Abstract
Objective: To compare a gram protein exchange system (1g=50-mg Phenylalanine) with a unit exchange system (1unit=15-mg Phenylalanine) and its effect on the blood Phenylalanine (Phe) levels and acceptance in the dietary management for children and adolescents with Phenylketonuria.
Methods: In Phase One, participants were randomised to continue counting Phe unit exchanges (n=8) or changed to counting gram protein exchanges (n=10), using a new diet chart developed in-house. Foods containing less than 20mg Phe per serve were now considered “free.” Interim data analysis confirmed no significant deterioration in Phe levels of the study group and the control group was changed to protein counting.
In Phase Two, 18 participants were educated to use an updated version of the in-house diet chart – in this version foods containing less than 50mg Phe per serve were considered “free.”
In both phases, attitudes to PKU and its management were evaluated at baseline and 6months. Phenylalanine and tyrosine levels were measured from filter paper blood spots by tandem mass spectrometry.
Results: Phase One: Phe levels over 6months were comparable to pre-study levels (mean Phe pre 366μmol/L+/− 169, mean Phe post change=388μmol/L+/− 160).
Phase Two: Four participants had a significant improvement in blood Phe levels, nine showed no significant change and one participant’s levels were significantly higher. There was incomplete data on four participants. All participants preferred the freer diet chart.
Conclusion: Protein exchanges (foods containing less than 50mg Phe/serve uncounted) are an alternative method of measuring Phe intake in the dietary management of Phenylketonuria.
Introduction
Compliance with dietary treatment for phenylketonuria (PKU; OMIM 261600) is a significant issue (MacDonald 2000) with parents reporting that managing the diet is the most significant problem related to parenting a child with PKU (Awiszus and Unger 1990). Current recommendations are for adherence to dietary treatment for life (Medical Research Council Working Party on PKU 1993). This further compounds the need to review dietary treatment recommendations to ensure that treatment is manageable for life and that optimal dietary control is achieved (Cockburn and Clark 1996). Dietary treatment should aim to be as least restrictive as possible, given that a simple approach is likely to be easier for patients to follow and hence more likely to be complied with (MacDonald 2000).
Traditional Australian dietary management of PKU consists of a medical formula (protein supplement) providing phenylalanine-free amino acids, vitamins, minerals, and trace elements. Special commercial low protein products and foods that contain minimal amounts of Phe such as fats and sugars are uncounted. Phenylalanine (Phe) is provided in carefully measured quantities of low Phe foods, (some cereals and most fruits and vegetables), using a 15 mg Phe unit exchange system. The number of units is based on blood Phe levels and varies between individuals and at different growth times. Our experience shows that this 15 mg Phe unit system enabled normal growth and development and helped achieve acceptable blood Phe levels (Australasian Society for Inborn Errors of Metabolism 1996).
There are, however, variations throughout the world for determining the Phe content of the diet used in the management of PKU, ranging from the 50 mg Phe exchange system in the UK, to the 15 mg Phe exchange system in the USA. (MacDonald 2000). It is acknowledged that the prescriptive nature of the PKU diet is challenging for the child and caregiver and how the family copes with these challenges influence the blood Phe levels (Ievers-Landis et al. 2005; Vetrone et al. 1989). MacDonald (2000) reviewed the current dietary management systems and concluded that “it is important that the diet is fully evaluated to ensure this regime is not stricter than necessary to achieve acceptable blood Phe control” (p.S141). Each dietary counting method has its own limitations and we were unable to locate publications that have compared dietary methods. Our study assessed the impact on metabolic control achieved using the unit exchange system (1 unit = 15 mg Phe) with a gram of protein exchange system (1 g = 50 mg Phe). Perceptions of the children and their parents were also sought. The new Australian food labelling standards provided the opportunity to directly read the protein content from the commercial foods. Our hypothesis was that this less rigid diet, offering “free fruit and vegetables” would improve dietary variety. There was a theoretical concern that the more relaxed diet adversely impact on Phe control.
It was predicted that (1) simplifying the protein counting system for children with PKU would not have an adverse metabolic effect, compared to the standard Australian PKU counting system (15 mg phe exchanges), and (2) parents and participants would find the new method simpler.
