Abstract
N-carbamylglutamate (NCG) has been reported to decrease ammonia levels in patients with propionic aciduria (PA) and methylmalonic aciduria (MMA), but reports on clinical outcomes remain scant. Here, we report a retrospective series of four patients with neonatal PA treated with NCG at presentation. Patients presented between 2 and 9 days of age and peak plasma ammonia ranged from 524 to 1,572 μM. Patients received bolus (30–200 mg/kg) and sustaining (115–300 mg/kg per day) doses of NCG in addition to a standard treatment regimen that included ammonia scavenger drugs. Ammonia levels decreased significantly in three of the four cases within 2 h after administration of NCG and fell below 100 μM in all within 12–29 h. Two patients received NCG (bolus 200 mg/kg) while ammonia was above 500 μM (740 and 1,572) and their levels fell below 500 μM by 4 and 8 h post-treatment, respectively. Outcomes of these NGC-treated patients were not improved over previously reported PA patients who did not receive NCG: two died during the initial episode and one after his third metabolic decompensation at 46 days. The survivor is now 3 years old and has a well-controlled seizure disorder and a mild developmental delay mostly in language. We conclude that despite a trial of NCG and a rapid fall in plasma ammonia, the short-term outcome of these patients was not improved.
Introduction
Propionic aciduria (PA) is a disorder of branched-chain amino acids caused by a deficiency of propionyl-CoA carboxylase, a hetero-oligomeric enzyme complex consisting of α- and β-subunits encoded by the PCCA and PCCB genes, respectively. Patients with neonatal onset of PA present with encephalopathy, ketoacidosis, and hyperammonemia. They may be symptomatic before the result of newborn screening is available. Mortality is significant in cases presenting in the neonatal period, with 30–36% dying at the initial decompensation or before 2 years of age (Dionisi-Vici et al. 2006; Surtees et al. 1992). Predictors of neurological outcomes include coma duration, and frequency and severity of metabolic decompensations. Various mechanisms have been proposed to explain the poor outcomes including inhibition of mitochondrial energy metabolism and direct ammonia toxicity (Picca et al. 2001; Schwab et al. 2006).
Hyperammonemia in PA is thought to be related to inhibition of N-acetylglutamate synthase (NAGS) enzyme by intramitochondrial accumulation of propionic acid (Coude et al. 1979), which results in decreased allosteric activation of the urea cycle enzyme, carbamoylphosphate synthetase I (CPSI). In fact, N-carbamylglutamate (NCG), an analog of N-acetylglutamate, can activate CPSI, and NCG treatment has been shown to reduce hyperammonemia in initial, as well as subsequent, PA decompensation episodes (Filippi et al. 2010; Gebhardt et al. 2003, 2005; Jones et al. 2008; Levrat et al. 2008; Schwahn et al. 2010). Moreover, increased ureagenesis has been demonstrated in PA patients after administration of NCG (Ah Mew et al. 2010). Other mechanisms have been proposed to explain hyperammonemia in organic aciduria including depletion of Krebs cycle intermediates, resulting in reduced alpha-ketoglutarate for glutamate and glutamine production, leading to altered synthesis of N-acetylglutamate. Indeed, glutamate and glutamine levels are reduced in PA (Al-Hassnan et al. 2003; Filipowicz et al. 2006). If the ammonia toxicity has a significant impact on the neurological outcomes of PA patients, then decreasing the hyperammonemia by NCG supplementation could reduce associated morbidity. However, reports on clinical outcomes of infants with PA treated with NCG are scant. Here, we report four neonatal cases of PA treated by NCG at presentation and their short-term clinical outcomes.
Case Reports
Between 2006 and 2009, in two tertiary pediatric hospitals in Montreal, Province of Quebec, Canada, a total of four cases of symptomatic neonatal-onset PA were treated by NCG. In Quebec, newborn screening for organic acidurias by tandem mass spectrometry is not yet available. NCG was obtained from Orphan Europe through a special program of Health Canada. Clinical characteristics, laboratory results, and management at presentation are shown in Table 1.
Table 1.
