Abstract
Mucopolysaccharidosis type VI, Maroteaux–Lamy syndrome is a lysosomal storage disorder with progressive, multisystem involvement caused by deficiency of the lysosomal enzyme N-acetylgalactosamine-4-sulfatase leading to accumulation of the glycosaminoglycan, keratan sulfate. Enzyme replacement therapy (ERT) has been shown to clinically benefit affected individuals. A combined treatment regime of ERT and hemopoietic stem cell transplantation (HSCT) has led to reduced morbidity and mortality in patients with MPS I. We have demonstrated that a treatment regime of ERT combined with HSCT in a 3-year-old girl with MPS VI provided similar benefit. This treatment regimen should be considered in the management of selected patients with MPS VI. Neither HSCT nor ERT can correct or completely prevent progression of the musculoskeletal complications. Long-term follow-up and regular assessments for these complications is necessary.
Introduction
Mucopolysaccharidosis type VI, Maroteaux–Lamy syndrome (MIM #253200) is a lysosomal storage disorder resulting from deficient activity of the enzyme N-acetylgalactosamine 4-sulfatase (Arylsulfatase B EC # 3.1.6.12), which impairs the stepwise degradation of the glycosaminoglycan, dermatan sulfate. Partially degraded dermatan sulfate accumulates in the lysosomes in a wide range of tissues, causing a chronic progressive disorder with shortened life span. Until recently, treatment has been symptomatic and supportive. Specific therapies are now available to provide deficient enzyme, including hematopoietic stem cell transplantation (HSCT) and enzyme replacement therapy (ERT). To date, a combined treatment regimen of ERT and HSCT has not been previously reported for this disorder.
Case Report
A 2-year 6-month-old girl was referred for evaluation of a possible lysosomal storage disorder. The patient was born in Saudi Arabia at term by cesarean section after a pregnancy complicated by gestational diabetes. There were no problems in the perinatal period; however in her first year, she suffered recurrent otitis media and upper respiratory tract infections. At 1 year of age, she was found to have an abnormal mitral valve.
Her early development was normal. She sat unsupported at 7 months, crawled at 8 months and learned to walk at 11 months. Her expressive and receptive speech development was normal and she developed a pincer grip at 12 months. When evaluated at our center, the patient was mildly developmentally delayed in her locomotor skills when scored using the Peabody two motor scales.
The patient’s height was just below the 50th percentile, weight was on the 50th percentile and head circumference was on the 75th percentile. She had coarsening of her facial features, macroglossia, prominent forehead, and had bilateral corneal opacification. She had upper airway obstruction, a small umbilical hernia and moderate hepatosplenomegaly. There was clawing of the distal and interphalangeal joints. In addition, she had restricted wrist flexion, radial deviation, and elbow extension bilaterally.
Initial screening suggested the possibility of upper airway obstruction with minimum oxygen saturation of 85% and evidence of hypercapnia on a morning (arterial) blood gas with pCO2 value of 54 mmHg. However, an overnight sleep study just 1 week later demonstrated severe obstructive sleep apnea with a total index of respiratory events of 19.5/h of sleep time, minimum oxygen saturation of 80% and a high baseline CO2 that was further increased by around 10 mmHg during periods of REM sleep when the index of obstructive respiratory events was 77.5/h. As a result of this study, nasal mask CPAP therapy was recommended to alleviate her upper-airway obstruction and although tolerated in hospital at pressures of 6 cm H2O to alleviate her upper airway obstruction, her parents had trouble maintaining this at home. Following commencement of enzyme therapy and before BMT, a follow-up sleep study showed considerable resolution of her airway obstruction with the total index of respiratory events falling to 8.2 events per hour of sleep time, minimum oxygen saturation 89% normal CO2 baseline values (39.2 mmHg) and the index of events in REM sleep had falling to 13.3 with only 3–4 mmHg increase in CO2 during REM sleep.
An echocardiogram showed mitral valve prolapse, moderate mitral valve regurgitation, dilated left ventricle and left atrium with normal ventricular function. She had moderately severe conductive hearing loss. A skeletal survey showed dysostosis multiplex with thickened calvarium, generalized osteopenia, broad long bones, flared ribs, bilateral coxa valga, and shallow acetabulae.
