhibitors of heme oxygenase (the rate-limiting enzyme in the production of bilirubin) have been shown to be rapidly efficient, following a single administration. However, in many countries, these new drugs are not available. Plasmapheresis has also been reported to be a useful tool when a rapid decrease in bilirubin serum concentration is mandatory.
We report on a 6-year-old CN-I patient who had developed kernicterus in the neonatal period, in whom very severe unconjugated hyperbilirubinemia occurred following a surgical procedure. Plasmapheresis was rapidly efficient in this patient, allowing a quick decrease in serum bilirubin concentration.
Case Report
The patient was a 6-year-old girl who was diagnosed with CN-I syndrome at the age of 3 months. The diagnosis was based on very high levels of serum-unconjugated bilirubin (621 μmol/l). She was the third child of consanguineous Tunisian parents. The first two children were healthy. She was born after an uneventful, full-term pregnancy, and was delivered vaginally. Weight and height were 3,950 g and 49 cm, respectively.
She had developed jaundice during the first 24 h of life and had been treated with classical phototherapy during 3 days, then discharged, after trans-cutaneous control of bilirubin serum concentration had shown an estimated value of 180 μmol/l. The infant was 3-months old when she was taken to the hospital for permanent and severe jaundice. Save marked jaundice, physical examination revealed peripheral hypertonia and axial hypotonia; the infant was reactive to sound stimulations, had an appropriate smile and the liver was not enlarged. Severe unconjugated hyperbilirubinemia was isolated, with neither evidence for hemolysis (normal RBC count, normal G6PD and pyruvate kinase activities, mother’s blood group A+, baby’s blood group A+) nor other hepatic dysfunction (normal levels of serum transaminases and γGT, PT and factor V at 100%). The serum bilirubin/albumin molar ratio was 1.15. The infant was placed under continuous intensive phototherapy and received albumin infusion (1 g/kg in 2 h).
Bilirubin serum concentrations quickly decreased to 500 (within 24 h), then 400 μmol/l. (within 48 h), while bilirubin/albumin ratio had the same evolution (0.8 at 24 h, 0.65 at 48 h). During the following weeks, the duration of phototherapy was progressively decreased, under both clinical and biological controls, to10 h a day with a good control of serum bilirubin concentrations (between 270 and 320 μmol/l). Brain MRI proved normal and EEG did not show any abnormality. Auditory function was tested and found to be normal. The child was then discharged with at-home phototherapy and regular follow-up. Molecular study showed that the child was homozygous for the c.1070A > G mutation within exon 3 of the UGT1A1 gene, a previously reported mutation in CN-I patients (Petit et al. 2008) thus confirming the diagnosis of CN-I syndrome.
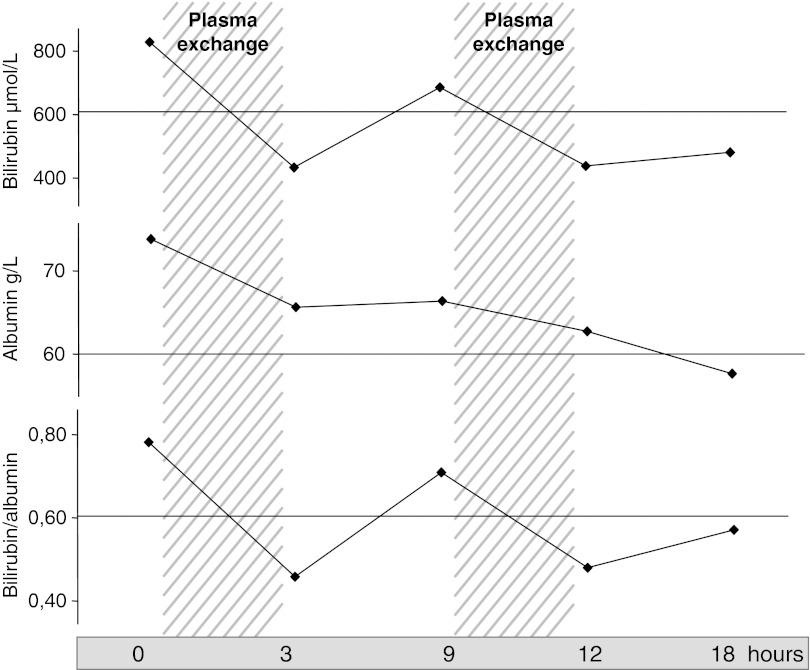
Unfortunately, the diagnosis of kernicterus, initially suspected, was confirmed in the following months with extrapyramidal hypertonia, axial hypotonia, swallowing troubles, and face and mouth dyspraxia. In spite of intensive physiotherapy, peripheral hypertonia continuously worsened resulting in partial dislocation of both hips that required surgery at the age of 6 years. After surgical procedure, both hips were immobilized by a hip-to-foot cast, thus reducing the efficiency of phototherapy, half of the body surface being hidden by the cast. Despite increasing the duration of phototherapy up to 18 h a day and the use of albumin infusions every 10 days (1 g/kg of body weight), serum bilirubin concentrations increased a week after surgery to reach 659, then 830 μmol/l (bilirubin albumin ratio at 1.2). The patient was transferred to a specialized unit and a central line was inserted in an internal jugular vein. The girl was treated with one session of plasmapheresis that resulted in a decrease of bilirubinemia to 430 μmol/l, followed, 24 h later by an increase of bilirubinemia to 630 μmol/l (Fig. 1). A second session of plasmapheresis was performed, allowing a rapid decrease of bilirubinemia to 420 μmol/l, while the bilirubin/albumin ratio decreased to 0.55 (Fig. 1). The evolution was then uneventful and the girl was able to come back to our unit.
