Abstract
Background: In Australia, enzyme replacement therapy (ERT) for Fabry Disease (FD), both Agalsidase alfa (Replagal, Shire HGT) and beta (Fabrazyme, Genzyme), is funded and monitored through a specific government program. Agalsidase beta supply has been rationed by Genzyme since 2009 due to manufacturing issues. Consequently, the Australian Fabry Disease Advisory Committee has treated patients on Agalsidase beta at 50% of their usual dose from mid-2009, with a further reduction to 30% for some patients from late 2009.
Aim: To determine the clinical effect of Agalsidase beta dose reduction in the Australian FD patient cohort.
Methods: A questionnaire assessing FD symptoms was administered to 40 patients on long-term ERT. Clinical data from The Fabry Registry for patients receiving Agalsidase alfa or beta, for at least 2 years prior to the time of enforced Agalsidase beta dose reduction, were reviewed. Disease burden and quality of life (QOL) were graded using the Disease Severity Scoring System, Mainz Severity Score Index, Brief Pain Inventory and Short Form 36 Health Survey at 2 years before dose reduction, at the time of dose reduction and at the most recent clinical review following dose reduction.
Results: Disease severity and QOL scores did not change between the ERT groups. Males on Agalsidase beta reported lower energy levels after dose reduction, while no change was reported by females on either product or by males on a stable dose of Agalsidase alfa.
Conclusion: This study suggests that energy levels in male patients worsen after dose reduction of Agalsidase beta.
Introduction
Fabry Disease (FD; MIM 301500) is associated with reduced quality of life (QOL) compared to the general population, particularly in affected males (Wilcox et al. 2004; Hoffmann et al. 2005). Consistent with the natural history of the disease, QOL continues to deteriorate in untreated FD patients with advancing age, independent of normal aging effect (Miners et al. 2002). We report on the course of FD symptoms and disease burden following an enforced dose reduction in Agalsidase beta therapy.
Two commercially available forms of human enzyme replacement therapy (ERT) are approved for use in FD in Australia: Agalsidase beta (Fabrazyme, Genzyme) at the approved dose of 1 mg/kg/2 weeks and Agalsidase alfa (Replagal, Shire HGT) at 0.2 mg/kg/2 weeks. In Australia, access to either Agalsidase alfa or beta is federally subsidized through the Life Saving Drugs Program (LSDP). Under this program, all patients have a structured review by a Fabry physician and undergo prescribed medical tests and QOL assessments every 6 months (LSDP 2010). This information is reviewed at least annually by the Fabry Disease Advisory Committee as part of the process for ongoing funding.
Since June 2009, a number of manufacturing problems have resulted in a worldwide shortage of Agalsidase beta. After Genzyme introduced dose-rationing measures in mid-2009, a decision was made by the Australian Fabry Disease Advisory Committee to initially continue treating FD patients with Agalsidase beta at 50% of their usual dose, with a further reduction in some patients to 30% from late 2009 until late 2010. The situation presented a unique opportunity to evaluate the impact of reduced Agalsidase beta dose on FD patients.
Systematic review of short-term randomized placebo controlled trials suggest benefit from treatment of FD with either Agalsidase alfa (improvement in pain and QOL scores) or Agalsidase beta (improved GL3 clearance from renal, cardiac, and skin biopsies) (El Dib and Pastores 2010). In uncontrolled studies, Agalsidase alfa improved QOL and pain, produced sustained reduction in left ventricular hypertrophy (LVH) and reduced the rate of decline of estimated glomerular filtration rate (eGFR) (Hoffmann et al. 2005; Mehta et al. 2009). Efficacy in improving pain, disease burden scores, and LVH reduction has been shown in female patients (Whybra et al. 2009). Agalsidase beta has been shown to improve QOL in male and female patients (Watt et al. 2010) and to slow progression to the combined outcome of death, renal, cardiac, and cerebrovascular events in patients with mild-to-moderate renal disease (Banikazemi et al. 2007).
Agalsidase alfa and beta differ only in glycosylation pattern: Agalsidase beta contains more mannose-6-phosphate (M6P), allowing greater M6P receptor mediated uptake by cultured human Fabry fibroblasts and in the kidney, spleen and heart cells of Fabry mice (Lee et al. 2003; Sakuraba et al. 2006). In vivo studies demonstrate similar antigenic profiles and the potency of both enzymes is dose-dependent (Lee et al. 2003; Sakuraba et al. 2006). It is unclear whether the two products differ in clinical efficacy or whether the approved dose of either product is optimal. One systematic review has suggested that Agalsidase beta at a dose of 1 mg/kg/2 weeks has more robust evidence for efficacy than Agalsidase alfa at 0.2 mg/kg/2 weeks, however, head-to-head studies between the two are lacking (Schaefer et al. 2009). Direct comparison of both ERT products at the same dose (0.2 mg/kg/2 weeks) in 34 patients over 24 months failed to show any difference, although the clinical benefit of either product was less than anticipated (Vedder et al. 2007). In 11 male FD patients with declining renal function on Agalsidase alfa 0.2 mg/kg/2 weeks, Agalsidase alfa at 0.2 mg/kg delivered weekly for 24 months significantly slowed the rate of eGFR decline (Schiffmann et al. 2007). Reduced proteinuria in patients receiving high dose (0.4 mg/kg/2 weeks) Agalsidase alfa has also been reported (Torra et al. 2008). Eighteen months of Agalsidase beta therapy at a dose of 0.3 mg/kg/2 weeks, after patients had received 1 mg/kg/2 weeks for 6 months, did not result in an increase in mean plasma globotriaosylceramide (GL3) levels, although some patients in whom plasma GL3 levels normalized recorded elevated levels after dose reduction (Lubanda et al. 2009). Clearance of GL3 from renal capillary endothelium was seen in 100% of patients after 6 months of ERT, and while no significant increase in GL3 accumulation was evident after 18 months at 0.3 mg/kg/2 weeks, small patient numbers limit the conclusions that can be made from this study (Lubanda et al. 2009). Thus, uncertainty remains as to the optimal dose of ERT relevant to clinical outcomes in FD.
