Abstract
The human HADH gene encodes the short-chain-L-3-hydroxyacyl-CoA dehydrogenase, the enzyme which catalyzes the third step of the β-oxidation of the fatty acids in the mitochondrial matrix. Loss-of-function mutations in the HADH gene lead to short-chain-L-3-hydroxyacyl-CoA dehydrogenase deficiency, an autosomal recessive genetic defect of unknown prevalence with a wide spectrum of phenotypic variability. As in other metabolic diseases, the diagnostic relevance of the biochemical evaluations, plasma acylcarnitines, and urinary organic acids, are crucially dependent on the clinical conditions of the patient during specimen collection.
This paper describes the eighth patient carrying a HADH gene mutation, a new homozygous deletion c.565delG leading to an early stop codon (p.V116Wfs124X), in an infant with hyperinsulininemic hypoglycemia, displaying abnormal patterns of plasma acylcarnitines and urinary organic acids. We conclude that, when the residual catalytic activity of the mutated enzyme is seriously reduced, the biochemical hallmarks of the disease, namely plasma 3-hydroxybutyrylcarnitine and urinary 3-hydroxyglutaric acid, are invariably present.
Introduction
The short-chain-L-3-hydroxyacyl-CoA dehydrogenase (EC 1.1.1.35) is an intramitochondrial homodimer enzyme, catalyzing the third of the four steps of the β-oxidation of the fatty acids, namely the NAD+-dependent dehydrogenation of the L-3-hydroxyacyl-CoA to the corresponding 3-ketoacyl-CoA. It is encoded by the HADH gene, mapped on human chromosome 4q22–q26 (Vredendaal et al. 1996). Loss-of-function mutations in the HADH gene lead to short-chain-L-3-hydroxyacyl-CoA dehydrogenase deficiency (Clayton et al. 2001), an autosomal recessive defect of the β-oxidation of fatty acids, the process providing energy during time of fasting, severe febrile illness or increased muscular activity (Roe and Ding 2001).
The first reported mutations in the HADH gene referred to a compound heterozygous patient affected by short-chain-L-3-hydroxyacyl-CoA dehydrogenase deficiency who presented at 3 years with fulminant hepatic failure, requiring prompt liver transplant (O’Brien et al. 2000).
Among subjects carrying a mutated HADH gene and affected by short-chain-L-3-hydroxyacyl-CoA dehydrogenase deficiency, a first group of three patients, with residual enzymatic activity less than 10% of controls, was reported as having a common feature of hyperinsulinemic hypoketotic hypoglycemia (Clayton et al. 2001; Molven et al. 2004; Hussain et al. 2005). The increased plasma level of 3-hydroxybutyryl-carnitine and the presence of 3-hydroxyglutaric acid in urine were considered informative biochemical hallmarks of the disease. 3-Hydroxybutyryl-carnitine, belonging stereochemically to the L-configuration, accumulates because of the defective fatty acid metabolism, on the contrary, the D-form would suggest a ketone body utilization defect. The latter could be due to the impairment of a minor pathway of degradation of the aminoacids lysine and tryptophan, in which the short-chain-L-3-hydroxyacyl-CoA dehydrogenase would catalyze the conversion of the 3-hydroxyglutaryl-CoA to 3-ketoglutaryl-CoA (Molven et al. 2004).
In all these patients, improvement of glucose homeostasis was obtained with diazoxide. The molecular action of the diazoxide involves opening of the ATP-sensitive potassium channels (KATP channels), with decreased insulin secretion. As the matter of fact, the physiological insulin secretion in the pancreatic β-cells is caused by an increase in ATP:ADP ratio, which leads to closure of the KATP channels, resulting in depolarization of the membrane, influx of calcium and ultimately insulin release (Palladino et al. 2008). The clinical observation of prompt glucose homeostasis improvement after diazoxide administration in these patients has led to the hypothesis that functional KATP channels were present. Therefore, mutations in the ABCC8 and KCNJ11 genes, encoding two subunits of the KATP channels, responsible of most cases, either recessive or dominant, of congenital hyperinsulinism (Tornovsky et al. 2004), were excluded.