Clinical presentation, diagnostic laboratory values, and management of propionic aciduria patients in this study
| Patients | A | B | C | D | |
|---|---|---|---|---|---|
| Initial presentation | Age at presentation | 2 days | 9 days | 2 days | 3 days |
| Symptoms | Respiratory distress Lethargy |
Vomiting Lethargy |
Respiratory distress | Feeding difficulties | |
| Hypothermia | Feeding difficulties | Lethargy | |||
| Hypothermia | |||||
| pH/Bicarbonate (mM) | 7.06/4 | 7.38/22 | 7.29/8 | 7.0/3 | |
| Anion gap (mM) | 34 | 9 | 21 | 42 | |
| Ammonia (μM) | 404 | 188 | 781 | 782 | |
| Diagnostic labs | Urine organic acids (μmol/mmol creatinine) | ||||
| 3-OH-propioniate (normal < 41) |
4,712 | 14,253 | 15,632 | 11,704 | |
| Propionylglycine (normal < 3) |
42 | 301 | 38 | 14 | |
| Methylcitrate (normal < 13) |
1,189 | 1,227 | 744 | 93 | |
| Plasma amino acids (μM) | |||||
| Glutamine (normal 474–736) |
450 | 457 | 504 | 1,085 | |
| Glutamate (normal 31–113) |
46 | 197 | 55 | 62 | |
| Glycine (normal 138–276) |
517 | 429 | 1,280 | 507 | |
| Molecular analysis | PCCB: | PCCB: | PCCA: | PCCB: | |
| Allele 1 | c.990dupT (p.E331X) | c.517_518delTT(p.L173GfsX56) | c.878A>G (p.Q293R) | c.331 C>T (p.R11X) | |
| Allele 2 | c.942 C>A (p.T314X) | c.183 + 3 G>C | c.878A>G (p.Q293R) |
c.415 C>T (p.Q139X) |
|
| Medication | Carnitine (mg/kg per day) | 100 | 100 | 100 | 100 |
| Ammonia scavengersa | |||||
| Arginine (mg/kg) | 600 | 250 | 600 | 600 | |
| Benzoate (mg/kg) | 250 | 250 | 250 | 250 | |
| Phenylacetate (mg/kg) | – | 250 | 250 | – | |
| NCG (nasogastric tube) | |||||
| Priming dose (mg/kg) | 75 | 30 | 200 | 200 | |
| Sustaining dose (mg/kg per day) | 300 (divided TID) | 115 (divided QID) | 200 (divided TID) | 200 (divided TID) | |
| Dialysis | No | No | No | Yes | |
aDosage for priming infusion provided. Sustaining infusion use same dosage over 24 h
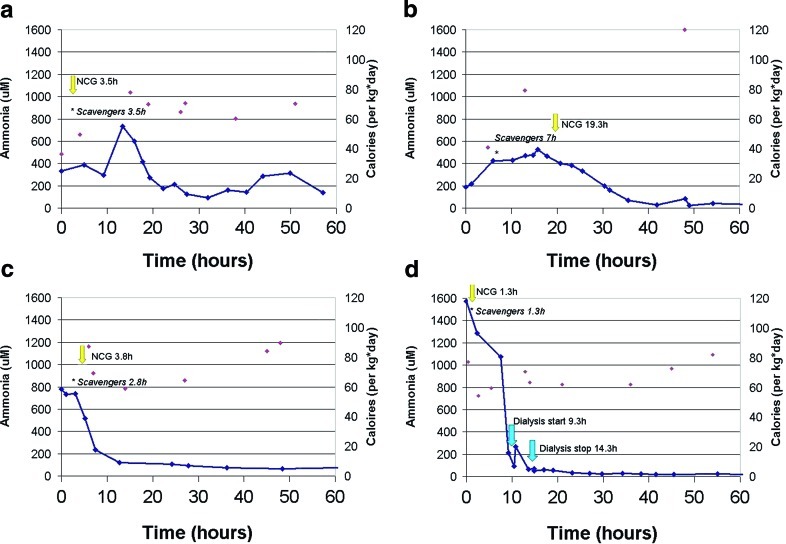
For all patients, pregnancy and delivery were unremarkable. Patients presented between 2 and 9 days of age and were from different ethnic backgrounds: Indian, Hispanic, Nigerian-Dominican, and Greek. Two patients (A and D) showed a more severe clinical picture with severe metabolic acidosis (pH < 7.1) and hypothermia. Three patients (A, C, and D) were transferred from a local hospital to the tertiary pediatric center where diagnostic investigations and metabolic treatment were promptly instigated. All patients showed characteristic metabolites on urine organic acid profiles by GC-MS, and diagnosis was confirmed by identification of mutations in either PCCA or PCCB genes (Table 1). Plasma glutamine levels were elevated only in patient D. Serial measurements of blood ammonia after admission to the tertiary hospital are shown in Fig. 1. Delay between reported first symptoms and