Methods
This study utilized The Fabry Registry data of all Australian FD patients receiving ERT, plus a brief custom-designed questionnaire regarding FD symptoms and attitude to changing ERT product. Specific data used in this study, prospectively collated in The Fabry Registry, are listed in Table 1.
Table 1.
Clinical information from The Fabry Registry used in this study
| Medical history, family pedigree, standardized neurological examination |
| Patient reports of sweating, gastrointestinal symptoms, pain |
| Pain and Quality of Life scores (Brief Pain Inventory-short form, Short Form-36 Health Survey) |
| Electrocardiogram, echocardiogram (angiogram and cardiac MRI results if available) |
| MRI/CT brain scan |
| Pulmonary function tests |
| Creatinine and estimated glomerular filtration (eGFR), timed urine collection (creatinine clearance, proteinuria, albumin excretion rate) or spot urine (albumin:creatinine ratio or protein:creatinine ratio), end stage renal failure requiring dialysis or renal transplantation |
| Genotype |
| ERT commencement date, current and previous administered doses Plasma GL3 levels and anti-α-galactosidase antibody titre (if available) Plasma GL3 levels (if available) |
Questionnaire
One year after the dose reduction of Agalsidase beta, all Australian patients who had already received Agalsidase beta for a minimum of 18 months, including 6 months of standard dose ERT before 12 months of reduced dose therapy, were invited to complete the questionnaire (Table 2).
Table 2.
Questionnaire administered to all ERT patients
| Are you receiving Fabrazyme or Replagal? |
| For patients on Agalsidase beta (Fabrazyme): We would like to find out about your attitude to changing to a different type of enzyme replacement therapy, Agalsidase alfa (Replagal). Do you wish to change from Fabrazyme to Replagal or do you wish to remain on Fabrazyme? What is the reason for wishing to change therapy/stay on your current therapy? |
| For all patients: Compared to this time last year, have you noticed a change in your: |
| Sweating? |
| Cold tolerance? |
| Heat tolerance? |
| Pain? |
| (If yes to any of these, please describe your symptoms). |
| On a scale of 0–10, (with 0 representing no energy and 10 representing feeling full of energy), how would you grade your energy at the moment? |
| On a scale of 0–10, how would you grade your energy this time last year? |
| Do you experience diarrhea? |
| If yes, how many bouts per month? |
| How many motions per day at the most? |
| Do you experience abdominal pain or cramping? |
| If yes, how many days per week? |
| Are you working? How many hours per week? What does your work involve? |
| Do you do any regular exercise? How many times per week? How long each time? |
| Do you notice any difference in how you feel before and after the infusions each fortnight? If yes, what have you noticed? |
| Have you ever had any infusion reactions? If yes, can you describe them? |
Serial Assessment of Disease Burden and Quality of Life
Data were collated for all Australian patients who had been on ERT, either Agalsidase alfa or beta, for at least 2 years before dose reduction and for whom Registry data from clinical review in 2010 was available. This data was graded using two tools (Table 3): the Mainz Severity Score Index and the Disease Severity Scoring System (DS3), validated to assess FD burden in classically affected male patients and in the general FD population, respectively (Whybra et al. 2004; Giannini et al. 2010). Disease severity was graded at 3 time points for each patient: 24 months prior to dose reduction, at the time of dose reduction and at the most recent clinical review. To assess the impact of Agalsidase beta dose reduction on patient QOL over this period, results of QOL assessment tools were collated at each of these 3 time points. These included the Brief Pain Inventory (BPI) “worst pain in the last 24 h” and “average pain” scores and Short Form 36 Health Survey (SF36) Mental Component Summary (MCS) and Physical Component Summary (PCS) scores. These tools and their domains have been previously validated and utilized to assess FD symptoms and response to therapy (Schiffmann et al. 2001; Cleeland 2002; Gold et al. 2002).
Table 3.
Disease burden scoring systems utilized in this study
| Scoring System | Score | |
|---|---|---|
| The Mainz Severity Score Index (MSSI) | ||
| General score | Characteristic facies, angiokeratoma, edema, musculoskeletal, cornea verticillata, diaphoresis, abdominal pain, diarrhea/constipation, hemorrhoids, pulmonary, NYHA | 18 |
| Neurological | Tinnitus, vertigo, acroparesthesia, fever pain crisis, cerebrovascular (ischemic lesions in MRI/CT, TIA, migraine, stroke), psychiatric/psychosocial (depression, fatigue, reduced activity level) | 20 |
| Cardiovascular | Changes in cardiac muscle thickness, valve insufficiency, ECG abnormalities, pacemaker, hypertension | 20 |
| Renal | Evidence of renal dysfunction (proteinuria, tubular dysfunction, serum creatinine levels > 3.5 mg/dL, dialysis) | 18 |
| Maximum score | 76 | |
| Disease severity: mild (<20), moderate (20–40) or severe (>40) | ||
| The Disease Severity Scoring System (DS3) | ||
| PNS | Sweating, gastrointestinal, pain | 4 |
| Renal | eGFR, proteinuria, eGFR slope | 8 |
| Cardiac | LVH, arrhythmia, NYHA score | 8 |
| CNS | White matter lesions, TIA/stroke | 8 |
| Self report of wellbeing | 4 | |
| Maximum score | 32 | |
CNS central nervous system, eGFR estimated glomerular filtration rate, LVH left ventricular hypertrophy, NYHA New York Heart Association score, PNS peripheral nervous system, TIA transient ischemic attack
The New York Heart Association (NYHA) class and the SF36 are valid criteria of heart failure symptoms (Garin et al. 2009). As neither NYHA symptom class nor specific patient symptoms (such as wellbeing, fatigue, and activity level) are currently collected by The Fabry Registry, surrogate measures for these parameters were used in estimating MSSI and DS3 scores. NYHA class I–IV was inferred from the SF36 Physical Functioning (PF) score (quartile 4 = NYHA I, 3 = NYHA II, 2 = NYHA III, 1 = NYHA IV). Reduced activity and fatigue levels (used in MSSI) were inferred if the SF36 PF and Vitality (VT) scores, respectively, were <50%. Assessment of pain in DS3 scoring covers 6 grades and was inferred from the SF36 Bodily Pain (BP) scores. Gastrointestinal (GI) symptoms were graded for the DS3 as shown in Table 4. The DS3 patient “self-report of wellbeing” (a domain of the DS3, scored 0–4) was inferred by averaging the SF36 PCS and MCS scores. These surrogate measurements represent 8% and 24% of the total MSSI and DS3 scores, respectively. The validity of these surrogate markers was confirmed not to affect the total MSSI or DS3 results, as scored using the primary data from 17 patients treated at a single centre where data for NYHA, activity and fatigue scores were all available. Data for all other parameters required by the MSSI and DS3 were obtained directly from the Registry.
