Abstract
Congenital disorders of glycosylation (CDG) are an expanding group of genetic diseases affecting protein and lipid glycosylation. These disorders have a variable presentation and are individually rare. DPAGT1-CDG is a protein N-glycosylation disorder with epilepsy, development delay, severe hypotonia, and dysmorphy, reported in a single patient. Here we present the second family with DPAGT1-CDG identified through homozygosity mapping in a large consanguineous family with 18 affected infants. The patients had severe hypotonia, global developmental delay, seizures, and microcephaly but no dysmorphy. In the index case, the brain MRI revealed delayed myelination, and there was fiber type disproportion on muscle biopsy. Homozygosity mapping identified a large block of homozygosity on chromosome 11p15.5-q25 where two known CDG-I causing genes, ALG9 and DPAGT1, are located. Sequencing ALG9 did not reveal any mutations while analysis of DPAGT1 identified a novel homozygous mutation c.902G>A (p.R301H) in two affected infants. The disorder was fatal in all affected cases and mostly in early infancy.
Introduction
Congenital disorders of glycosylation (CDG) are an expanding group of genetic diseases due to defects in the synthesis of the glycan moiety of glycoproteins or glycolipids and in the attachment of these glycans to proteins and lipids (Freeze 2006; Jaeken 2010). More than 45 CDG have been reported. DPAGT1-CDG is a protein N-glycosylation disorder with only one patient reported showing epilepsy, developmental delay, severe hypotonia, and dysmorphy (Wu et al. 2003; OMIM # 608093).
In a highly consanguineous Saudi family with several affected infants, we identified DPAGT1-CDG through homozygosity mapping. We describe detailed clinical, neuroradiological, and muscle morphology data in the index case. Our report provides a more comprehensive delineation of the phenotype of a very rare type of CDG.
Patients and Methods
Patients
The index case was the product of full term Cesarean section (C-section) due to two previous C-sections. His Apgar scores were 7, 7, and 8 at 1, 5, and 10 min, respectively. He was admitted to the neonatal intensive care unit for 4 days due to respiratory distress that resolved without the need for mechanical ventilation. He was presented to our Medical Genetics clinic at the age of 8 months with a history of developmental delay and hypotonia. He was not able to roll over, sit, or raise his head on prone position. In addition, he started to have episodes of cyanotic spells with brief cessation of breathing. Physical examination revealed a head circumference of 41.5 cm (1 SD below the 5th percentile), weight of 8.2 kg (at the 25th percentile), and length of 76 cm (at the 95th percentile). There was no dysmorphy. He was not able to fix and follow properly but fundoscopy was normal. There was no tongue fasciculation. He had generalized hypotonia, head lag, and depressed deep tendon reflexes. Cardiovascular, respiratory, and abdominal examination was unremarkable.
Investigations revealed the following: complete blood count, renal profile, serum ammonia and lactate, tandem mass spectrometry for acylcarnitines, plasma very long chain fatty acids, urine organic acids, and cerebrospinal fluid glucose, lactate and amino acids were all normal. Liver function test showed elevated alanine aminotransferase at 74 U/L (normal 10–45). Creatine kinase was elevated at 354 U/L (normal 24–195). Ultrasound abdomen and echocardiogram were normal. Brain magnetic resonance imaging (MRI) showed normal appearance of the posterior fossa, of the midline, and of the ventricular system. There was diffuse paucity of the myelin. The cerebral white matter exhibited a hypointensive appearance with respect to the cortex in the temporal polar area which suggested a delay in myelination. No apparent diffusion abnormality was identified. The MRI spectra showed a slight increase of the choline peak and a decrease in N-acetylaspartate and creatinine peaks with normal lactate suggesting increased myelin turnover. Electroencephalography revealed multiple spikes, polyspikes, and waves in multiple regions involving right and left temporal lobes. Visual evoked potentials were normal. Muscle biopsy showed two populations of muscle fibers: uniformly smaller type-1 and normal-sized type-2 fibers. Gomori stain revealed no evidence of mitochondrial abnormalities or abnormal cytoplasmic inclusions. There was no evidence of glycogen or lipid storage. Cytochrome c oxidase was present in all fibers. Acid and alkaline phosphatases were unremarkable. Nonspecific esterase revealed scattered highly atrophic angulated fibers consistent with denervation. Immunohistochemistry revealed normal dystrophins 1, 2 and 3, sarcoglycans (α, β, γ, and δ), β dystroglycan, merosin, and spectrin. Isoelectric focusing of serum transferrin showed a type-I pattern. Phosphomannomutase and phosphomannose isomerase activities in fibroblasts were normal.