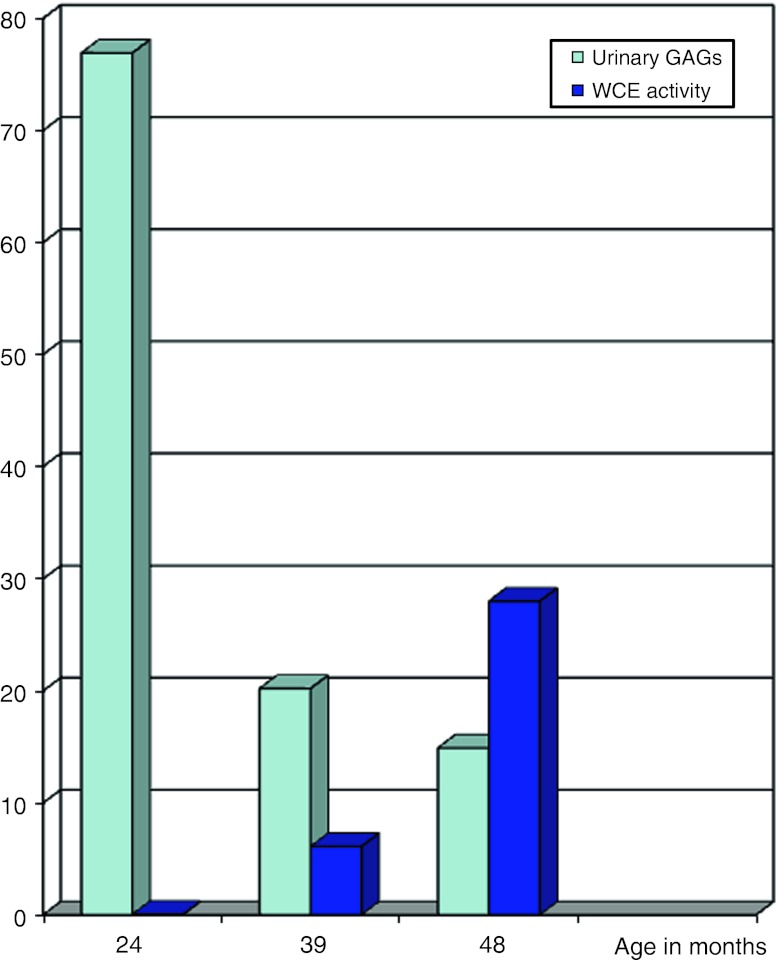
The diagnosis of MPS VI was established at age 2 years and 6 months by the finding of elevated urinary glycosaminoglycans, 76.9 mg/mM (normal range 5.4–26.6 mg/mM creatinine) and a reduced level of the lysosomal enzyme N-acetylgalactosamine-4-sulfate sulfatase activity, 0.05 pmol/min per mg protein (normal range 1.5–21.3 pmol/min per mg protein).
The patient was subsequently found to be homozygous for the p.Y251X mutation in the ARSB gene.
Combined Enzyme Replacement Therapy: Hematopoietic Progenitor Stem Cell Transplantation
ERT was commenced at 2 years and 8 months of age, 16 weeks prior to HSCT, providing weekly intravenous infusions of recombinant human enzyme Galsulfase at a dose of 1 mg/kg per dose. This was continued for 12 weeks after HSCT, providing a total of 28 weekly infusions. HSCT was performed using matched, T-cell depleted unrelated donor peripheral blood stem cells following conditioning with fludarabine, busulfan, and cyclophosphamide.
Combined ERT/HSCT was well tolerated by the patient. The post HSCT course was complicated by mild skin graft versus host disease, treated with steroids.
She required some physiotherapy treatment whilst an inpatient to maintain joint range of movement, muscle strength, and maintenance of developmental milestones in keeping with HSCT and graft versus host disease rather than a result of the MPS.
Follow-Up 1 Year Post HSCT
The response to ERT was rapid with softening of cutaneous features, improved breathing, regression of tonsillar and adenoidal hypertrophy, resolution of obstructive sleep apnea and hepatosplenomegaly. The umbilical hernia was surgically repaired 8 months after the transplantation.