Table 4.
Scoring of gastrointestinal symptoms (including abdominal pain and diarrhea) for the DS3
| Score | Abdominal pain | Diarrhea |
|---|---|---|
| 0 | None | None |
| 1 | None | Monthly or weekly |
| 2 | None | Daily |
| OR | ||
| Monthly | None or monthly | |
| 3 | Monthly | Weekly or daily |
| OR | ||
| Weekly | None or monthly | |
| 4 | Weekly | Daily or Weekly |
| 5 | Weekly or daily | Daily |
Statistical analyses were conducted using Graphpad Prism v5.03 (1992–2010 GraphPad Software Inc) and included Mann Whitney tests, Fisher’s exact t-test, Wilcoxon matched pairs signed rank tests for nonparametric data and one- and two-way ANOVA for group comparisons over time.
Results
Questionnaire
Of 43, 40 eligible patients completed the questionnaire (93%): 28 Agalsidase beta patients (23 males, 5 females) and 12 Agalsidase alfa patients (9 males, 3 females). Table 5 indicates the mutation class responsible for FD in the patients completing the questionnaire. At the time of the questionnaire, the median age of Agalsidase beta and alfa patients was 46 years (range 25–71 years) and 40 years (range 22–64 years), respectively; this difference was not statistically significant (Mann Whitney p = 0.29). Median duration of ERT prior to the time of Agalsidase beta dose reduction was 73.5 months (range 16–138) for Agalsidase beta patients and 100 months (range 38–118) for Agalsidase alfa patients (Mann Whitney p = 0.06). Median doses of Agalsidase beta, administered every 2 weeks, before and after the initial 50% dose reduction were 70 mg (range 50–90 mg) and 35 mg (range 15–65 mg), respectively. Of the 28, 14 Agalsidase beta patients completing the questionnaire had a second dose reduction to 0.3 mg/kg, but this did not significantly affect the final median dose in the whole group of 28 patients (35 mg, range 10–65 mg).
Table 5.
Mutations for male and female FD patients on ERT
| Mutation | Agalsidase beta | Agalsidase alfa | ||
|---|---|---|---|---|
| Males (n = 23) | Females (n = 5) | Males (n = 9) | Females (n = 3) | |
| Missense | 15 | 5 | 4 | 1 |
| Intronic splice site | 1 | 0 | 0 | 0 |
| Nonsense | 5 | 0 | 0 | 0 |
| Deletion | 2 | 0 | 4 | 2 |
| Insertion | 0 | 0 | 1 | 0 |
Self-Reported Energy Levels
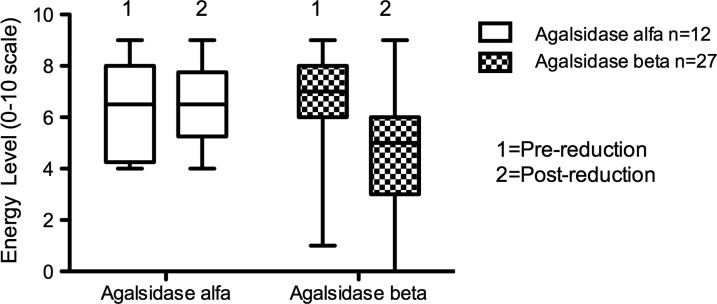
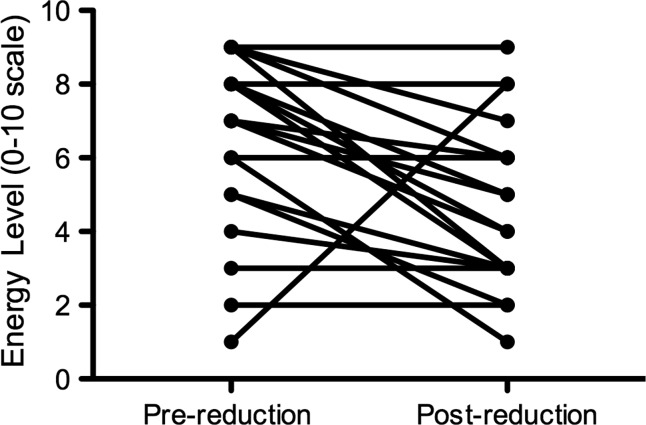
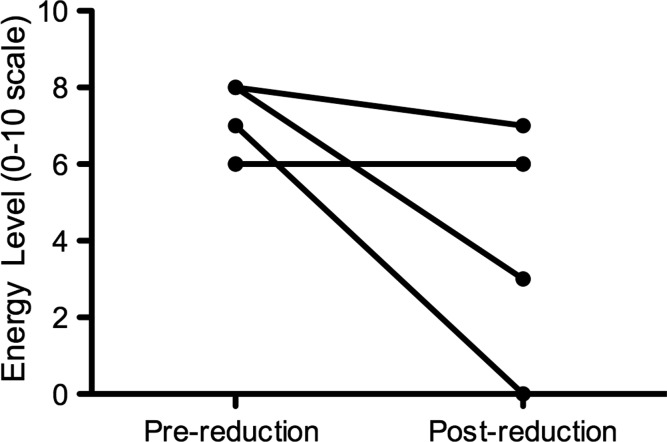
Of the 28, 27 Agalsidase beta patients were able to describe their energy levels at the time of the questionnaire compared to 12 months earlier. There was a significant change in energy levels reported in Agalsidase beta patients between 2009 and 2010 (ANOVA p = 0.03; see Fig. 1). The decrease in energy levels (scored 0–10) over this 12-month period was significant in males receiving Agalsidase beta (median scores of 7 in 2009 and 5 in 2010, range 1–9 for both; Wilcoxon signed rank test p = 0.007), but not in females (median scores of 7 in 2009 and 6 in 2010, ranges 6–8 and 0–9, respectively; Wilcoxon signed rank test p = 0.25; Figs. 2 and 3). There was no significant change in reported energy score in patients on Agalsidase alfa: male (median scores of 8 in 2009 and 7 in 2010, ranges 4–9 and 5–9, respectively, Wilcoxon signed rank test p = 0.77) and female (median scores of 4 in 2009 and 2010, ranges 4–6 and 4–7, respectively, Wilcoxon signed rank test p = 1.0).