Methods
Study Design
The study was conducted in two phases. In the first phase, an interim diet chart based on 1 g of protein = 50 mg Phe was introduced, “free” foods were defined as those with a cut off point less than 20 mg Phe/serve, resulting in a small number of free fruits and vegetables. The second phase diet chart defined foods with less than 50 mg Phe/serve as “free.” The new definition resulted in an extensive list of free foods, similar to that already in place in the UK (NSPKU 2005/2006).
Measurement of Blood Phe Levels
Blood Phe levels were measured using a weekly home-collected filter paper blood spot and measured by tandem mass spectrometry. Dietary management aimed for blood Phe levels within the range recommended by the Medical Research Council Working Party on Phenylketonuria (1993).
PKU Diet Attitudes Questionnaire
A questionnaire was designed for this study to evaluate the attitudes of young people with PKU and their parents about the preparation of foods, monitoring of the diet, collecting blood tests, metabolic control, dietary variety, stressfulness of the diet, and quality of life. The main caregiver and school aged children and adolescents completed the PKU Diet Attitudes Questionnaire. The questionnaire comprised 18 questions, answered on a five-point Likert scale and three open-ended questions. A parent version and an equivalent version for young people with PKU were used.
Assessment of Dietary Intake
A 3-day diet diary, with a follow up interview was used to assess dietary and supplement intake in both phases (Hackett et al. 1983). The quality of 3-day dietary information was not of sufficient quality to formally report results; however, it was used during consultations with the dietitian.
Phase One
Diet Chart Development
An interim diet chart was developed, before moving to the final and freer counting system in phase 2, to confirm that metabolic control would not be compromised. The phase 1 diet chart was developed in-house using the basis of 1 g of protein as equivalent to 50 mg Phe and permitted all foods that contained less than 20 mg Phe/serve to be uncounted. Where the Phe content per serve was greater than 20 mg the protein content was counted using 0.5 g increments. This was in contrast to the usual 15 mg Phe unit system where foods containing 7.5 mg Phe per serve were counted as a 0.5 unit. The change in cut-off value resulted in new foods, particularly fruit and vegetables becoming “free.” The new “protein” diet chart was written in the same format as the old unit diet chart. Food was listed in grams of protein per serve of a particular food and amount of the food in grams that contained 1 g of protein. Parents and children were taught to use values directly from the nutritional panel for commercial foods and to round protein values up and down. This differed from the unit system where parents and children had to be taught to multiply the protein value on the nutritional panel by 3 to obtain the number of units in a particular food contained. Free commercial snack foods were limited to <0.3 g of protein/serve, due to the ease of being able to eat large serves of these foods compared to fruit and vegetables. Nutritional information was sourced from food tables with amino acid composition information (NUTTAB 1995; Holland et al. 1991)
Participants
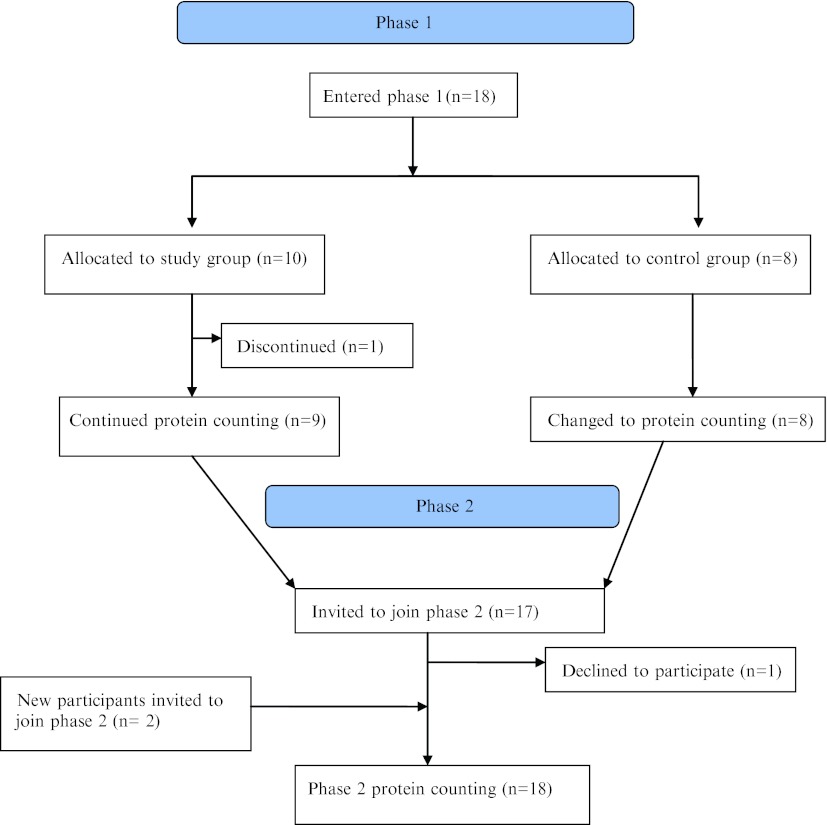