Congenital hyperinsulinism, characterized by the dysregulated secretion of insulin from pancreatic β-cells (Stanley 1997) is a major cause of persistent hypoglycemia in the neonatal and infancy period. The genetic basis involves defects in key genes regulating insulin secretion such as the above-mentioned ABCC8 and KCNJ11. In addition, several other dominant forms are due to activating mutations in GLUD1 (encoding glutamate dehydrogenase type I) (Stanley et al. 1998), GCK (glucokinase) (Glaser et al. 1998), HNF4A (hepatocyte nuclear factor 4a) (Kapoor et al. 2008) and SLC16A1 (monocarboxylate transporter 1) (Otonkoski et al. 2007). Recessive forms are ascribed to mutations in HADH (Clayton et al. 2001), with similar prevalence to congenital hyperinsulinism attributable to HNF4A or GLUD1 (Flanagan et al. 2011).
A functional genomics approach (Hardy et al. 2007) and a study of the effects of siRNA-mediated HADH silencing (Martens et al. 2007) led to the conclusion that in case of short-chain-L-3-hydroxyacyl-CoA dehydrogenase deficiency, the mutation in the HADH gene is actually the cause of hyperinsulinism.
In a second group of two patients, carrying mutations in the HADH gene with residual enzymatic activity in the 35–50% of the control range, the plasma acylcarnitine and the urinary organic acid profiles were either completely normal (Kapoor et al. 2009), or remarkable for increased plasma C4-carnitine and urinary glutaric acid (but not 3-hydroxyglutaric acid) (Bennett et al. 2006). In the former case, the hyperinsulinemic hypoglycemia was triggered by dietary protein load, whereas in the latter case, the patient was never hypoglycemic, but presented at 10 months of age with hepatomegaly and coagulopathy.
One last patient, carrying a homozygous nonsense mutation in the HADH gene, first hospitalized at 2 months of age for hypoketotic hyperinsulinemic hypoglycemia, displayed normal plasma acylcarnitine and urinary organic acid profiles, but, upon reevaluation at 7 years of age, he developed elevated levels of urinary 3-hydroxybutyric acid, and slightly increased dicarboxylic aciduria (Di Candia et al. 2009).
Still too few cases are reported in literature to clearly define the clinical and biochemical spectrum of the disease, which is likely influenced both by the residual catalytic activity of the short-chain-L-3-hydroxyacyl-CoA dehydrogenase and the environmental conditions. In particular, the plasma acylcarnitine and the urinary organic acid profiles are informative only in few cases, likely under acute conditions.
In this report, we describe a patient affected by short-chain-L-3-hydroxyacyl-CoA dehydrogenase deficiency, carrying a severe mutation in the HADH gene. The homozygous deletion of one base pair leads to the formation of an early stop codon within exon 3, in the NAD+-binding domain of the enzyme. The nonsense mutation located at the beginning of the mRNA leads to the prediction of complete absence of the protein. The clinical and biochemical presentation is consistent with hyperinsulinemic hypoketotic hypoglycemia, with informative plasma acylcarnitines and urinary organic acids.
Case Report
The patient was born in Morocco from consanguineous Moroccan parents. In the maternal family, two cases of neonatal death of unknown causes are reported. The pattern of transmission of the disease is consistent with an autosomal recessive inheritance (see Fig. 1).
Fig. 1.
Pedigree and details of the HADH sequences of the patient’s family. Half-filled symbols denote heterozygous carrier subjects. Filled symbol represents the homozygous propositus. Automated direct sequencing of PCR amplified genomic DNA of the propositus and his parents evidenced the deletion c.565delG within exon 3 of the NAD+-binding domain of the enzyme
He was born full-term after an uneventful gestation. Maternal anamnesis was negative for gestational diabetes. He was breast-fed till the last day before hospital admission, when he was also bottle-fed because of breast-milk paucity.
At 3 months of age, he started to show signs of seizures with hypertonia, staring gaze, cyanosis, and frothing at the mouth. The seizures were described by the parents as multiple in a single day, however slight, and spontaneously resolving in a few seconds.
When the baby was brought to the emergency room, he was having seizures and was hypoglycemic (blood glucose 1.3 mmol/L), with no detectable ketonuria. Other biochemical routine tests were in the normal range for age. EEG showed no alteration of electrical activity and cerebral ultrasound scan excluded congenital malformations. The baby received an intravenous glucose infusion, in addition to enteral feed, to maintain normoglycemia (6 mg/kg/min to maintain plasma glucose between 2.6 and 3.0 mmol/L) (Aynsley-Green et al. 2000). During the subsequent days of hospitalization, hypoglycemia occurred whenever a reduction in the glucose infusion rate was attempted: sometimes after a few hour-fast, sometimes in the first 2 h after a meal.