Fig. 1.
Energy level for patients receiving enzyme replacement therapy. Anova p = 0.03. Note: One patient in the Agalsidase beta group has been excluded from this analysis, as this patient was unable to recall their energy levels 12 months earlier
Fig. 2.
Energy level in male Agalsidase beta patients (n = 22). Wilcoxon matched-pairs signed rank test p = 0.007
Fig. 3.
Energy level in female Agalsidase beta patients (n = 5). Wilcoxon matched-pairs signed rank test. p = 0.25. Note: 2 patients recorded the same energy level scores pre- and postreduction
Impact on Symptoms of Sweating, Heat and Cold Tolerance, Pain, Diarrhea and Abdominal Pain and Differences in Wellbeing After Each Infusion
No significant changes in sweating, heat, or cold tolerance, pain or post-infusion symptoms were identified in any patient group during this period. At the time of the questionnaire, there was no difference in diarrhea or abdominal pain between those on reduced dose Agalsidase beta and standard dose Agalsidase alfa (Fisher’s exact t-test p = 0.16 and p = 0.49, respectively).
Attitude to Changing ERT to Agalsidase Alfa
At the time of questionnaire, 8 of the 23 male Agalsidase beta patients were considering changing product, 1 was uncertain and 14 wished to remain on Agalsidase beta. Two of the 5 female patients were considering changing and 3 did not wish to change. Reasons given for wishing to change ERT from Agalsidase beta to alfa included concern over ongoing supply issues (n = 5), perceived worsening of symptoms or change in test results since dose reduction (n = 3), immediate home infusion availability (n = 1) and because a relative was changing (n = 1).
Of the 17 patients who did not wish to change product, a dominant reason was provided by 13:8 were content with current therapy, 3 expressed concerns of infusion reactions if therapy was changed, 1 perceived the alternative therapy as inferior, and 1 wished to defer the decision pending physician’s review of test results.
Self-Report of Infusion Reactions
More patients on Agalsidase beta (14 of 28) recalled having infusion reactions at the start of their ERT compared to none of the 12 patients on Agalsidase alfa (Fisher’s exact t-test p = 0.003), but there was no statistically significant difference in reported infusion reactions between male and female patients receiving Agalsidase beta (13 of 23 Vs 1 of 5, Fisher’s exact t-test p = 0.33).
Disease Burden and Quality of Life
Data were available to allow DS3 and MSSI scoring at all time points in 26 Agalsidase beta patients (23 males, 3 females) and 7 Agalsidase alfa patients (6 males, 1 female), all of whom had received full-dose ERT for at least 2 years prior to any dose reduction. Table 6 reports the baseline characteristics of these patients; 79% of patients on Agalsidase beta who had antibody testing performed were seropositive. As the 6-month interval data was provided to the Registry asynchronously for each patient, it was not possible to grade disease severity at the same time point for each patient in relation to dose reduction. Follow-up data was graded at a median of 7.5 months (range 3–12) post-dose reduction for Agalsidase beta patients and at a median of 9 months (range 8–12) follow-up for Agalsidase alfa patients.
Table 6.
Baseline characteristics of patients receiving Agalsidase alfa or beta included in the assessment of disease burden and quality of life
| Agalsidase alfa | Agalsidase beta | Mann Whitney test (p value) | |
|---|---|---|---|
| Males | n = 6 | n = 23 | |
| Age at ERT commencement (yrs) | 30 (14–54) | 38 (21–69) | 0.12 |
| ERT duration (months) | 114 (22–118) | 87 (20–138) | 0.03 |
| LVH (mm) | 12.5 (9–20) | 14 (7.1–27) | 0.64 |
| Proteinuria (g/24 h) | 0.1 (0.06–0.63) | 0.23 (0–3.1) | 0.51a |
| eGFR (mL/min/1.73 m2) | 84 (49–137) | 69 (40–125) | 0.68a |
| Number with ESRF requiring RRT (%) | 1 | 6 | 1.00b |
| Number with antibody testing performed | 1 | 14 | |
| Number who were antibody positive (%) | 0 | 11 | |
| Peak antibody titre | – | 1:600 (0-1:3200) | |
| Females | n = 1 | n = 3 | |
| Age at ERT commencement (yrs) | 54 | 49 (43–62) | |
| ERT duration (months) | 114 | 36 (16–74) | |
| LVH (mm) | 11 | 16 (11–19) | |
| Proteinuria (g/24 h) | 1.2 | 0.27 (0.11–0.69) | |
| eGFR (mL/min/1.73 m2) | 63 | 76 (73–85) |
eGFR estimated glomerular filtration rate, ESRF end-stage renal failure, LVH left ventricular hypertrophy, RRT renal replacement therapy
Note: Results are presented as median with range in parentheses. No female patient had ESRF requiring RRT or assessment of their antibody status performed.