All parents and participants over 1 year of age currently attending the Women’s and Children’s Hospital Metabolic Unit for early treated PKU, were invited by telephone, letter or personal approach to participate in the study. Eighteen of 21 parents gave informed consent for their child to participate, 5 males and 13 females, with a median age of 10 years 1 month (range 2 years 5 months to 17 years 6 months) (Fig. 1). Sixteen participants had the classic PKU phenotype and two had the moderate PKU phenotype. (Medical Research Council Working Party on PKU 1993). All participants and parents had been previously educated on the 15 mg Phe unit system and were on phe-free protein supplement for the treatment of PKU. The study was approved by the Women’s and Children’s Hospital Research Ethics Committee.
Fig. 1.
Methodology of allocation of participants in phases 1 and 2
Study Protocol
Before randomization all participants completed a baseline 3-day diet diary, including protein supplement intake and our PKU Diet Attitudes Questionnaires. Participants with classic PKU were randomly assigned to two groups using a computer generated randomization schedule with balanced blocks of 4 to two groups: either to continue their current Phe exchange system (control group) or change to counting protein exchanges (study group). Two participants with moderate PKU who had difficulty with the PKU diet were allocated to the study group.
All participants had an individual appointment with the same dietitian, separate from their regular clinic appointment, for re-education on dietary management. The study group were educated on the gram protein counting system at this time.
Clinic visits were unchanged – at these visits weight and height, protein supplement, and dietary information were recorded and appropriate changes made. The protein supplement is recommended to be taken throughout the day, as timing of taking the supplement has been shown to affect plasma Phe (MacDonald et al. 1996; MacDonald et al. 2004). Weekly home blood phe monitoring, at a regular time that suited the family, continued as usual (van Spronsen et al. 1993).
There was no change in the method of reporting blood results (phone call, letter, or email) and no other changes were made to the management in either group of patients.
Six months after the initial appointment, or when families had been using the new counting method for at least 6 months, questionnaires and diet diaries were completed for both groups and were analysed along with blood Phe results. The control group were then educated on the phase 1 diet counting method (Fig. 1). Six months after the control group had used the new counting method, questionnaires and diet diaries were completed.
Phase Two
At the conclusion of phase 1 of the study, the second phase of the study commenced, to assess the impact on blood Phe of a further liberalization of uncounted foods.
Diet Chart Development
The study diet chart used in Phase 1 was modified to test whether a further liberalisation of uncounted foods to 50 mg Phe per serve would have a detrimental effect on blood Phe levels. In addition,
Foods containing 40–50 mg Phe/serve were considered to be free but were given a serve size limit
Foods containing more than 50 mg Phe/serve were counted in 0.5 g increments
The Phase 2 diet chart therefore had an extensive list of free foods, particularly for fruits and vegetables. The phase 2 diet chart was written in the same format as the phase one diet chart. Information on reading nutritional panels on commercial foods was given and commercial snack foods were again limited to those <0.3 g of protein/serve considered free. Nutritional information was again sourced from food tables with amino acid composition information, (NUTTAB 1995; Holland et al. 1991).