The patient was started on phenobarbitone which controlled convulsions. He continued to show severe global developmental delay with frequent episodes of aspiration. He succumbed during one of these episodes at the age of 5 years.
His brother (individual V12, Fig. 1) was presented to our clinic at the age of 2 months with a similar presentation. His mother reported decreased fetal movement. He was noticed to have hypotonia with poor feeding and recurrent choking since birth. His head circumference was 1 SD below the 5th percentile, while weight was at the 10th percentile and length at the 50th percentile. He was not dysmorphic. His pupils were equal and reactive to light. Extraocular movements were intact. He had head lag, generalized hypotonia, and depressed deep tendon reflexes. Cardiovascular, respiratory, and abdominal examination was unremarkable. Relevant investigations revealed elevated alanine aminotransferase at 101 U/L (normal 10–45). Abdominal ultrasound was unremarkable. Brain MRI at the age of 3 months showed no visible myelination of the splenium and anterior limb of the internal capsule but discrete myelination of the white matter in the centrum semiovale. The cerebellum was normally myelinated. There were no focal changes and the ventricular system was normal in size with no evidence for atrophy. The posterior fossa was unremarkable. Mildly delayed myelination could not be excluded due to the age of the patient. Immunonephelometry assay for transferrin was abnormal at 24.5% (normal ≤ 2.47).
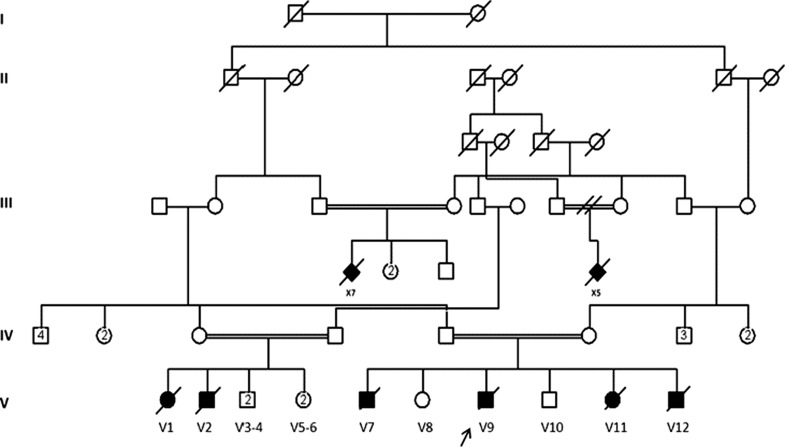
Fig. 1.
The family pedigree showing high degree of consanguinity and multiple affected family members. Arrow indicates index patient
The patient died at the age of 7 months with aspiration pneumonia. His consanguineous parents lost two other children (individuals V7 and V11, Fig. 1) with similar presentations. They had frequent aspirations requiring intensive care unit admission in the local hospital and both died at the age of 8 months before being able to refer them to our hospital for evaluation. The family pedigree was remarkable for 14 cousins in three sibships who died in infancy with severe hypotonia and developmental delay of unknown etiology.
Methods
Sample Collection and DNA Extraction
Whole blood samples were obtained from the two affected patients and their parents and genomic DNA was extracted for each sample by standard salt-precipitation methods (Miller et al. 1988).
Homozygosity Mapping
SNP-based genotyping was performed on the index case and his parents using the Affymetrix® GeneChip Human Mapping 6.0 Array (Affymetrix, Santa Clara, CA, USA). The genotypes of SNPs were called using Affymetrix GCOS 1.4 software, which generated an overall average SNP call rate of 97% and was further analyzed to detect regions of homozygosity using the GTConsole (Affymetrix, Santa Clara, CA, USA) software package. Conventionally, regions or blocks of homozygosity are defined as fragments where SNPs are homozygous for a stretch of consecutive alleles, in this study defined as 2 Mb or longer in length, in affected individuals and heterozygous or homozygous for the other allele in unaffected members of the same family. This genome-wide homozygosity mapping analysis approach was used in this family as it assumes that individuals affected with an autosomal recessive disease, born from parents of a consanguineous marriage, are very likely to be homozygous for the pathogenic mutation and for a substantial number of SNPs surrounding it (Woods et al. 2006).