In Table 1, the results of plasma insulin, C-peptide, ammonia, lactate and blood ketone bodies when the patient was hypoglycemic are reported. GH, IGF-1, ACTH, cortisol, and thyroid hormones were all in the reference ranges. After glucagon injection (0.1 mg/kg), an appropriate raise in glucose plasma concentration was obtained.
Table 1.
Patient’s biochemical evaluations corresponding to four different specimen collections. Reference values are reported in italics between square brackets (bold types refer to pathological or inappropriate values)
| Analyte | Sample 1 | Sample 2 | Sample 3 | Sample 4 |
|---|---|---|---|---|
| Patient’s age, months+days,(time of withdrawal) | 3+18 | 4+10(9:30 am) | 4+10(6:40 pm) | 4+17 |
| Plasma glucose (mmol/L) [3.3–5.6] | 2.1 | 2.5 | 2.7 | 2.3 |
| Serum insulin (mU/L) [4.0–24.0] | <2.0 | 4.7 | 22 | 16 |
| Serum C-peptide (ng/mL) [0.90–7.19] | 0.97 | NA | NA | NA |
| Plasma ammonia (μmol/L) [13.0–42.0] | 84 | 44 | 56 | NA |
| Plasma lactate (mmol/L) [0.5–2.2] | 1.5 | NA | NA | 2 |
| Capillary blood D-3-OH-butyrate (mmol/L) [<0.6] | 0.2 | 0.2 | 0.2 | 0.0 |
NA not analyzed
On the basis of persistent hyperinsulinemic hypoglycemia without evidence of ketosis or metabolic acidosis, we collected capillary blood spots during fasting hypoglycemia to perform acylcarnitine analysis.
Therapy with diazoxide at 5 mg/kg/day was instituted, obtaining prompt clinical response with maintenance of normoglycemia with usual enteral feeding, without the need of intravenous glucose infusion.
The parents denied consent for biopsy and for any further investigation; therefore, no evaluations of transcription and enzymatic activity were carried out.
Materials and Methods
Biochemical Evaluation
For analysis of acylcarnitine and aminoacid profiles, a 3 mm punch of blood spot sample was first processed using a NeoBase Non-derivatized MSMS Kit (PerkinElmer Wallac Oy) and analyzed by multiple reaction monitoring (MRM) in a tandem mass spectrometer TQD Detector (Waters), equipped with an ESI source positively charged.
Unlike the derivatized procedure, the nonderivatized assay cannot distinguish between the two isomers malonylcarnitine (C3DC) and 3-hydroxy-butyrylcarnitine (C4OH), the latter being the hallmark of the short-chain-L-3-hydroxyacyl-CoA dehydrogenase deficiency. Upon detection of an increase in the corresponding mass transition, in order to evidence which of the two analytes was actually increased (De Jesús et al. 2010), a derivatized procedure was also carried out (Turgeon et al. 2008).
Organic acid profile in urine was obtained according to the conventional method as previously reported (Rinaldo 2008).
HADH Sequencing
Genomic DNA was extracted from peripheral leukocytes using QIAamp DNA Mini Kit (QIAGEN). The eight exons of the HADH gene were amplified individually by PCR using intronic primers (Clayton et al. 2001). The amplicons were purified using the Exosap-IT clean up Kit (USB) and then submitted to sequence in both directions (GenomeLabTM Dye Terminator Cycle Sequencing with Quick Start Kit, Beckman Coulter). The sequencing reactions were analyzed on a CEQTM 8800 capillary sequencer (Beckman Coulter) and results were compared with the NCBI Reference Sequences NC_000004.11 and NM_001184705.2.
Results
Biochemical Findings
During hypoglycemia, two blood spots were collected and acylcarnitines’ analyses were undertaken (see Table 2), displaying persistently elevated levels of 3-hydroxybutyryl-carnitine (>0.94 μmol/L). Slightly abnormal values of some aminoacids were detected as well, but they are likely due to the metabolic decompensation and hence without diagnostic relevance.
Table 2.