Participants
Participants (n = 17) from phase 1 were invited by telephone, letter or personal approach to participate in phase 2 of the study; one declined. Two participants with early treated classical PKU (previously educated on the 15 mg Phe unit system) were now eligible to participate as they were over 1 year of age. In total, 18 participants (16 classic PKU, 2 moderate PKU) were enrolled in phase 2; 13 females and 5 males with a median age of 11 years, 6 months, (range 1 year 7 months to 20 years 3 months) (Fig. 1).
Study Protocol
All participants completed a baseline 3 day diet diary, including protein supplement intake and the PKU Diet Attitudes Questionnaires prior to re-education on the updated counting method at an individual appointment, separate from their regular clinic appointment, by the same dietician. As in phase 1, no other changes were made to the management of the participants. Six months after the education session questionnaires and diet diaries were completed.
Statistics
For both phases 1 and 2, all blood tests results and questionnaires for the 6 months prior to the education session and the first 6 months after the educations session were analysed using a non-parametric statistic, the Wilcoxon Signed Rank Test. SPSS for WINDOWS version 10.0 (SPSS Inc., Chicago, IL, USA) at a statistical significance of p < 0.05.
Results
Phase One
Of the 18 phase 1 participants, 10 were allocated to the study group, [3 males, 7 female, median age 10 years 5 months, (range 2 years 5 months to 15 years)]. One young mother of a toddler declined the allocation to the new diet because of lack of confidence. Data was analysed from the remaining 9 study subjects (2 males, 7 females, median age 11 years 5 months, range 2 years 5 months to 15 years) and 8 control subjects. (Fig. 1)
Blood Phe Levels
Table 1 summarises the Phe results for the nine participants in the initial study group. For each participant, there was no significant difference between the Phe levels in the 6 months prior to the study and the 6 months of the new counting method. Participants 8 and 9 had moderate PKU and did not currently use the unit system of counting but avoided animal protein and limited bread and cereal serves. They reported that they found the protein counting system easier than just avoidance of foods as they now had a guideline of the quantity they should be eating of certain foods and felt more confident in their foods choices.
Table 1.
Blood Phe results for the nine participants, pre and post change to the phase one new counting method
| No. | Age | Prescribed units | Prescribed dietary Phe (mg) | Phe prea 6/12 Mean +/− SD |
Prescribed protein (g) | Phe posta 6/12 Mean +/− SD |
Sign |
|---|---|---|---|---|---|---|---|
| 1 | 9.5 | 17.0 | 255 | 517 +/− 270 | 5.0 | 660 +/− 244 | 0.108 |
| 2 | 12 | 24.0 | 360 | 570 +/− 371 | 7.0 | 728 +/− 363 | 0.149 |
| 3 | 12.5 | 17.0 | 255 | 723 +/− 166 | 5.0 | 737 +/− 351 | 0.776 |
| 4 | 15 | 25.0 | 375 | 463 +/− 136 | 7.5 | 516 +/− 136 | 0.173 |
| 5 | 5 | 25.0 | 375 | 251 +/− 57 | 7.5 | 307 +/− 131 | 0.272 |
| 6 | 7.5 | 20.0 | 300 | 359 +/− 255 | 6.0 | 425 +/− 208 | 0.449 |
| 7 | 2.5 | 21.0 | 315 | 202 +/− 117 | 6.0 | 247 +/− 99 | 0.126 |
| 8 | 12 | u/c | u/c | 463 +/− 100 | 28.0 | 488 +/− 64 | 0.583 |
| 9 | 11 | u/c | u/c | 499 +/− 104 | 25.0 | 505 +/− 87 | 0.893 |
aμmol/l
Table 2 summarises the Phe results for the eight participants in the control group for whom there was no change to their dietary counting method in the pre- to post -6 month periods There was no significant difference in blood Phe levels during these two 6 month periods.
Table 2.