initiation of treatment ranged from 8 to 23 h (Table 2). Upon admission to tertiary center, calories were provided in the form of intravenous dextrose and lipids. In addition, the good neurological status of patient B permitted the early introduction of enteral nutrition with protein-free formula. Protein was reintroduced in parenteral nutrition for patients B, C, and D at, respectively 48 h (0.6 g/kg per day), 48 h (0.5 g/kg per day) and 62 h (0.9 g/kg per day) after admission. The total caloric intake for each patient during the course of the admission is shown in Fig. 1. Relevant medications provided are listed in Table 1 and include l-carnitine, ammonia scavenger drugs (priming and sustaining infusion), and NCG (priming and sustaining oral doses by nasogastric tube). Sustaining doses of NCG and ammonia scavengers were provided until ammonemia normalized. Timing of ammonia scavenger drugs, NCG and dialysis, if performed, is indicated in Fig. 1.
Fig. 1.
Decrease in ammonia blood level in propionic aciduria patient treated with N-Carbamylglutamate (NCG). Solid lines represent ammonia blood level and single points, the total amount of calories provided during the time course. NCG administration is indicated by yellow arrow, blue arrow represents dialysis, and asterisks indicates the start of ammonia scavengers
Table 2.
Patients outcomes
| Patient | Initial decompensation | Follow up | |||||
|---|---|---|---|---|---|---|---|
| Onset treatment (h) | Hyperammonemia | Coma (days) | ICU days | Survival | Outcomes (age) | ||
| Peak (μM) | Duration > 500 μM (h) | ||||||
| A | 23 | 735 | 6 | 2 | – | Deceased 6 days |
– |
| B | 13 | 524 | 2 | 0 | 1 | Alive | Seizure, mild hepatomegaly, mild developmental delay (3 years) |
| C | 8 | 781 | 5 | 1 | 2 | Deceased 46 days | Hypotonia, delayed myelination on MRI(1 month) |
| D | 18 | 1,572 | 23 | 4 | – | Deceased 8 days | – |
Coma defined as Glasgow score less than 8. ICU intensive care unit
Administration of NCG was concomitant to the scavenger drugs, except for patient B where NCG was given 12 h after starting the scavenger drugs. Plasma ammonia levels decreased significantly in three of the four patients within 2 h after administration of NCG. The nonresponder (patient A) had a transient elevation of ammonia lasting ~10 h. The ammonia level in Patient B showed a downward trend just prior to NCG administration. Blood ammonia levels fell below 100 μM in all patients within 12–29 h of treatment initiation. For patients C and D, whose blood ammonia was above 500 μM prior to NCG bolus (C: 740 μM, D: 1,572 μM), levels fell below 500 μM in 4 and 8 h post-treatment, respectively. Among the four patients, hemodialysis was performed in patient D only, and was begun after a dramatic decrease of blood ammonia had occurred.