aPatients with ESRF were excluded from proteinuria and eGFR comparisons
bAssessed by Fisher’s exact t-test
Disease Burden
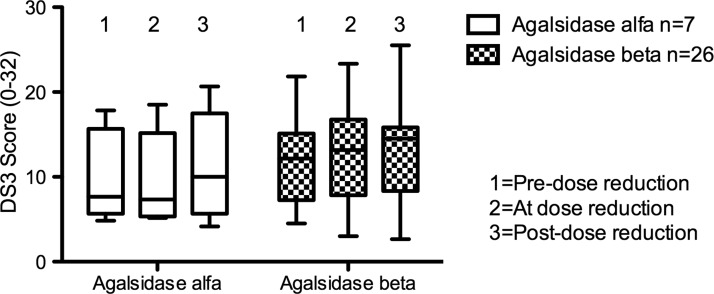
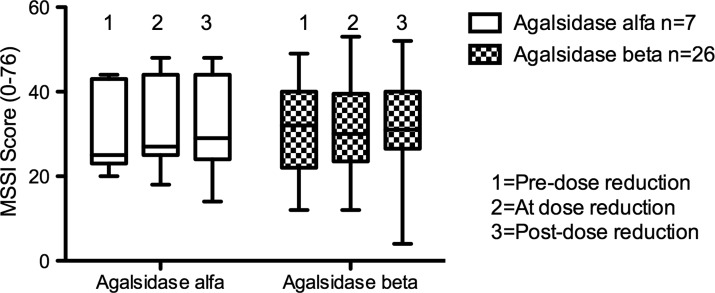
Two years prior to dose reduction, median MSSI scores between ERT groups were similar: 26 (range 12–49) in Agalsidase beta patients and 25 (range 20–44) in Agalsidase alfa patients (Mann Whitney p = 0.89). This represents a “moderate” burden of disease in both groups of patients. Similarly, median DS3 scores of Agalsidase beta patients 2 years before dose reduction (12, range 5–22) were not different from those of Agalsidase alfa patients (8, range 5–18; Mann Whitney p = 0.42). There was no significant change in DS3 or MSSI scores (Figs. 4 and 5), or in any of the individual domain scores on either tool, between patients receiving either ERT over the period of the study. The “peripheral nervous system” domain of the DS3 includes a score for GI symptoms. The median score for GI symptoms in those receiving Agalsidase beta after dose reduction was 2 (range 0–5), compared to a median score of 1 (range 0–4) for Agalsidase alfa patients; however, this failed to reach statistical significance (ANOVA p = 0.55). There was no difference in GI symptom score between males receiving either form of ERT or between males and females treated with Agalsidase beta.
Fig. 4.
Disease Severity Scoring System (DS3) total scores over time for patients receiving enzyme replacement therapy. ANOVA p = 0.8
Fig. 5.
Mainz Severity Score Index (MSSI) total scores over time for patients receiving enzyme replacement therapy. ANOVA p = 0.92
Quality of Life
Average and worst pain BPI scores 24 months before dose reduction were 1 (range 0–7) and 2 (range 0–8), respectively, for Agalsidase beta patients, and 1 (range 0–4) and 2 (range 0–8), respectively, for Agalsidase alfa patients. Over the study period, no significant change between the ERT groups was found in average or worst pain scores (ANOVA p = 0.27 and p = 0.05, respectively). There was no significant change in worst pain scores for male and female patients receiving Agalsidase beta (ANOVA p = 0.69 and p = 0.65 respectively), or in the six male Agalsidase alfa patients (ANOVA p = 0.46). Using the MCS and PCS results of the SF36, neither score changed significantly over the study period for patients receiving Agalsidase beta and alfa (MCS: ANOVA p = 0.3; PCS: ANOVA p = 0.22).
Plasma GL3
Levels of GL3 were measured before commencement of ERT in 10 male and 2 female patients receiving Agalsidase beta. No GL3 levels prior to ERT commencement for patients receiving Agalsidase alfa were recorded in The Fabry Registry. The pre-ERT median GL3 level for males on Agalsidase beta was 6.37 μg/mL (range 2.7–10.3); in the case of the 2 female patients, pre-ERT levels were 3 and 3.5 μg/mL. There was no correlation between pre-ERT baseline GL3 levels and change in energy level after dose reduction in the 10 males on Agalsidase beta therapy (Spearman correlation r = 0.2). The change in energy level reported by males on Agalsidase beta did not differ between those with a GL3 measurement prior to ERT commencement and those without a baseline GL3 level (Mann Whitney p = 0.97).
Discussion
Our male FD patients reported reduced energy levels 1 year after reduction in Agalsidase beta dose. The five female patients treated with Agalsidase beta did not report decreased energy levels after dose reduction, but female FD patients might be expected to be less severely affected by ERT dose reduction, given their higher level of endogenous α-galactosidase. It is certainly possible that the psychological effects of dose reduction impact on subjective symptoms, although we could not detect changes in mental health scores within the SF36. “Energy level” is not captured in either of the disease severity scores used in this study, only in our questionnaire. “Self report of wellbeing” in the DS3 did not change over the study period, suggesting that short-term dose reduction does not affect patient well-being, or that the surrogate used to measure well-being (average of the SF36 MCS and PCS results) inadequately assesses this parameter. Neither MCS nor PCS scores changed in either ERT group over the study period. Our patients reported no significant change in “average pain” or higher “worst pain scores” after Agalsidase beta dose was reduced. Ongoing clinical evaluation will be important to verify these effects, as there is considerable variability in pain patterns between patients and over time for a single FD patient, whether or not on ERT.
The unusual supply problem of Agalsidase beta for FD patients on long-term ERT has presented a unique opportunity to assess clinical efficacy at reduced dose. While the glycosylation pattern of the two ERT products varies, their amino acid sequences are identical. If the products are equipotent, it is interesting that Australian patients receiving Agalsidase beta at 0.5 mg/kg/2 weeks report worse energy levels over the study period compared to patients receiving a stable dose of Agalsidase alfa at 0.2 mg/kg/2 weeks. Various factors determine the product choice of individual Australian physicians, including previous patient participation in clinical trials, unit workload, disease phenotype in relation to published data, and perceptions by patients, families, or physicians that the chosen product, at its approved dose, is superior. There are limitations and biases within this study. Agalsidase beta patients were not blinded to their ERT dose, which may induce anxiety and influence symptom perception. The retrospective nature of the questionnaire has relied on patient’s recall of energy levels, which may not be accurate. The SF36, DS3, and MSSI may not be sensitive tools to allow accurate longitudinal assessment of energy levels. Other variables affecting perceived energy levels, such as concurrent illness or depression (possibly more common in male FD patients than females) (Cole et al. 2007), have not been evaluated in this study.