Blood Phe results for the eight control group participants during the phase one initial study (no change made to diet chart)
| No. | Age | Prescribed units | Prescribed Phe (mg) |
Phe prea 6/12 Mean +/− SD |
Phe posta 6/12 Mean =/− SD |
Sign |
|---|---|---|---|---|---|---|
| 10 | 11 | 38.0 | 570 | 357 +/− 288 | 341 +/− 233 | 0.693 |
| 11 | 12.5 | 36.0 | 540 | 321 +/− 121 | 270 +/− 132 | 0.300 |
| 12 | 18.5 | 20.0 | 300 | 450 +/− 189 | 512 +/− 183 | 0.249 |
| 13 | 11 | 26.0 | 390 | 552 +/− 151 | 557 +/− 183 | 0.460 |
| 14 | 5.5 | 16.0 | 240 | 231 +/− 240 | 119 +/− 68 | 0.104 |
| 15 | 3 | 14.0 | 210 | 460 +/− 226 | 390 +/− 261 | 0.322 |
| 16 | 4 | 14.0 | 210 | 252 +/− 131 | 275 +/− 166 | 0.477 |
| 17 | 6.5 | 20.0 | 300 | 249 +/− 167 | 254 +/− 94 | 0.705 |
aμmol/l
As there was no detrimental effect on blood Phe and there was positive acceptance of the new counting method by participants and their families the control group were educated on the new diet counting method. At the time of change from control to phase 1 diet chart the median age of the group was 9 years 11 months (range 4 years to 18 years 2 months) (Fig. 1). Table 3 summarises the Phe levels for these participants, pre and post the change to the protein counting method. One participant had a significant improvement in their Phe levels and the other seven participant’s Phe levels were unchanged.
Table 3.
Blood Phe results for the eight (initial control) participants, pre and post change to the new counting method
| No. | Age | Prescribed units | Prescribed Phe (mg) |
Phe prea 6/12 Mean +/− SD |
Prescribed protein (g) |
Phe posta 6/12 Mean =/− SD |
Sign |
|---|---|---|---|---|---|---|---|
| 10 | 11 | 38.0 | 570 | 402 +/− 207 | 11 | 237 +/− 181 | 0.008* |
| 11 | 14 | 36.0 | 540 | 350 +/− 132 | 11 | 405 +/− 197 | 0.300 |
| 12 | 18 | 20.0 | 300 | 561 +/− 158 | 6 | 505 +/− 80 | 0.799 |
| 13 | 12 | 26.0 | 390 | 609 +/− 199 | 7.5 | 553 +/− 111 | 0.056 |
| 14 | 6.5 | 16.0 | 240 | 67 +/− 66 | 5 | 81 +/− 73 | 0.289 |
| 15 | 4 | 14.0 | 210 | 318 +/− 434 | 4 | 178 +/− 123 | 0.465 |
| 16 | 4.5 | 14.0 | 210 | 274 +/− 155 | 3.5–4 | 215 +/− 145 | 0.260 |
| 17 | 8 | 20.0 | 300 | 232 +/− 104 | 6 | 275 +/− 88 | 0.778 |
*p = <0.05
aμmol/l
Questionnaire Results
Every participant from the first group of 8 families, children, and parents indicated that they preferred the protein counting method. Written comments from the first group of 8 parents and children to change counting method described the protein counting method as easier, with smaller numbers to count and thus considered easier to track. Comments were also made that they didn’t need to be as precise with all foods, that “free” foods were used particularly as snacks, with counted protein often kept for meals. Parents noted that commercial products were easier to use and that their children could now understand and read labels now, and were more interested in their diet.
Total scores for all phase one participants on the PKU Diet Attitudes Questionnaire for both parents and children were not significantly different pre and post change to the protein counting method (parents pre change mean = 51.6, SD = 6.5, post change mean = 53.8, SD = 5.6, Z = −1.1, p = 0.25; children pre change mean = 30.7, SD = 5.8, post change mean = 33.1, SD = 5.9, Z = −1.3, p = 0.20). Parent responses to the question “to what extent is there variety of foods in your child’s diet?” indicated a trend towards reporting more variety in their children’s diet post change in counting method, (Z = 0.48, p = 0.06) while children’s own report was of significantly more variety in their diet (Z = −2.45, p = 0.1). All phase one participants preferred the protein counting method.