One year after transplantation, she was growing and developing well. There was an improvement in her joint range of movement, gross motor abilities and independence, although remains delayed for her age. She had only mild progression of metatarsus adductus and did not display calf tightness despite growth.
Marrow engraftment was achieved with 100% donor cell line by chimerism studies at 10 months after transplantation.
A follow-up sleep study (10 months after the HSCT) showed that her respiratory indices had now fallen into the normal range, with a total index of respiratory events of 3.3/h, but obstructive events were 0.9/h. Minimum oxygen saturation was 84.0% (after a central apnea). However, she had tachypnea with respiratory rates in the 30s and continued to have mild CO2 retention (4 mmHg) during REM sleep periods.
A skeletal survey showed that the severity of the gibbus deformity had only minimally progressed since commencing therapy. There was some improvement of her hearing. An echocardiogram showed mitral valve prolapse and moderate mitral regurgitation, dilated left ventricle with normal ventricular function.
Urinary glycosaminoglycans fell to within the normal range. The level of N-acetylgalactosamine-4-sulfate sulfatase activity increased to be within the normal range. See Fig. 1.
Fig. 1.
Urinary GAGS and leucocyte N-acetylgalactosamine-4-sulfate sulfatase activity
Discussion
Maroteaux–Lamy syndrome (MPS VI) is an autosomal recessive disorder caused by the deficiency of the lysosomal enzyme N-acetylgalactosamine-4-sulfatase (arylsulfatase B). It is a progressive multisystem disorder with a wide phenotypic spectrum and normal intelligence. Pathogenic mutations in the arylsulfatase B (ARSB) gene lead to incomplete degradation, cellular accumulation, and increased urinary excretion of the glycosaminoglycan, dermatan sulfate. The estimated incidence of MPS VI is approximately 1:300,000 (Giugliani et al. 2007).
The disorder shows a wide spectrum of clinical manifestations with slowly to rapidly progressive forms. The characteristic skeletal dysplasia includes short stature, dysostosis multiplex and degenerative joint disease. Rapidly progressive forms may have onset from birth, severe dysotosis multiplex and death before the second or third decades. A more slowly progressive form has been described having a later onset with milder musculoskeletal complications and death in the fourth or fifth decades. Other clinical features include cardiac valve disease, hepatosplenomegaly, hearing loss, recurrent otitis media, obstructive sleep apnea, corneal clouding, carpal tunnel disease, umbilical and/or inguinal hernia. Central nervous system manifestations include slowly progressive cervical cord compression caused by, accumulation of T2 negative on MRI fibrogelatinous material, circumferential meningeal thickening and/or bony stenosis, rarely cervical spine instability, communicating hydrocephalus, optic nerve atrophy, and blindness. Most MPS VI patients have relatively normal intellectual development (Valayannopoulos et al. 2010).
Until recently, supportive care and bone marrow transplantation were the only therapies available for MPS VI patients. HSCT restores endogenous production of inherently deficient enzyme. The morbidity and mortality associated with HSCT in patients with MPS has improved since HSCT was introduced but the general results have been poor compared to the reduction in morbidity and mortality seen in patients receiving HSCT for hemopoietic malignancies (Turbeville et al. 2011). ERT with Galsulfase®, which became available with the first trials commencing in 2002, resulted in significant improvement in endurance, respiratory, cardiovascular, and musculoskeletal function in patients with MPS VI (Hamartz et al. 2008; Giugliani et al. 2007).
A number of recent publications have demonstrated reduced HSCT morbidity and mortality from preconditioning with ERT and support during the first 120 days post-transplant in infants with MPS I (Hurler). Supplementary ERT before HSCT rapidly reduces upper airway obstruction, and improves cardiorespiratory capacity thereby improving patient fitness for HSCT (Grewal et al. 2005; Cox-Brinkman et al. 2006; Muenzner et al. 2009; Wynn et al. 2009; Tolar et al. 2008; Bijarnia et al. 2009; Clarke et al. 2009). We have demonstrated that a treatment regimen of ERT combined with HSCT in our patient with MPS VI provided similar benefit. This treatment regimen should be considered in the management of selected patients with MPS VI. Long-term follow-up studies comparing the morbidity and mortality of HSCT and ERT combined with HSCT are necessary to evaluate the overall benefits of each treatment regime.