To assess the impact of a reduction in Agalsidase beta dose on clinical disease state, we have relied on clinical tools to assess disease load. The size of the patient cohort in this study and the slow, progressive nature of disease manifestations in FD means detecting early progression (or regression) of disease in response to changes in therapy is challenging and unlikely to be identified on studies such as this one. Ideally, a biomarker correlating with clinical disease state or overall disease burden would be helpful for the assessment of therapies and the prediction of prognosis. Elevated plasma and urinary GL3 typically reduce to normal levels after initiation of ERT (Young et al. 2005) and urinary GL3 levels increased when FD patients (already treated with 1 year of standard dose Agalsidase beta therapy) were given only 0.3 mg/kg/2 weeks of Agalsidase beta (Lubanda et al. 2009). However, urinary and plasma GL3 have not been shown to be optimal biomarkers; indeed, there is no validated biomarker to monitor response to therapy in this disease at present (Aerts et al. 2011). Plasma globotriaosylsphingosine (lysoGb3) is elevated in male and female FD patients (Aerts et al. 2008; Togawa et al. 2010). van Breemen et al. have demonstrated that plasma lysoGb3 levels decrease after 3 months of ERT (and these lower levels are sustained after 12 months of ERT) and that a dose-response exists, as patients given Agalsidase beta 1 mg/kg/2 weeks had a greater reduction in lysoGb3 levels than those treated with Agalsidase alfa or beta at 0.2 mg/kg/2 weeks (van Breemen et al. 2011). Plasma lysoGb3 has also been identified as an independent risk factor for white matter lesions in males and LVH in females, with lifetime exposure to plasma lysoGb3 correlating with MSSI score, and may therefore prove to be a clinically useful biomarker in FD (Rombach et al. 2010).
Patients treated with ERT frequently develop IgG antibodies to the recombinant α-galactosidase protein, especially male FD patients who have little or no endogenous production of this enzyme. The delivered dose of enzyme and the type of recombinant enzyme may be important factors in the generation of antibodies, with a number of studies showing a higher prevalence of seropositivity among patients treated with Agalsidase beta (in the order of 80–90%) compared with Agalsidase alfa (~40–60%) (Eng et al. 2001; Linthorst et al. 2004; Schiffmann et al. 2006; Vedder et al. 2008; van Breemen et al. 2011). The prevalence of seropositivity for anti-α-galactosidase antibodies within our Australian cohort is consistent with that described. When Agalsidase alfa and beta are administered at 0.2 mg/kg/2 weeks, some authors still found a higher prevalence of anti-α-galactosidase IgG antibodies in patients treated with Agalsidase beta (van Breemen et al. 2011), citing differences in the cell lines used in the production of these recombinant enzymes as a possible explanation (Pastores and Thadhani 2001; Beck 2002). However, others have not demonstrated this finding (Vedder et al. 2008). The presence of antibodies has been shown to bind circulating enzyme, inhibiting its enzymatic activity in vitro and in vivo, with the neutralizing capacity of antibodies to one recombinant form of α-galactosidase being cross-reactive toward the alternative form of ERT (Linthorst et al. 2004; Ohashi et al. 2008). Therefore, concern exists that these antibodies may interfere with the clinical benefit that is hoped to be gained through ERT; the antibody level and the dose of ERT administered to seropositive patients may be important factors to consider in treatment. Increased urinary GL3 levels have been demonstrated in seropositive patients compared to antibody negative patients, although some seropositive patients do demonstrate normalization of urinary GL3 levels after 12 months of ERT (Linthorst et al. 2004; Ohashi et al. 2007). Fabry mice had reduced α-galactosidase activity within their major organs (including heart and kidneys) after infusion of Agalsidase beta incubated with serum from seropositive patients, compared to those treated with an equal dose of enzyme pre-incubated with serum from seronegative patients (Ohashi et al. 2008). However, a tenfold increase in enzyme dose resulted in increased enzyme activity in all tissues and enzyme activity was similar between seropositive and seronegative patients (Ohashi et al. 2008). Anti-α-galactosidase IgG titer was not found to correlate with a rate of change in eGFR, onset of clinical events or plasma GL3 elevations after 5 years of Agalsidase beta therapy in 134 male and female FD patients, although patients with a high antibody titer were found to have significantly increased GL3 deposition within dermal capillary endothelial cells during this study, suggesting that plasma GL3 clearance is impaired in the presence of a high antibody titer (Benichou et al., Mol Genet Metab 2009). The shortage of Agalsidase beta has raised practical questions regarding dose of ERT in patients who have been recipients of long-term therapy, the majority of whom have antibodies to the enzyme. What is the clinical implication of a reduction in ERT dose after a patient has received standard dose ERT for many years? Is the background burden of disease an important factor in determining suitability for dose reduction? Should males receiving Agalsidase beta with anti-α-galactosidase antibodies be excluded from a reduction in ERT dose? When supply is restored, should they be given a higher dose of ERT than the “standard” therapeutic dose? Will there be an increase in antibody titer when full dose Agalsidase beta therapy is reinstituted? Are they at greater risk of disease deterioration with prolonged dose reduction? These questions draw attention to the concept of individualized ERT for FD patients to ensure they obtain maximal clinical benefit from this treatment, and warrants ongoing research.
Consistent supply of ERT was a concern for Agalsidase beta patients and a reason for consideration of changing product. However, most patients on Agalsidase beta preferred to continue at the reduced dose, 12 months after dose reduction. A minority feared infusion reactions if their ERT product was changed. The incidence of infusion reactions with Agalsidase beta recalled by patients in this study was consistent with that reported in other work (Wilcox et al. 2004; Keating and Simpson 2007) and lower than in other reports of Agalsidase beta therapy (Ries et al. 2006; Pastores et al. 2007).