Phase Two
There were 18 participants enrolled in this phase, 4 participants (3 classic PKU, 1 moderate PKU) have been excluded as they failed to neither provide regular blood tests nor complete all the questionnaires. Complete data was available on 14 participants (9 females, 5 males; median age 9 years 6 months, range 1 year 7 months to 20 years 3 months).
Both parents and children unanimously preferred the freer diet chart.
Blood Phe Levels
Table 4 summarises the blood Phe results for participants in phase 2. In summary, four participant’s blood Phe levels were significantly better following the change in diet chart while nine participants showed no significant difference in blood Phe levels. Participant 18’s blood Phe levels were significantly higher in the second 6 months period; he was a young child who had a number of illnesses during this period of time. He has continued with the new counting method and subsequent results have been acceptable.
Table 4.
Blood Phe results for all study participants, pre and post change to the phase two new counting method
| No.a | Age | Prescribed grams of protein | Pre 6/12b Mean +/− SD |
Post 6/12b Mean =/− SD |
Sign |
|---|---|---|---|---|---|
| 4 | 18 | 5 | 604 +/− 133 | 663 +/− 95 | 0.262 |
| 5 | 8.5 | 7.5 | 279 +/− 101 | 218 +/− 127 | 0.114 |
| 6 | 10.5 | 6 | 657 +/− 120 | 546 +/− 238 | 0.272 |
| 7 | 6 | 7 | 370 +/− 124 | 392 +/− 112 | 0.889 |
| 8 | 15.5 | 15 | 605 +/− 146 | 503 +/− 108 | 0.029*a |
| 10 | 15 | 11 | 528 +/− 316 | 328 +/− 167 | 0.004*a |
| 11 | 16 | 10 | 493 +/− 118 | 343 +/− 99 | 0.002*a |
| 12 | 20.5 | 5 | 550 +/− 128 | 658 +/− 153 | 0.401 |
| 14 | 9 | 5–6 | 285 +/− 145 | 185 +/− 234 | 0.071 |
| 15 | 6.5 | 5 | 418 +/− 485 | 324 +/− 340 | 0.679 |
| 16 | 7.5 | 4 | 327 +/− 298 | 374 +/− 165 | 0.477 |
| 17 | 10 | 6 | 495 +/− 174 | 318 +/− 159 | 0.001*a |
| 18 | 1.5 | 6 | 299 +/− 160 | 456 +/− 151 | 0.001*b |
| 19 | 2.5 | 6 | 274 +/− 252 | 277 +/− 178 | 0.886 |
*a = p < 0.05, based on positive ranks, *b = p < 0.05, bases on negative ranks
aNumber = participant number in phase one
bμmol/l
Discussion
Phase One
Researchers and participants were not prepared to change from a strict 15 mg Phe unit system to a free 50 mg Phe exchange system in one step and a two phase study was designed. There was no significant deterioration in the metabolic control of the interim protein counting group compared to their pre-study levels. Qualitative reports were also positive. The protein-counting group reported ease of dietary management, as the number of exchanges was smaller to count, easier to grasp and made using commercial products easier. In addition, some extended family members reported more confidence in assisting with the management of the child with PKU. During phase 1, the dietitian received feedback from some families who had previously counted all foods very accurately, that they found it hard to try the new system and were very anxious about the possible adverse effect on metabolic control. Other families had already not been counting many of the low Phe foods such as salad vegetables and fruits but had been feeling guilty about this. At the end of phase one, the anxious families were reassured enough to try the more liberal diet.
Phase Two
The change to more “free foods” with a protein prescription resulted in stable blood Phe levels of 13 of the 14 participants compared to their previous levels. One participant, who was ill frequently during this period, had worsening metabolic control while four participant’s levels improved. All reported improved ease of dietary management. Qualitative feedback was also positive. Many parents reported a reduction in the burden of food preparation time, as fewer foods needed to be weighed. Most children were reported to have become more involved in their diets. The older children reported using the extensive list of free fruits and vegetables for their snacks, counting protein only at mealtimes which they reported they did more accurately as there were less foods to measure and count. By allowing “free foods” the children were theoretically consuming more Phe than prescribed in the strict unit system. However, as others have reported (MacDonald et al 2003), we found no negative effect on Phe levels. Some children’s blood Phe levels remained above the target range; however, they reported they found this gram protein exchange system easier to use.