Mutation Screening in ALG9 and DPAGT1
Genomic DNA of the proband and his parents was amplified by PCR using intronic primers that were designed to flank (50–100 bp) the coding exons (as defined by Ensembl Genome Browser; http://www.ensembl.org/index.html) of ALG9 (NM_001077690.1) and DPAGT1 (NM_001382.3). PCR was performed in a final volume of 20 μL containing approximately 10 ng of genomic DNA, using standard conditions (primer sequences and conditions are available on request).
Automated Sequencing
Purified PCR amplicons covering the entire coding region of each gene were directly sequenced with the dideoxy chain-termination method using an ABI Prism Big Dye Terminator v3.1 Cycle Sequencing Kit following the manufacturer’s instructions and processed on a MegaBACE 1000 DNA Analysis System (Molecular Dynamics; Sunnyvale, CA, USA). Sequence analysis was performed using the SeqMan 6.1 module of the Lasergene (DNA Star Inc., WI, USA) software package, then compared to the reference GenBank sequence. Numbering commenced with the A of the ATG initiation codon as +1.
Results
Homozygosity Mapping
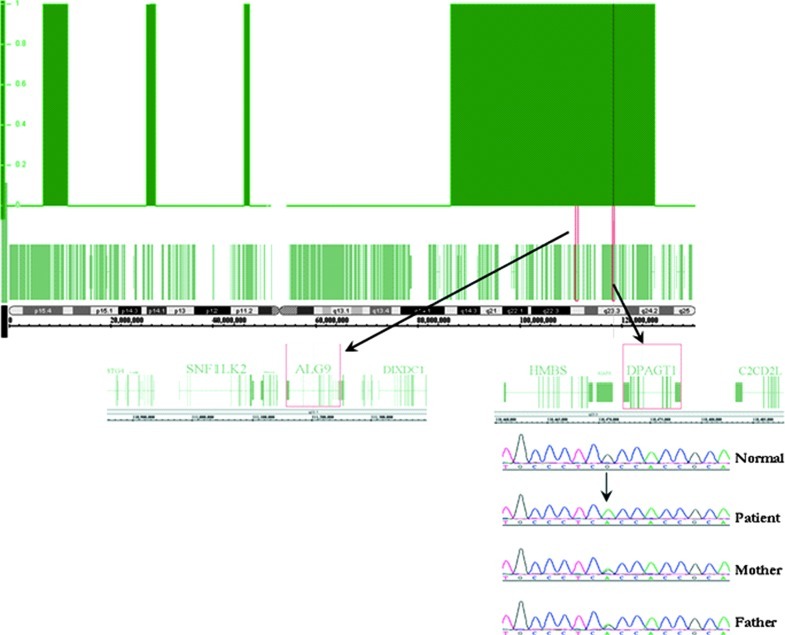
Analysis of the SNP-based genotyping identified a large block of homozygosity (~85 Mb) on chromosome 11p15.5-q25 that was unique to the index case and not shared by his parents (Fig. 2). The known CDG-I genes, ALG9 and DPAGT1, were directly sequenced in this family as they were located in the homozygous block on chromosome 11p15.5-q25. No other CDG-I genes that have been reported previously were found in any other regions of homozygosity that fit the aforementioned criteria.
Fig. 2.
Block of homozygosity on chromosome 11p15.5-q25 in the affected patient harboring both of the known CDG-I causing ALG9 and DPAGT1 genes. Mutation analysis showed the presence of a novel pathogenic c.902G>A (p.R301H) mutation in DPAGT1, segregating with the disease phenotype in this family
Mutation Detection in ALG9 and DPAGT1
Direct sequencing in both the forward and reverse directions for ALG9 did not show any variation from the reference sequence. However, subsequent analysis of the DPAGT1 gene in the proband revealed the presence of a homozygous c.902G>A mutation, resulting in the substitution of arginine to histidine at position 301 (p.R301H). This novel missense mutation segregated with the disease phenotype; both parents were heterozygous carriers, and the individual V11 was homozygous for the mutation. The mutation was not found in 200 ethnically matched normal controls, which indicated that this variant was pathogenic. Protein sequence alignment of DPAGT1 orthologs demonstrated that the arginine residue is highly conserved across 42 different species from human to zebrafish (UCSC Genome Browser Vertebrate Multiz Alignment and Conservation Tool; http://genome.ucsc.edu/). In addition, both Polyphen (http://genetics.bwh.harvard.edu/pph/) and SIFT (http://sift.jcvi.org) programs predicted the variant to be probably damaging lending further support to the pathogenicity of this novel mutation.