Notwithstanding its limitations, our study found that male Australian FD patients report lower energy levels on reduced dose Agalsidase beta therapy. Clinical disease burden and QOL, as measured by the SF36 Health Survey, did not change during the study period. Close monitoring of clinical parameters will be required to identify if QOL deteriorates further and if disease progression has been affected. Currently, patients on either product are administered a single dose irrespective of the phase or severity of their condition or duration of ERT. The analysis of FD patients on lower doses due to supply problems, together with data from long-term trials directly comparing Agalsidase alfa and beta therapy, may help define optimal ERT doses in patients receiving ERT over many years.
Acknowledgements
We thank our Fabry disease coordinators and nurse specialists at all Australian treatment centers for their commitment to retrieving data, in maintaining The Fabry Registry and in assisting with this study.
Synopsis
Australian male Fabry disease patients receiving long-term enzyme replacement therapy (ERT) with Agalsidase beta report reduced energy levels (without a change in disease severity or other quality of life measures) following a 50% reduction in Agalsidase beta dose, as a consequence of the global shortage of this product.
Declarations
All authors have received travel support from Genzyme and/or Shire HGT. Dr Nicholls has received research support from Genzyme and Shire HGT. Dr Fletcher and Prof Sillence have received speaker honoraria from Genzyme. Dr Denaro and Prof Sillence have received coordinator support from Genzyme. Genzyme supports Registry data entry for all centers.
Disclaimer
This paper has been produced with the cooperation of the Australian Government Department of Health and Ageing, which funds and administers the Life Saving Drugs Program (LSDP). The analysis which forms the basis of this paper was conducted by the authors without the use or disclosure of personal information of any patient of the LSDP. Any views expressed or conclusions drawn in the paper are entirely those of the authors and do not reflect any views of the Department or the Australian Government.
Footnotes
Competing interests: None declared.
References
- Aerts JM, Groener JE, Kuiper S, Donker-Koopman WE, Strijland A, Ottenhoff R, van Roomen C, Mirzaian M, Wijburg FA, Linthorst GE, Vedder AC, Rombach SM, Cox-Brinkman J, Somerharju P, Boot RG, Hollak CE, Brady RO, Poorthuis BJ. Elevated globotriaosylsphingosine is a hallmark of Fabry disease. Proc Natl Acad Sci U S A. 2008;105(8):2812–2817. doi: 10.1073/pnas.0712309105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aerts JM, Kallemeijn WW, Wegdam W, Joao Ferraz M, van Breemen MJ, Dekker N, Kramer G, Poorthuis BJ, Groener JE, Cox-Brinkman J, Rombach SM, Hollak CE, Linthorst GE, Witte MD, Gold H, van der Marel GA, Overkleeft HS, Boot RG. Biomarkers in the diagnosis of lysosomal storage disorders: proteins, lipids, and inhibodies. J Inherit Metab Dis. 2011;34:605–619. doi: 10.1007/s10545-011-9308-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banikazemi M, Bultas J, Waldek S, Wilcox WR, Whitley CB, McDonald M, Finkel R, Packman S, Bichet DG, Warnock DG, Desnick RJ. Agalsidase-beta therapy for advanced Fabry disease: a randomized trial. Ann Intern Med. 2007;146(2):77–86. doi: 10.7326/0003-4819-146-2-200701160-00148. [DOI] [PubMed] [Google Scholar]
- Beck M. Agalsidase alfa–a preparation for enzyme replacement therapy in Anderson-Fabry disease. Expert Opin Investig Drugs. 2002;11(6):851–858. doi: 10.1517/13543784.11.6.851. [DOI] [PubMed] [Google Scholar]
- Beck M. Agalsidase alfa–a preparation for enzyme replacement therapy in Anderson-Fabry disease. Expert Opin Investig Drugs. 2002;11(6):851–858. doi: 10.1517/13543784.11.6.851. [DOI] [PubMed] [Google Scholar]
- Cleeland CS. Pain assessment: the advantages of using pain scales in lysosomal storage diseases. Acta Paediatr Suppl. 2002;91(439):43–47. doi: 10.1111/j.1651-2227.2002.tb03109.x. [DOI] [PubMed] [Google Scholar]
- Cole AL, Lee PJ, Hughes DA, Deegan PB, Waldek S, Lachmann RH. Depression in adults with Fabry disease: a common and under-diagnosed problem. J Inherit Metab Dis. 2007;30(6):943–951. doi: 10.1007/s10545-007-0708-6. [DOI] [PubMed] [Google Scholar]
- El Dib RP, Pastores GM. Enzyme replacement therapy for Anderson-Fabry disease. Cochrane Database Syst Rev. 2010;5:CD006663. doi: 10.1002/14651858.CD006663.pub2. [DOI] [PubMed] [Google Scholar]
- Eng CM, Guffon N, Wilcox WR, Germain DP, Lee P, Waldek S, Caplan L, Linthorst GE, Desnick RJ. Safety and efficacy of recombinant human alpha-galactosidase A–replacement therapy in Fabry's disease. N Engl J Med. 2001;345(1):9–16. doi: 10.1056/NEJM200107053450102. [DOI] [PubMed] [Google Scholar]
- Garin O, Ferrer M, Pont A, Rue M, Kotzeva A, Wiklund I, Van Ganse E, Alonso J. Disease-specific health-related quality of life questionnaires for heart failure: a systematic review with meta-analyses. Qual Life Res. 2009;18(1):71–85. doi: 10.1007/s11136-008-9416-4. [DOI] [PubMed] [Google Scholar]