We did not find any significant changes in the attitudes of young people with PKU and their parents about the preparation of foods, monitoring of the diet, collecting blood tests, metabolic control, stressfulness of the diet, and quality of life, as a result of changes in dietary management, except for a small increase in reports of dietary variety.
It is possible that the imposition on a child and their family’s life of a low Phe diet in an Australian setting is so significant that ratings of the overall impact of the diet did not change, although qualitative comments indicated some acknowledgement that this new counting system represented a small but helpful improvement.
A strength of this research was the high participation rate of the families approached, which may have been the result of the close and longstanding relationship the researchers have with the children and parents with PKU who attend this clinic, as well as the interest families have in simplifying dietary management.
The U.K. clinics have been using the 50 mg Phe exchange system for many years and these research findings have validated their method of counting Phe in PKU diets. Following this study, many PKU treatment centres in Australia have changed to counting protein.
As this research was carried out in only one centre, participant numbers were low. Future work should aim for collaborative work across centres. This would also enable consideration of a possible impact of the age of children on the effect of changes to diet management. Although food diaries were completed, they were not accurate enough for quantitative analysis. Future work will need to consider how food diaries are collected and examined. Further research should aim to establish whether these findings are replicated in other cultures and countries and should also aim to evaluation of longer term changes to blood Phe levels.
Since completion of this study, further information has been published by English researchers on Phe content of foods and the effect on Phe levels of allowing these foods freely in the PKU diet (Weetch and MacDonald 2006). Updated information on the Phe content of foods (NUTTAB 2006) continues to be published and emphasises the need for dietitians to continually update and review diet charts.
In our centre, the dietary management of PKU during pregnancy has been informed by these findings and three recent pregnancies in our Unit, for women with classic PKU, have been managed using the phase two diet chart with good metabolic control achieved (Sweeney et al. 2006).
Conclusion
Protein exchanges (foods containing less than 50 mg Phe/serve uncounted) are an alternative method of measuring Phe intake in the dietary management of PKU. All participants found protein counting easier to manage compared to the traditional Australian 15 mg Phe exchange system and continue to use this method of counting the Phe in the PKU diet. Some participants have continued to count protein for 8 years with good metabolic control, indicating sustainability of this counting method. Counting protein exchanges is now is now used for all PKU patients including maternal PKU in South Australia.
Acknowledgements
The authors are very grateful and thank the children and their parents from the W.C.H. Metabolic Clinic for participating in the 2 phases of this study.
We also thank Dr. Maria Makrides (Child Health Research Institute, WCH Adelaide) for advice on study design and for the randomization schedule.
This research was supported by Nutricia Australia; none of the researchers had a financial or personal interest in Nutricia Australia
Take Home Message
Protein exchanges with foods containing less than 50 mg Phe/serve uncounted is an alternative method of measuring Phe intake in the dietary management of Phenylketonuria and is preferred by participants and their families when compared with a 15 mg Phe unit system.
Details of the Contributions of Individual Authors
A. Sweeney: Study design, planning and conduct, analysis and interpretation of data, and writing of manuscript.
R. Roberts: Study planning and conduct, interpretation of data, and writing of manuscript.
J. Fletcher: Study design, planning and conduct, interpretation of results, and writing of manuscript.
Author Serving as Guarantor for Article
A. Sweeney accepts full responsibility for the work, had access to the data, and controlled the decision to publish.
Details of Funding
This research was supported by SHS Nutricia Australia. The authors confirm independence from SHS Nutricia Australia. There was no control over design of study, interpretation or reporting of results. The content of the article has not been influenced by the sponsor.
Details of Ethics Approval
This study was approved by the Women’s and Children’s Hospital Research Ethics committee. Approval number 1270/12/2007.
Patient Consent Statement
Informed consent to participate in this study was obtained for all participants (or parents of participants if participant was younger than 18 years).
Footnotes
Competing interests: None declared.