Fig. 1.
Changes in bilirubin and albumin serum concentration during plasmapheresis. Plasma exchanges were performed on a Hématé machine, using a Gambro PF 1000 filter. Each exchange was performed during 120 min, with a volume of 60 ml/kg body weight, against albumin (50 g/l). The flow of extracorporal circulation was 100 ml/min. Anticoagulation with enoxaparine (50 U/kg body weight) was used
Two months later, when the cast was taken off, the duration of phototherapy was reduced to 10–12 h a day. Neurological examination proved unchanged, thus suggesting that plasmapheresis possibly prevented further worsening of kernicterus.
Discussion
Kernicterus is a neurological syndrome that is due to the accumulation of unconjugated bilirubin in the brain, and more precisely in the subcortical nuclei, e.g., subthalamic nuclei, globus pallidus, and brainstem cranial nerve nuclei. Kernicterus is a well-known complication of severe unconjugated hyperbilirubinemia in infants and young children (Wang et al. 2008), especially in patients with CN-I (Blaschke et al. 1974; Chalasani et al. 1997; Labrune et al. 1992; Shapiro 2010). Our patient, when admitted at 3 months of age, already had clinical manifestations of kernicterus, obviously related to the very high levels of bilirubin serum concentrations she had had since birth.. This complication should have been prevented and thus avoided, should this neonate have been carefully followed up after being discharged from the hospital at 4 days (Ahlfors et al. 2009; Strauss et al. 2006). Once the diagnosis had been ascertained, usual daily phototherapy allowed a good control of serum bilirubin concentration, as it is the case in most patients with CN-I.
The production of bilirubin physiologically increases during fasting periods, infectious episodes. Thus, patients with CN-I and their families are told to increase the duration of phototherapy should such events occur. Despite these precautions, kernicterus has been reported in such patients, following infections (Labrune et al. 1992), or surgical procedures (Walmsley et al. 2010), even in adults. In our patient, we tried to forecast the postoperative evolution by trying to reproduce the consequences of the hip-to-foot cast (we covered the lower half of the patient’s body with a sheet, thus reducing the available skin surface for phototherapy), with a careful monitoring of bilirubin serum concentrations. We had concluded that (data not shown) increasing the duration of daily phototherapy up to 16–18 h combined with regular albumin infusions should be efficient for preventing the risks related to increased bilirubin serum levels. This therapeutic management was enough during a week, and then failed to control the severe and rapid increase of bilirubinemia (there was no clear explanation for such a rapid increase in bilirubin serum concentration, but several factors may have contributed such as reduction of food intakes, physical stress, …). Few reports had shown the efficiency of plasmapheresis for extreme hyperbilirubinemia. A few years ago, a paper reported the successful use of plasmapheresis in an 18-year-old patient who developed acute EBV infection with a peak bilirubin level of 75.7 mg/dl (1,287 μmol/l, 50% of unconjugated and 50% of conjugated bilirubin). Twenty-four hours after plasmapheresis, the bilirubin concentration had decreased to 24.4 mg/dl (Place et al. 2007). In patients with CN-I syndrome, plasmapheresis has been successfully used in three adults (48, 18, and 25 years old) who developed hyperbilirubinemia after surgical procedures (Blaschke et al. 1974; Chalasani et al. 1997; Walmsley et al. 2010).