Discussion
Congenital defect of glycosylation type DPAGT1-CDG (CDG-Ij) was identified for the first time in a female patient who was presented at the age of 4 months with infantile spasms (Wu et al. 2003). The patient showed global developmental retardation, microcephaly, severe hypotonia, and intractable seizures. Dysmorphic features were also apparent with arched palate, micrognathia, esotropia, fifth finger clinodactyly, single flexion creases, and skin dimples on the upper thigh. Her brain MRI was normal. To our knowledge, no other cases of DPAGT1-CDG (CDG-Ij) have been reported since then. We present a detailed description of the phenotype of DPAGT1-CDG (CDG-Ij) in a large consanguineous Saudi family. The neurodevelopmental phenotype in our patients was similar to the original case described by Wu et al. (2003) with respect to the severe hypotonia, global developmental delay, and microcephaly. Dysmorphic features, however, were not observed in our patients. The brain MRI in our index case revealed delayed myelination, a nonspecific neuroradiological finding that was previously detected in ALG2-CDG (CDG-Ii) (Thiel et al. 2003). Wu et al. (2003) reported that their patient had minimal speech at 6 years of age but survival beyond that age was not reported. In our family, the disorder was fatal in all affected members (18 individuals) and mostly in early infancy.
The muscle biopsy in our patient revealed fiber type disproportion (FTD) which is typically diagnosed when type-1 fibers are consistently smaller than type-2 fibers. This condition, however, is genetically heterogeneous and has been observed in several forms of congenital myopathies, neuromuscular disorders, and muscular dystrophies (Clarke and North 2003). Mutations in ACTA1 (Laing et al. 2004), SEPN1 (Clarke et al. 2006), TPM3 (Clarke et al. 2008), RYR1 (Wilmshurst et al. 2010), and more recently MYH7 (Ortolano et al. 2011) have also been identified in patients with FTD. To add to its nonspecific nature, this morphological finding has been reported in association with several inherited metabolic disorders including Pompe disease (Martin et al. 1976), multiple sulfatase deficiency (Tachi et al. 1984), congenital lactic acidosis (Iso et al. 1993), metachromatic leukodystrophy (Krendel et al. 1994), carnitine palmitoyltransferase deficiency (Shintani et al. 1995), and Krabbe disease (Marjanovic et al. 1996). To our knowledge, FTD has never been described in patients with CDG. Supported clinically and biochemically with the presence of hypotonia and elevated creatine kinase, our case expands the list of genetic conditions that are known to be associated with FTD to include DPAGT1-CDG (CDG-Ij).
The homozygous mutation in the gene DPAGT1 in our family was identified after homozygosity mapping, an approach that we have adopted in this case to overcome the tremendous locus heterogeneity of CDG. In the presence of an abnormal CDT assay, consanguinity, and multiple affected family members, homozygosity mapping is potentially a powerful, and likely cost-effective, approach to identify the specific defect in contrast to the laborious biochemical assays of the glycosylation pathways or to sequential sequencing of genes implicated in the various types of CDG.
In summary, we describe a detailed phenotype of DPAGT1-CDG in a large consanguineous family. This family includes CDG as a cause of FTD.
Acknowledgements
We thank members of the Saudi Diagnostics Laboratory sequencing core facility for technical assistance. This work reports findings identified during the course of routine molecular diagnostic services undertaken by the Research Centre of King Faisal Special Hospital and Research Centre.
Synopsis
Better understanding of the phenotype of DPAGT1-CDG (CDG-Ij) defect. Utilization of homozygosity mapping to identify the specific gene defect in consanguineous families suspected to have an undefined type of CDG.
Footnotes
Competing interests: None declared.