References
- Awiszus D, Unger I. Coping with PKU: results of narrative interviews with parents. Eur J Pediatr. 1990;149(Suppl 1):S45–51. doi: 10.1007/BF02126299. [DOI] [PubMed] [Google Scholar]
- PKU Handbook. Surry Hills, NSW, Australia: Human Genetic Society of Australasia; 1996. [Google Scholar]
- Cockburn F, Clark BJ. Recommendations for protein and amino acid intake in PKU patients. Eur J Pediatr. 1996;155(Suppl 1):S125–129. doi: 10.1007/PL00014228. [DOI] [PubMed] [Google Scholar]
- Hackett AF, Rugg-Gunn AJ, Appleton DR. Use of dietary diary and interview to estimate the food intake of children. Hum Nutr Appl Nutr. 1983;37A:293–300. [PubMed] [Google Scholar]
- Holland B, Welch AA, Unwin ID, Buss DH, Paul AA, Southgate DAT. McCance and Widdowson’s the composition of foods. 5. Cambridge: Royal Society of Chemistry and Ministry of Agriculture, Fisheries and Food; 1991. [Google Scholar]
- Ievers-Landis C, Hoff AL, Brez C, Cancilliere MK, McConnell J, Kerr D. Situational analysis of dietary challenges of the treatment regimen for children and adolescents with phenylketonuria and their primary caregivers. J Dev Behav Paediatr. 2005;26:186–193. doi: 10.1097/00004703-200506000-00004. [DOI] [PubMed] [Google Scholar]
- MacDonald A, Rylance G, Hall SK, Asplin A, Booth IW. Factors affecting the variation in plasma phenylanaline in patients with phenylketonuria on diet. Arch Dis Child. 1996;74:412–417. doi: 10.1136/adc.74.5.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald A. Diet and compliance in phenylketonuria. EurJ Pediatr. 2000;159(Suppl 2):S136–S141. doi: 10.1007/PL00014375. [DOI] [PubMed] [Google Scholar]
- MacDonald A, Rylance G, Davies P, Asplin D, Hall SK, Booth IW. Free use of fruit and vegetables in phenylketonuria. J Inherit Metab Dis. 2003;26:327–338. doi: 10.1023/A:1025150901439. [DOI] [PubMed] [Google Scholar]
- MacDonald A, Dayly A, Davies P, et al. Protein substitutes for PKU: what’s new? J Inherit Metab Dis. 2004;27:363–371. doi: 10.1023/B:BOLI.0000031099.79046.65. [DOI] [PubMed] [Google Scholar]
- Medical Research Council Working Party on PKU (1993) Arch Dis Child 68:426–427 [DOI] [PMC free article] [PubMed]
- NSPKU (2005/2006) The National Society for Phenylketonuria (United Kingdom) Ltd. Dietary information for the treatment of phenylketonuria
- NUTTAB (1995) Nutrient tables for use in Australia. Food Standards Australia and New Zealand (www.foodstandards.gov.au)
- NUTTAB (2006) Nutrient tables for use in Australia. Food Standards Australia and New Zealand (www.foodstandards.gov.au)
- Sweeney AL, Ketteridge DB, Ranieri E, Fletcher JM. Managing PKU pregnancies using a low protein diet (abstract) J Inherit Metab Dis. 2006;29(Suppl 1):39. [Google Scholar]
- van Spronsen FJ, van Rijn M, van Dijk T, et al. Plasma phenylananine and tyrosine responses to different nutritional conditions (fasting/postprandial) in patients with phenylketonuria: effect of sample timing. Pediatrics. 1993;92:570–573. [PubMed] [Google Scholar]
- Vetrone P, Leuzzi V, Zazzara V, Antonozzi I. Psychological effects on parents of children with early detected Phenylketonuria. J Inher Metab Dis. 1989;12:345–6. doi: 10.1007/BF01799238. [DOI] [PubMed] [Google Scholar]
- Weetch E, MacDonald A. The determination of phenylalanine content of foods suitable for phenylketonuria. J Hum Nutr Diet. 2006;19:229–236. doi: 10.1111/j.1365-277X.2006.00696.x. [DOI] [PubMed] [Google Scholar]