Patient’s blood spot acylcarnitines’ analysis. Two blood spots, collected during hypoglycemia, were tested with two procedures (derivatized and nonderivatized) in different laboratories
| Analyte | Procedure | Sample 1 | Sample 2 | Sample 3 |
|---|---|---|---|---|
| Patient’s age, months+ days,(time of withdrawal) | 3+18 (9:00 am) |
3+18 (6:45 pm) |
4+17 | |
| Plasma glucose (mmol/L) [3.3–5.6] | 2.1 | 2.5 | 2.3 | |
| C4-OH (μmol/L) | Derivatized | 0.94 | NA | 1.72 |
| Nonderivatized (isomers: C4-OH + C3DC)a | 2.39 | 1.99 | NA | |
| C0 (μmol/L) | Derivatized | 43 | NA | 51 |
| Nonderivatized | 37 | 43 | NA |
C4-OH 3-hydroxybutyrylcarnitine, C3DC malonylcarnitine, C0 free carnitine, NA not analyzed
aWhen the patient’s blood spots were assayed, the internal standard for the determination of the isomers (C4-OH + C3DC) of the NeoBase Non-derivatized MSMS kit by PerkinElmer was the 2H6-C5DC. A more recent version of the kit uses for the determination of the same analytes the internal standard 2H3-C4
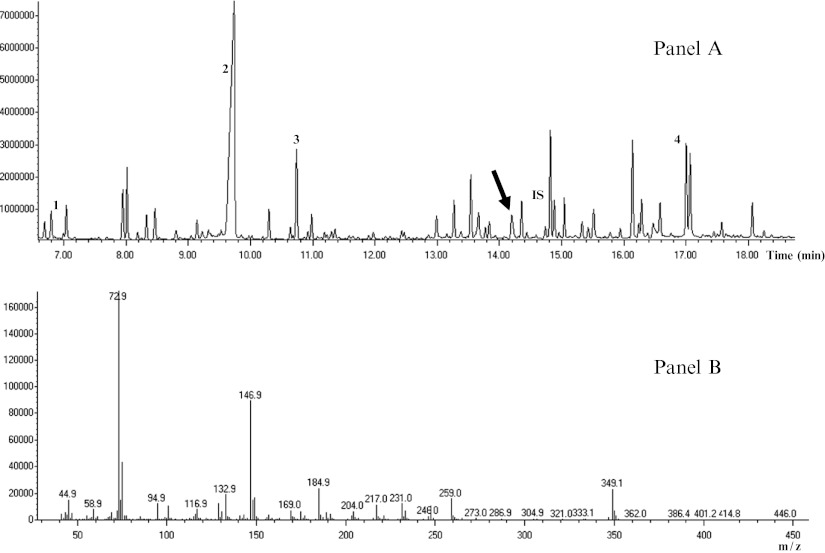
Urine organic acid analysis performed for diagnostic confirmation at time of fasting, evidenced increased quantities of 3-hydroxyglutaric acid (see Fig. 2).
Fig. 2.
Total-ion chromatogram of the trimethylsilyl derivatives of organic acids extracted from urine of the patient. Panel a: Diagnostic peak of 3-hydroxyglutaric acid is indicated with an arrow. For orientation, additional peaks without diagnostic significance are labeled as follows: (1) lactic acid; (2) urea; (3) succinic acid; (4) hippuric acid; (IS) tropic acid (internal standard). Panel b: Mass spectra of 3-hydroxyglutaric acid
Mutational Analysis
Direct sequencing of the patient’s HADH gene revealed a new homozygous deletion, c.565delG (reference sequence NM_001184705.2), located within exon 3 in the NAD+-binding domain of the enzyme (Fig. 1). The deletion leads to an inserted frame-shift sequence of eight aminoacids (WWKPSWRI), followed by the formation of a stop at codon 124.
The early upstream location along the gene of a stop codon leads to the reasonable conclusion that a complete nonfunctional enzyme is produced. The possible presence of the transcript was not investigated due to the parent refusal to biopsy and to any further investigation.
Both the parents are heterozygous carriers of the same deletion (Fig. 1).
Discussion
In this paper, we present the eighth case reported in literature of short-chain-L-3-hydroxyacyl-CoA dehydrogenase deficiency with mutation in the HADH gene. At 3 months of age, the patient required hospitalization for severe hypoglycemia, with the need of a glucose infusion rate of at least 6 mg/kg/min to maintain normoglycemia. Hypoglycemia always occurred in the absence of ketonemia or acidosis. Plasma insulin concentration, measured several times during hypoglycemia, was often inappropriately elevated for the glycemia. GH, IGF-1, ACTH, cortisol, and thyroid hormones concentration were in the normal ranges, excluding congenital pituitary hormones deficiency. The mobilization of hepatic glycogen deposits was not impaired, given the normal glycemic response to glucagon injection. During hypoglycemia, ammonia plasma concentration was slightly above the normal range, but never reached levels suggestive of hyperinsulinemia-hyperammonemia syndrome (Stanley et al. 1998). Accordingly, a leucine-sensitivity test was not performed.