In our case, as heme oxygenase inhibitors are currently unavailable in our country (Abraham and Kappas 2008; Drummond and Kappas 2004; Kappas 2004) plasmapheresis appeared to be the only way for a rapid biological efficiency. Two sessions had to be performed for correct control of hyperbilirubinemia. We believe that this treatment has been efficient and possibly prevented the development of new sequellae in our patient in whom physical and neurological examinations remained unchanged.
The prevention of such situations is mandatory. Intensification of phototherapy, avoiding fasting periods (and thus providing regular and increased caloric intakes) and contraindicated drugs (Strauss et al. 2006) is highly important. However, prevention may not be efficient enough, as in our case. Liver transplantation, which is, to date, the only “radical” treatment of CN-I had been discussed in this patient but, owing to kernicterus, its indication had been rejected.
In conclusion, plasmapheresis may be a useful treatment for extreme acute unconjugated hyperbilirubinemia occurring in children with CN-I syndrome when phototherapy is transiently impaired for any adverse complication.
Footnotes
Competing interests: None declared.
References
- Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev. 2008;60:79–127. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- Ahlfors CE, Wenneberg RP, Ostrow JD, Tiribelli C. Unbound (free) bilirubin: improving the paradigm for evaluating neonatal jaundice. Clin Chem. 2009;55:1288–1299. doi: 10.1373/clinchem.2008.121269. [DOI] [PubMed] [Google Scholar]
- Blaschke TF, Berk PD, Scharschmidt BF, Guyther JR, Vergalla JM, Waggoner JG. Crigler-Najjar syndrome: an unusual course with development of neurologic damage at age eighteen. Pediatr Res. 1974;8:573–590. doi: 10.1203/00006450-197405000-00006. [DOI] [PubMed] [Google Scholar]
- Chalasani N, Roy Chowdhury N, Roy Chowdhury J, Boyer TD. Kernicterus in an adult who is heterozygous for Crigler-Najjar syndrome and homozygous for Gilbert-type genetic defect. Gastroenterology. 1997;112:2099–2103. doi: 10.1053/gast.1997.v112.pm9178703. [DOI] [PubMed] [Google Scholar]
- Drummond GS, Kappas A. Chemoprevention of severe neonatal hyperbilirubinemia. Semin Perinatol. 2004;28:365–368. doi: 10.1053/j.semperi.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Kappas A. A method for interdicting the development of severe jaundice in newborns by inhibiting the production of bilirubin. Pediatrics. 2004;113:119–123. doi: 10.1542/peds.113.1.119. [DOI] [PubMed] [Google Scholar]
- Labrune P, Myara A, Francoual J, Trivin F, Odièvre M. Cerebellar symptoms as the presenting manifestations of bilirubin encephalopathy in children with Crigler-Najjar type I disease. Pediatrics. 1992;89:768–770. [PubMed] [Google Scholar]
- Petit FM, Bézieau S, Gajdos V, Parisot F, Scoul C, Capel L, Stozinic V, Khrouf N, M'Rad R, Koshy A, Mollet-Boudjemline A, Francoual J, Labrune P. The Tunisian population history through the Crigler-Najjar type I syndrome. Eur J Hum Genet. 2008;16:848–853. doi: 10.1038/sj.ejhg.5201989. [DOI] [PubMed] [Google Scholar]
- Place E, Wenzel JE, Arumugam R, Belani K, Messinger Y. Successful plasmapheresis for extreme hyperbilirubinemia caused by acute Epstein-Barr virus. J Pediatr Hematol Oncol. 2007;29:323–326. doi: 10.1097/MPH.0b013e3180590c11. [DOI] [PubMed] [Google Scholar]
- Shapiro SM. Chronic bilirubin encephalopathy: diagnosis and outcome. Semin Fetal Neonatal Med. 2010;15:157–163. doi: 10.1016/j.siny.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Strauss KA, Robinson DL, Vreman HJ, Puffenberger EG, Hart G, Morton DH. Management of hyperbilirubinemia and prevention of kernicterus in 20 patients with Crigler-Najjar disease. Eur J Pediatr. 2006;165:306–319. doi: 10.1007/s00431-005-0055-2. [DOI] [PubMed] [Google Scholar]
- Walmsley D, Alzaharani K, Coke WJ, Gandhi R. Total knee arthroplasty and Crigler-Najjar syndrome: a case report. Knee. 2010;17:252–254. doi: 10.1016/j.knee.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Wang X, Wu W, Hou BL, Zhang P, Chineah A, Liu F, Liao W. Studying neonatal bilirubin encephalopathy with conventional MRI, MRS, and DWI. Neuroradiology. 2008;50:885–893. doi: 10.1007/s00234-008-0423-5. [DOI] [PubMed] [Google Scholar]