References
- Clarke NF, North KN. Congenital fiber type disproportion–30 years on. J Neuropathol Exp Neurol. 2003;62:977–989. doi: 10.1093/jnen/62.10.977. [DOI] [PubMed] [Google Scholar]
- Clarke NF, Kidson W, Quijano-Roy S, et al. SEPN1: associated with congenital fiber-type disproportion and insulin resistance. Ann Neurol. 2006;59:546–552. doi: 10.1002/ana.20761. [DOI] [PubMed] [Google Scholar]
- Clarke N, Kolski H, Dye D, et al. Mutations in TPM3 are a common cause of congenital fiber type disproportion. Ann Neurol. 2008;63:329–337. doi: 10.1002/ana.21308. [DOI] [PubMed] [Google Scholar]
- Freeze HH. Genetic defects in the human glycome. Nat Rev Genet. 2006;7:537–551. doi: 10.1038/nrg1894. [DOI] [PubMed] [Google Scholar]
- Iso A, Murakami N, Yoneyama H, Hanaoka S, Kurokawa T, Nonaka I. Idiopathic lactic acidemia with developmental delay and type 1 muscle fiber atrophy: report of two patients. Brain Dev. 1993;15:384–386. doi: 10.1016/0387-7604(93)90127-T. [DOI] [PubMed] [Google Scholar]
- Jaeken J. Congenital disorders of glycosylation. Ann NY Acad Sci. 2010;1214:190–198. doi: 10.1111/j.1749-6632.2010.05840.x. [DOI] [PubMed] [Google Scholar]
- Krendel DA, Shutter LA, Holt PJ. Fiber type disproportion in metachromatic leukodystrophy. Muscle Nerve. 1994;17:1352–1353. [PubMed] [Google Scholar]
- Laing NG, Clarke NF, Dye DE, et al. Actin mutations are one cause of congenital fibre type disproportion. Ann Neurol. 2004;56:689–694. doi: 10.1002/ana.20260. [DOI] [PubMed] [Google Scholar]
- Marjanovic B, Cvetkovic D, Dozic S, Todorovic S, Djuric M. Association of Krabbe leukodystrophy and congenital fiber type disproportion. Pediatr Neurol. 1996;15:79–82. doi: 10.1016/0887-8994(96)00092-6. [DOI] [PubMed] [Google Scholar]
- Martin JJ, Clara R, Ceuterick C, Joris C. Is congenital fiber type disproportion a true myopathy? Acta Neurol Belg. 1976;76:335–344. [PubMed] [Google Scholar]
- Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16(3):1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortolano S, Tarrío R, Blanco-Arias P, et al. A novel MYH7 mutation links congenital fiber type disproportion and myosin storage myopathy. Neuromuscul Disord. 2011;21(4):254–262. doi: 10.1016/j.nmd.2010.12.011. [DOI] [PubMed] [Google Scholar]
- Shintani S, Shiigai T, Sugiyama N. Atypical presentation of carnitine palmitoyltransferase (CPT) deficiency as status epilepticus. J Neurol Sci. 1995;129:69–73. doi: 10.1016/0022-510X(94)00223-B. [DOI] [PubMed] [Google Scholar]
- Tachi N, Fujibayashi S, Wagatsuma K, Minami R, Imamura S. A case of multiple sulfatase deficiency with fiber type disproportion. No To Hattatsu. 1984;16:205–209. [PubMed] [Google Scholar]
- Thiel C, Schwarz M, Peng J, et al. A new type of congenital disorders of glycosylation (CDG-Ii) provides new insights into the early steps of dolichol-linked oligosaccharide biosynthesis. J Biol Chem. 2003;278:22498–22505. doi: 10.1074/jbc.M302850200. [DOI] [PubMed] [Google Scholar]
- Wilmshurst JM, Lillis S, Zhou H, et al. RYR1 mutations are a common cause of congenital myopathies with central nuclei. Ann Neurol. 2010;68(5):717–726. doi: 10.1002/ana.22119. [DOI] [PubMed] [Google Scholar]
- Woods CG, Cox J, Springell K, et al. Quantification of homozygosity in consanguineous individuals with autosomal recessive disease. Am J Hum Genet. 2006;78(5):889–896. doi: 10.1086/503875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Rush JS, Karaoglu D, et al. Deficiency of UDP-GlcNAc:dolichol phosphate N-acetylglucosamine-1 phosphate transferase (DPAGT1) causes a novel congenital disorder of glycosylation type Ij. Hum Mutat. 2003;22:144–150. doi: 10.1002/humu.10239. [DOI] [PubMed] [Google Scholar]