The blood spot acylcarnitine profile showed consistently increased concentration of 3-hydroxybutyryl-carnitine, urinary excretion of 3-hydroxyglutaric acid was also elevated. Given the suggestive combination of results, we resolved to investigate the HADH gene of the patient, and a new homozygous deletion, c.565delG, was identified in exon 3. The mutation leads to the formation of an early stop codon, within the NAD+-binding domain of the enzyme. The actual presence of the mRNA and the residual catalytic activity were not assessed due to parent refusal to perform a skin biopsy or any further evaluation as soon as the diagnosis was established. Nevertheless, on the basis of the early location along the gene of the stop codon, it is reasonable to conclude that the corresponding enzyme is virtually absent.
The molecular mechanism of how defects in HADH gene lead to unregulated insulin secretion remains unclear. A recent study identified a high expression of HADH and a low expression of other β-oxidation enzymes in rat pancreatic β-cells (Martens et al. 2007). In addition, in the same cells of the mouse, the short-chain-L-3-hydroxyacyl-CoA dehydrogenase proved to be a negative regulator of insulin secretion through a mechanism independent of KATP channel, suggesting a molecular explanation for the diazoxide responsiveness of the hyperinsulinemic hypoglycemia in patients affected by short-chain-L-3-hydroxyacyl-CoA dehydrogenase deficiency (Hardy et al. 2007). Particularly relevant in this view is the in vitro observation that in human liver mitochondrial extracts, the short-chain-L-3-hydroxyacyl-CoA dehydrogenase specifically interacts with glutamate dehydrogenase type I (GLUD1), known to play an important role in the amino acid induced insulin secretion (Filling et al. 2008). Further studies on the molecular interaction between the two proteins in short-chain-L-3-hydroxyacyl-CoA dehydrogenase deficiency affected patients could partially explain the unpredictable and intermittent nature of the hyperinsulinemia (Clayton et al. 2001; Hussain et al. 2005), sometimes triggered by a protein dietary load (Kapoor et al. 2009), sometimes even absent (Bennett et al. 2006). Notably, in the hadh knockout mouse, an animal model of the hyperinsulinemic hypoglycemia reported in children with recessive inactivating mutation of the HADH gene, the insulin secretion dysregulation is due to the loss of an inhibitory protein–protein interaction of the short-chain-L-3-hydroxyacyl-CoA dehydrogenase upon glutamate dehydrogenase (Li et al. 2010).
The diagnostic relevance of the biochemical evaluations, plasma acylcarnitines, and urinary organic acids is known to be crucially dependent on the clinical conditions of the patient during specimen collection. However, when the residual catalytic activity of the mutated enzyme is seriously reduced, the hallmarks of the disease, namely plasma 3-hydroxybutyrylcarnitine and urinary 3-hydroxyglutaric acid, are present in all reported cases.
In conclusion, we have described the eighth case of short-chain-L-3-hydroxyacyl-CoA dehydrogenase deficiency, with a homozygous deletion in the HADH gene leading to an early stop codon, characterized by hyperinsulinemic hypoketotic hypoglycemia with enhanced 3-hydroxybutyryl-carnitine in plasma and 3-hydroxyglutaric acid in urine.
Acknowledgements
Partial of the funding required for this study was kindly provided by Associazione Italiana Sostegno Malattie Metaboliche Ereditarie (AISSME).
Synopsis
Particularly reduced catalytic activity of the short-chain-L-3-hydroxyacyl-CoA-dehydrogenase leads to hyperinsulinemic hypoketotic hypoglycemia with enhanced plasma 3-hydroxybutyryl-carnitine and urine hydroxyglutaric acid.
Details of the Contributions of Individual Authors
Florina Ion Popa performed the molecular analysis of HADH gene.
Silvia Perlini followed the clinical case since patient hospitalization.
Francesca Teofoli followed the clinical case and was responsible for diagnostic confirmation.
Daniela Degani followed the clinical case since patient hospitalization.
Silvia Funghini carried out the urine organic acids’ analysis.