The outcomes of our four patients are shown in Table 2. Two patients (A and D) died at the initial metabolic decompensation. Patient C survived the initial episode and was subsequently treated with a protein-restricted diet and carnitine supplementation. Axial hypotonia was noted at 1 month of age and brain MRI showed delayed myelination. He was re-admitted to the ICU for two additional metabolic decompensations; on the second, he presented with lethargy, pH 6.92 and died during the course of the hospitalization at 46 days of life. Patient B, the only survivor, is now 3 years of age. She receives protein restriction and l-carnitine supplementation, without further use of NCG. At 6 months of age, overnight gavage was introduced to improve metabolic control. The patient was admitted once to the ICU in the context of a rotavirus gastroenteritis and dehydration. Mild hepatomegaly was observed early in the course and remained stable without evidence of liver dysfunction. A seizure disorder was diagnosed at age 10 months and remains well controlled with anticonvulsant monotherapy. Head computed tomography and brain MRI were normal. Delayed gross motor milestones were apparent by age 6 months. At 3 years old, she has a mild developmental delay mainly in language. She plays interactive games, copy circles, combines 3–4 words and walk up steps. Although our small sample size prevents a formal data analysis to identify prognosis markers, it is noteworthy that the survivor (patient B) had the lowest peak ammonia level, the shortest duration of hyperammonemia, and was never in coma. Furthermore, this patient had a splice site mutation on one allele, which was not studied but could lead to residual wild type transcript and protein.
Discussion
We report the outcomes of four patients with neonatal PA that were treated with NCG at the initial metabolic decompensation. Previous reports have suggested a role for NCG in the treatment of hyperammonemia in PA (Filippi et al. 2010; Gebhardt et al. 2003, 2005; Jones et al. 2008; Levrat et al. 2008; Schwahn et al. 2010). Since inhibition of N-acetylglutamate synthase may be a contributing factor to the hyperammonemia in PA, the use of NCG, a compound analogous to N-acetylglutamate, could stimulate CPSI in lieu of N-acetylglutamate (Hall et al. 1958; Rubio and Grisolía 1981). Recently, other investigators provided support for this idea by showing increased ureagenesis and decreased ammonia levels following NCG administration in PA patients (Ah Mew et al. 2010; Tuchman et al. 2008). However in all reports, including ours, evaluation of NCG efficacy remains limited by concomitant dialysis and /or the co-administration of ammonia scavenger drugs. The efficacy of ammonia scavengers for controlling hyperammonemia in organic acidurias also remains controversial, given the low or normal glutamine plasma levels typically found in PA patients (Al-Hassnan et al. 2003; Filipowicz et al. 2006). Indeed, glutamine was not elevated in three of our four patients. Thus, the contribution of ammonia scavengers to the decrease in ammonia observed is expected to be less robust than that observed in urea cycle defects.
In our series, three out of four patients showed decrease in ammonemia concomitant with NCG administration. Patient B received NCG 12 h after ammonia scavengers alone had not shown a definitive decrease in ammonia levels, thus arguing for a direct response to NCG. Although reversal of catabolism could have caused the decrease in ammonia, calories were increased from only 44 Kcal/kg per day to 80 Kcal/kg per day just 6 h prior the first NCG dose. In patients C and D, the ammonia decreased more rapidly with NCG, but this was concomitant to the administration of the scavenger drugs. Again, reversal of catabolism as the only factor contributing to the reduction in ammonia is unlikely, given the relatively low total calories provided. We suggest that the combination of NCG and scavengers, or possibly NCG alone, was sufficient to lower ammonia below 500 μM within 6 h, which is a time frame required to start dialysis. Thus, NCG treatment may avoid dialysis in PA when the only indication is hyperammonemia.
The response rate to NCG is unknown in PA, as there is no systematic study of its administration. In this study, one out of four PA patients (patient A) did not initially respond to NCG. An insufficient NCG dose (75 mg/kg) may explain this finding, although one patient with organic aciduria responded to an initial dose of 70 mg/kg (Gebhardt et al. 2003). Others reported initial doses ranging from 100 mg/kg to 250 mg/kg, all associated with reduction in ammonemia (Filippi et al. 2010; Gebhardt et al. 2003, 2005; Jones et al. 2008; Levrat et al. 2008; Schwahn et al. 2010). One reported patient had no response to a dose of 25 mg/kg (Jones et al. 2008).
Outcomes observed in our series of NCG-treated patients were not improved over those with classical treatment without NCG (Dionisi-Vici et al. 2006; Surtees et al. 1992). Mortality of 30–36% has been reported before 2 years of age and development delay is commonly observed. In our series, three patients (A, C, D) out of four were deceased before 2 years of age and the survivor (B) has mild developmental delay. In the latter, it is uncertain whether NCG had a significant role in controlling hyperammonemia and influencing the outcome. Compared to other patients, the lower peak ammonia level, the shorter duration of hyperammonemia and absence of coma could be related possibly to higher residual enzyme activity given presence of a splice site mutation.