Neither HSCT nor ERT can correct or completely prevent progression of the musculoskeletal complications of mucopolysaccharidosis, carpal tunnel syndrome, or corneal changes. Moreover, progressive kyphosis and scoliosis are common complications. Regular, at least annual assessment for these complications is necessary and surgical interventions are indicated and usually successful.
Acknowledgments
The authors thank Dr Michael Fietz, National Reference laboratory for Lysosomal Disorders, Adelaide, South Australia for Enzyme and Mutation Analysis and Dr Kevin Carpenter, Biochemical Genetics, Sydney Children’s Hospital Network (Westmead) for serial GAG analysis.
Abbreviations
- CPAP
Continuous positive airway pressure
- ERT
Enzyme replacement therapy
- GAGS
Glycosaminoglycans
- HSCT
Hematopoietic stem cell transplantation
- MPS
Mucopolysaccharidosis
Footnotes
Competing interests: None declared.
References
- Bijarnia S, Shaw P, Vimpani A, et al. Combined enzyme replacement and haematopoietic stem cell transplantation in Hurler syndrome. J Paediatr Child Health. 2009;45:469–472. doi: 10.1111/j.1440-1754.2009.01537.x. [DOI] [PubMed] [Google Scholar]
- Clarke L, Wraith J, Beck M, et al. Long-term efficiacy and safety of laronidase in the treatment of mucopolysacchariodosis I. Pediatrics. 2009;123:229–240. doi: 10.1542/peds.2007-3847. [DOI] [PubMed] [Google Scholar]
- Cox-Brinkman J, Boelens J, Wraith J, et al. Haematopoietic stem cell transplantation (HCT) in combination with enzyme replacement therapy (ERT) in patients with Hurler syndrome. Bone Marrow Transplant. 2006;38:17–21. doi: 10.1038/sj.bmt.1705401. [DOI] [PubMed] [Google Scholar]
- Grewal S, Wynn R, Abdenur J, et al. Safety and efficacy of enzyme replacement therapy in combination with hematopoietic stem cell transplantation in hurler syndrome. Genet Med. 2005;7(2):143–146. doi: 10.1097/01.GIM.0000154299.22120.6A. [DOI] [PubMed] [Google Scholar]
- Giugliani R, Hamartz P, Wraith J. Management guidelines for mucopolysaccharidosis VI. Pediatrics. 2007;120:405–418. doi: 10.1542/peds.2006-2184. [DOI] [PubMed] [Google Scholar]
- Harmatz P, Giugliani R, Schwartz I, et al. Long-term follow-up of endurance and safety outcomes during enzyme replacement therapy for mucopolysaccharidodis VI: final results of three clinical studies of recombinant human N-acetylgalactosamine 4-sulfatase. Mol Genet Metab. 2008;98:469–475. doi: 10.1016/j.ymgme.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Muenzner J, Wraith J, Clarke L. The international consensus panel on the management and treatment of mucopolysaccharidosis type I. Mucopolysaccharodosis I: management and treatment guideline. Pediatrics. 2009;123:19–29. doi: 10.1542/peds.2008-0416. [DOI] [PubMed] [Google Scholar]
- Tolar J, Grewal S, Bjoraker K, et al (2008) Combination of enzyme replacement and hematopoietic stem cell transplantation as therapy for Hurler syndrome. Bone Marrow Transplantation 41:531–535 [DOI] [PubMed]
- Turbeville S, Nicely H, Rizzo JD, et al. Clinical outcomes following hematopoietic stem cell transplantation for the treatment of mucopolysaccharidosis VI. Mol Genet Metab. 2011;102(2):111–115. doi: 10.1016/j.ymgme.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valayannopoulos V, Nicely H, Harmatz P, Turbeville S. Mucopolysaccharidosis VI. Orphanet J Rare Dis. 2010;5:5. doi: 10.1186/1750-1172-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn R, Mercer J, Page J, et al. Use of enzyme replacement therapy (laronidase) before hematopoietic stem cell transplantation for mucopolysaccharidosis I: experience in 18 patients. J Pediatr. 2009;154:135–139. doi: 10.1016/j.jpeds.2008.07.004. [DOI] [PubMed] [Google Scholar]