Giancarlo La Marca performed the derivatized tandem mass spectrometry analysis.
Piero Rinaldo performed the derivatized tandem mass spectrometry analysis and reviewed the paper.
Monica Vincenzi carried out the nonderivatized tandem mass spectrometry analysis.
Franco Antoniazzi is responsible for clinical follow up.
Attilio Boner coordinated the whole diagnostic/therapeutic pathway, which consisted in connecting and keeping in touch different laboratories and clinics.
Marta Camilot planned and conducted the work described in the article. She wrote the paper.
All Authors edited and approved the final version of the manuscript.
Marta Camilot is the guarantor for the article. She accepts full responsibility for the work, has access to the data and controlled the decision to publish.
All authors have nothing to declare about competing interests.
Part of the funding required for this study was provided by Associazione Italiana Sostegno Malattie Metaboliche Ereditarie (AISSME). The authors confirm independence from the sponsors. The content of the article has not been influenced by the sponsors.
Ethics approval was not required. The patient’s parents signed an informed consent that is available upon request.
Footnotes
FIP and SP equally contributed to this paper and should be considered co-first authors.
Competing interests: None declared.
References
- Aynsley-Green A, Hussain K, Hall J, et al. Practical management of hyperinsulinism in infancy. Arch Dis Child Fetal Neonatal Ed. 2000;82:F98–F107. doi: 10.1136/fn.82.2.F98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MJ, Russell LK, Tokunaga C, et al. Reye-like syndrome resulting from novel missense mutations in mitochondrial medium- and short-chain L-3-hydroxyacyl-CoA dehydrogenase. Mol Genet Metab. 2006;89:74–79. doi: 10.1016/j.ymgme.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Clayton PT, Eaton S, Aynsley-Green A, et al. Hyperinsulinism in short-chain L-3-hydroxyacyl-CoA dehydrogenase deficiency reveals the importance of β-oxidation in insulin secretion. J Clin Invest. 2001;108:457–465. doi: 10.1172/JCI11294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jesús V, Chace DH, Lim TH, Mei JV, Hannon WH. Comparison of amino acids and acylcarnitines assay methods used in newborn screening assays by tandem mass spectrometry. Clin Chim Acta. 2010;411:684–689. doi: 10.1016/j.cca.2010.01.034. [DOI] [PubMed] [Google Scholar]
- Di Candia S, Gessi A, Pepe G, et al. Identification of a diffuse form of hyperinsulinemic hypoglycemia by 18-fluoro-L-3,4 dihydroxyphenylalanine positron emission tomography/CT in a patient carrying a novel mutation of the HADH gene. Eur J Endocrinol. 2009;160:1019–1023. doi: 10.1530/EJE-08-0945. [DOI] [PubMed] [Google Scholar]
- Filling C, Keller B, Hirschberg D, et al. Role of short-chain hydroxyacyl CoA dehydrogenases in SCHAD deficiency. Biochem Biophys Res Commun. 2008;368:6–11. doi: 10.1016/j.bbrc.2007.10.188. [DOI] [PubMed] [Google Scholar]
- Flanagan ES, Patch AM, Locke JM et al (2011) Genome-wide homozygosity analysis reveals HADH mutations as a common cause of diazoxide-responsive hyperinsulinemic-hypoglycemia in consanguineous pedigrees. J Clin Endocrinol Metab. doi:10.1210/jc.2010-1906 [DOI] [PMC free article] [PubMed]
- Glaser B, Kesavan P, Heyman M, et al. Familial hyperinsulinism caused by an activating glucokinase mutation. N Engl J Med. 1998;338:226–230. doi: 10.1056/NEJM199801223380404. [DOI] [PubMed] [Google Scholar]
- Hardy OT, Hohmeier HE, Becker TC, et al. Functional genomics of the beta-cell: short-chain 3-hydroxyacyl-coenzyme A dehydrogenase regulates insulin secretion independent of K+ currents. Mol Endocrinol. 2007;21:765–773. doi: 10.1210/me.2006-0411. [DOI] [PubMed] [Google Scholar]