There are only five other cases reported in the literature on the outcome of neonatal PA treated by NCG, which are summarized in Table 3. Other reports of use of NCG in PA are limited to the description of the trend of ammonia levels (Gebhardt et al. 2003, 2005; Levrat et al. 2008). Publication bias for successful NCG trials, various NCG doses, and concomitant dialysis and/or ammonia scavengers obscure the ability to draw accurate conclusions. The long-term benefits of NCG in treatment of PA, thus remain unknown. It should be noted that NCG targets only one potential mechanism of neurotoxicity, which is hyperammonemia. Currently, there is no strong evidence that actively treating hyperammonemia improves outcomes in PA, and other mechanisms, such as inhibition of the mitochondrial respiratory chain, may play a more determining role. Better assessment of long-term outcomes of patients treated with NCG is currently needed. This could be facilitated by longitudinal multicenter studies involving a registry and a standard administration protocol.
Table 3.
Previously published outcomes of propionic aciduria patients treated with N-Carbamylglutamate (NCG)
| Onset of symptoms (days) | NGC priming dose | Dialysis | Outcome (age) | Reference |
|---|---|---|---|---|
| 2 | 250 mg/kg | Yes (before NCG) | Microcephaly, sits, polysyllabic (14 months) | Jones et al. (2008) |
| 11 | 250 mg/kg | Yes (after NCG) | Sits, few words (3.5 years old) | Jones et al. (2008) |
| 3 | 400 mg | No | Global developmental delay (age?) | Schwahn et al. (2010) |
| 3 | 400 mg | No | Motor delay, feeding difficulties (age?) | Schwahn et al. (2010) |
| 2 | 150 mg/kg | No | Diffuse cerebral atrophy, axial hypotonia (2 months old) | Filippi et al. (2010) |
Conclusion
In summary, we report the use of NCG in the treatment of neonatal PA in four patients. As previously reported, interpretation of NCG efficacy was limited by concomitant use of ammonia scavengers. Nevertheless, a rapid and sustained decrease in ammonia level was observed in three out of four cases. However, short-term outcome was not improved compared to that reported in cases who did not receive NCG. More data is needed to evaluate the long-term benefit of NCG in treatment of hyperammonemia in PA.
Synopsis
While a trial of NCG may be useful in controlling hyperammonemia in PA, the evidence for improved long-term outcomes are still lacking.
Footnotes
Competing interests: None declared.
References
- Ah Mew N, McCarter R, Daikhin Y, Nissim I, Yudkoff M, Tuchman M. N-carbamylglutamate augments ureagenesis and reduces ammonia and glutamine in propionic acidemia. Pediatrics. 2010;126:208–214. doi: 10.1542/peds.2010-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hassnan ZN, Boyadjiev SA, Praphanphoj V, Hamosh A, Braverman NE, Thomas GH, Geraghty MT. The relationship of plasma glutamine to ammonium and of glycine to acid-base balance in propionic acidaemia. J Inherit Metab Dis. 2003;26:89–91. doi: 10.1023/A:1024048118294. [DOI] [PubMed] [Google Scholar]
- Coude FX, Sweetman L, Nyhan WL. Inhibition by propionyl-coenzyme A of N-acetylglutamate synthetase in rat liver mitochondria. A possible explanation for hyperammonemia in propionic and methylmalonic acidemia. J Clin Invest. 1979;64:1544–1551. doi: 10.1172/JCI109614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionisi-Vici C, Deodato F, Röschinger W, Rhead W, Wilcken B. 'Classical' organic acidurias, propionic aciduria, methylmalonic aciduria and isovaleric aciduria: long-term outcome and effects of expanded newborn screening using tandem mass spectrometry. J Inherit Metab Dis. 2006;29:383–389. doi: 10.1007/s10545-006-0278-z. [DOI] [PubMed] [Google Scholar]
- Filipowicz HR, Ernst SL, Ashurst CL, Pasquali M, Longo N. Metabolic changes associated with hyperammonemia in patients with propionic acidemia. Mol Genet Metab. 2006;88:123–130. doi: 10.1016/j.ymgme.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Filippi L, Gozzini E, Fiorini P, Malvagia S, la Marca G, Donati MA. N-carbamylglutamate in emergency management of hyperammonemia in neonatal acute onset propionic and methylmalonic aciduria. Neonatology. 2010;97:286–290. doi: 10.1159/000255168. [DOI] [PubMed] [Google Scholar]
- Gebhardt B, Vlaho S, Fischer D, Sewell A, Böhles H. N-carbamylglutamate enhances ammonia detoxification in a patient with decompensated methylmalonic aciduria. Mol Genet Metab. 2003;79:303–304. doi: 10.1016/S1096-7192(03)00095-7. [DOI] [PubMed] [Google Scholar]
- Gebhardt B, Dittrich S, Parbel S, Vlaho S, Matsika O, Bohles H. N-carbamylglutamate protects patients with decompensated propionic aciduria from hyperammonaemia. J Inherit Metab Dis. 2005;28:241–244. doi: 10.1007/s10545-005-5260-7. [DOI] [PubMed] [Google Scholar]
- Hall LM, Metzenberg RL, Cohen PP. Isolation and characterization of a naturally occurring cofactor of carbamyl phosphate biosynthesis. J Biol Chem. 1958;230:1013–1021. [PubMed] [Google Scholar]
- Jones S, Reed CA, Vijay S, Walter JH, Morris AA (2008) N-Carbamylglutamate for neonatal hyperammonaemia in propionic acidaemia. J Inherit Metab Dis. doi:10.1007/s10545-008-0777-1 [DOI] [PubMed]
- Levrat V, Forest I, Fouilhoux A, Acquaviva C, Vianey-Saban C, Guffon N. Carglumic acid: an additional therapy in the treatment of organic acidurias with hyperammonemia? Orphanet J Rare Dis. 2008;3:2. doi: 10.1186/1750-1172-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picca S, Dionisi-Vici C, Abeni D, Pastore A, Rizzo C, Orzalesi M, Sabetta G, Rizzoni G, Bartuli A. Extracorporeal dialysis in neonatal hyperammonemia: modalities and prognostic indicators. Pediatr Nephrol. 2001;16:862–867. doi: 10.1007/s004670100702. [DOI] [PubMed] [Google Scholar]
- Rubio V, Grisolía S. Treating urea cycle defects. Nature. 1981;292:496. doi: 10.1038/292496a0. [DOI] [PubMed] [Google Scholar]
- Schwab MA, Sauer SW, Okun JG, Nijtmans LG, Rodenburg RJ, van den Heuvel LP, Dröse S, Brandt U, Hoffmann GF, Ter Laak H, Kölker S, Smeitink JA. Secondary mitochondrial dysfunction in propionic aciduria: a pathogenic role for endogenous mitochondrial toxins. Biochem J. 2006;398:107–112. doi: 10.1042/BJ20060221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwahn BC, Pieterse L, Bisset WM, Galloway PG, Robinson PH. Biochemical efficacy of N-carbamylglutamate in neonatal severe hyperammonaemia due to propionic acidaemia. Eur J Pediatr. 2010;169:133–134. doi: 10.1007/s00431-009-1036-7. [DOI] [PubMed] [Google Scholar]
- Surtees RA, Matthews EE, Leonard JV. Neurologic outcome of propionic acidemia. Pediatr Neurol. 1992;8:333–337. doi: 10.1016/0887-8994(92)90085-D. [DOI] [PubMed] [Google Scholar]
- Tuchman M, Caldovic L, Daikhin Y, Horyn O, Nissim I, Korson M, Burton B, Yudkoff M. N-carbamylglutamate markedly enhances ureagenesis in N-acetylglutamate deficiency and propionic acidemia as measured by isotopic incorporation and blood biomarkers. Pediatr Res. 2008;64:213–217. doi: 10.1203/PDR.0b013e318179454b. [DOI] [PMC free article] [PubMed] [Google Scholar]