- Hussain K, Clayton PT, Krywawych S, et al. Hyperinsulinism of infancy associated with a novel splice site mutation in the SCHAD gene. J Pediatr. 2005;146:706–708. doi: 10.1016/j.jpeds.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Kapoor RR, Locke J, Colclough K, et al. Persistent hyperinsulinaemic hypoglycaemia and maturity onset diabetes of the young (MODY) due to heterozygous HNF4A mutations. Diabetes. 2008;57:1659–1663. doi: 10.2337/db07-1657. [DOI] [PubMed] [Google Scholar]
- Kapoor RR, James C, Flanagan SE, Ellard S, Eaton S, Hussain K. 3-Hydroxyacyl-coenzyme A dehydrogenase deficiency and hyperinsulinemic hypoglycemia: characterization of a novel mutation and severe dietary protein sensitivity. J Clin Endocrinol Metab. 2009;94:2221–2225. doi: 10.1210/jc.2009-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Chen P, Palladino A, et al. Mechanism of hyperinsulinism in short-chian-3-hydroxyacyl-CoA dehydrogenase deficiency involves activation of glutamate dehydrogenase. J Biol Chem. 2010;285:31806–31818. doi: 10.1074/jbc.M110.123638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens GA, Vervoort A, Van de Casteele M, et al. Specificity in beta cell expression of L-3-hydroxyacyl-CoA dehydrogenase, short chain, and potential role in down-regulating insulin release. J Biol Chem. 2007;282:21134–21144. doi: 10.1074/jbc.M700083200. [DOI] [PubMed] [Google Scholar]
- Molven A, Matre GE, Duran M, et al. Familial hyperinsulinemic hypoglycemia caused by a defect in the SCHAD enzyme of mitochondrial fatty acid oxidation. Diabetes. 2004;53:221–227. doi: 10.2337/diabetes.53.1.221. [DOI] [PubMed] [Google Scholar]
- O’Brien LK, Rinaldo P, Sims HF, et al. Fulminant hepatic failure associated with mutations in the medium and short chain L-3-hydroxyacyl-CoA dehydrogenase gene. J Inherit Metab Dis. 2000;23(Suppl. 1):127. [Google Scholar]
- Otonkoski T, Jiao H, Kaminen-Ahola N, et al. Physical exercise-induced hypoglycemia caused by failed silencing of monocarboxylate transporter 1 in pancreatic β cells. Am J Hum Genet. 2007;81:467–474. doi: 10.1086/520960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palladino AA, Bennett MJ, Stanley CA. Hyperinsulinism in infancy and childhood: when an insulin level is not always enough. Clin Chem. 2008;54:256–263. doi: 10.1373/clinchem.2007.098988. [DOI] [PubMed] [Google Scholar]
- Rinaldo P. Organic acids. In: Blau N, Duran M, Gibson KM, editors. Laboratory guide to the methods in biochemical genetics. Berlin/Heidelberg: Springer; 2008. pp. 137–169. [Google Scholar]
- Roe CR, Ding J. Mitochondrial fatty acid oxidation disorders. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited disease. 8. New York: McGraw-Hill; 2001. pp. 2297–2326. [Google Scholar]
- Stanley CA. Hyperinsulinism in infants and children. Pediatr Clin North Am. 1997;44:363–374. doi: 10.1016/S0031-3955(05)70481-8. [DOI] [PubMed] [Google Scholar]
- Stanley CA, Lieu YK, Hsu BY, et al. Hyperinsulinism and hyperammonemia in infants with regulatory mutations of the glutamate dehydrogenase gene. N Engl J Med. 1998;338:1352–1357. doi: 10.1056/NEJM199805073381904. [DOI] [PubMed] [Google Scholar]
- Tornovsky S, Crane A, Cosgrove KE, et al. Hyperinsulinism of infancy: novel ABCC8 and KCNJ11 mutations and evidence for additional locus heterogeneity. J Clin Endocrinol Metab. 2004;89:6224–6234. doi: 10.1210/jc.2004-1233. [DOI] [PubMed] [Google Scholar]
- Turgeon C, Magera MJ, Allard P, et al. Combined newborn screening for succinylacetone, amino acids, and acylcarnitines in dried blood spots. Clin Chem. 2008;54:657–664. doi: 10.1373/clinchem.2007.101949. [DOI] [PubMed] [Google Scholar]
- Vredendaal PJ, van den Berg IE, Malingré HE, Stroobants AK, Olde Weghuis DE, Berger R. Human short-chain L-3- hydroxyacyl-CoA dehydrogenase: cloning and characterization of the coding sequence. Biochem Biophys Res Commun. 1996;223:718–723. doi: 10.1006/bbrc.1996.0961. [DOI] [PubMed] [Google Scholar]