- Giannini EH, Mehta AB, Hilz MJ, Beck M, Bichet DG, Brady RO, West M, Germain DP, Wanner C, Waldek S, Clarke JT, Mengel E, Strotmann JM, Warnock DG, Linhart A. A validated disease severity scoring system for Fabry disease. Mol Genet Metab. 2010;99(3):283–290. doi: 10.1016/j.ymgme.2009.10.178. [DOI] [PubMed] [Google Scholar]
- Gold KF, Pastores GM, Botteman MF, Yeh JM, Sweeney S, Aliski W, Pashos CL. Quality of life of patients with Fabry disease. Qual Life Res. 2002;11(4):317–327. doi: 10.1023/A:1015511908710. [DOI] [PubMed] [Google Scholar]
- Hoffmann B, Garcia de Lorenzo A, Mehta A, Beck M, Widmer U, Ricci R. Effects of enzyme replacement therapy on pain and health related quality of life in patients with Fabry disease: data from FOS (Fabry Outcome Survey) J Med Genet. 2005;42(3):247–252. doi: 10.1136/jmg.2004.025791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating GM, Simpson D. Agalsidase Beta: a review of its use in the management of Fabry disease. Drugs. 2007;67(3):435–455. doi: 10.2165/00003495-200767030-00007. [DOI] [PubMed] [Google Scholar]
- Lee K, Jin X, Zhang K, Copertino L, Andrews L, Baker-Malcolm J, Geagan L, Qiu H, Seiger K, Barngrover D, McPherson JM, Edmunds T. A biochemical and pharmacological comparison of enzyme replacement therapies for the glycolipid storage disorder Fabry disease. Glycobiology. 2003;13(4):305–313. doi: 10.1093/glycob/cwg034. [DOI] [PubMed] [Google Scholar]
- Linthorst GE, Hollak CE, Donker-Koopman WE, Strijland A, Aerts JM. Enzyme therapy for Fabry disease: neutralizing antibodies toward agalsidase alpha and beta. Kidney Int. 2004;66(4):1589–1595. doi: 10.1111/j.1523-1755.2004.00924.x. [DOI] [PubMed] [Google Scholar]
- LSDP (2010) Guidelines for the treatment of Fabry Disease through the Life Saving Drugs Program, August 2010
- Lubanda JC, Anijalg E, Bzduch V, Thurberg BL, Benichou B, Tylki-Szymanska A. Evaluation of low dose, after a standard therapeutic dose, of agalsidase beta during enzyme replacement therapy in patients with Fabry disease. Genet Med. 2009;11(4):256–264. doi: 10.1097/GIM.0b013e3181981d82. [DOI] [PubMed] [Google Scholar]
- Mehta A, Beck M, Elliott P, Giugliani R, Linhart A, Sunder-Plassmann G, Schiffmann R, Barbey F, Ries M, Clarke JT. Enzyme replacement therapy with agalsidase alfa in patients with Fabry's disease: an analysis of registry data. Lancet. 2009;374(9706):1986–1996. doi: 10.1016/S0140-6736(09)61493-8. [DOI] [PubMed] [Google Scholar]
- Miners AH, Holmes A, Sherr L, Jenkinson C, MacDermot KD. Assessment of health-related quality-of-life in males with Anderson Fabry Disease before therapeutic intervention. Qual Life Res. 2002;11(2):127–133. doi: 10.1023/A:1015009210639. [DOI] [PubMed] [Google Scholar]
- Ohashi T, Sakuma M, Kitagawa T, Suzuki K, Ishige N, Eto Y. Influence of antibody formation on reduction of globotriaosylceramide (GL-3) in urine from Fabry patients during agalsidase beta therapy. Mol Genet Metab. 2007;92(3):271–273. doi: 10.1016/j.ymgme.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Ohashi T, Iizuka S, Ida H, Eto Y. Reduced alpha-Gal A enzyme activity in Fabry fibroblast cells and Fabry mice tissues induced by serum from antibody positive patients with Fabry disease. Mol Genet Metab. 2008;94(3):313–318. doi: 10.1016/j.ymgme.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Pastores GM, Thadhani R. Enzyme-replacement therapy for Anderson-Fabry disease. Lancet. 2001;358(9282):601–603. doi: 10.1016/S0140-6736(01)05816-0. [DOI] [PubMed] [Google Scholar]
- Pastores GM, Boyd E, Crandall K, Whelan A, Piersall L, Barnett N. Safety and pharmacokinetics of agalsidase alfa in patients with Fabry disease and end-stage renal disease. Nephrol Dial Transplant. 2007;22(7):1920–1925. doi: 10.1093/ndt/gfm096. [DOI] [PubMed] [Google Scholar]
- Ries M, Clarke JT, Whybra C, Timmons M, Robinson C, Schlaggar BL, Pastores G, Lien YH, Kampmann C, Brady RO, Beck M, Schiffmann R. Enzyme-replacement therapy with agalsidase alfa in children with Fabry disease. Pediatrics. 2006;118(3):924–932. doi: 10.1542/peds.2005-2895. [DOI] [PubMed] [Google Scholar]
- Rombach SM, Dekker N, Bouwman MG, Linthorst GE, Zwinderman AH, Wijburg FA, Kuiper S, Vd Bergh Weerman MA, Groener JE, Poorthuis BJ, Hollak CE, Aerts JM. Plasma globotriaosylsphingosine: diagnostic value and relation to clinical manifestations of Fabry disease. Biochim Biophys Acta. 2010;1802(9):741–748. doi: 10.1016/j.bbadis.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Sakuraba H, Murata-Ohsawa M, Kawashima I, Tajima Y, Kotani M, Ohshima T, Chiba Y, Takashiba M, Jigami Y, Fukushige T, Kanzaki T, Itoh K. Comparison of the effects of agalsidase alfa and agalsidase beta on cultured human Fabry fibroblasts and Fabry mice. J Hum Genet. 2006;51(3):180–188. doi: 10.1007/s10038-005-0342-9. [DOI] [PubMed] [Google Scholar]
- Schaefer RM, Tylki-Szymanska A, Hilz MJ. Enzyme replacement therapy for Fabry disease: a systematic review of available evidence. Drugs. 2009;69(16):2179–2205. doi: 10.2165/11318300-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Schiffmann R, Kopp JB, Austin HA, III, Sabnis S, Moore DF, Weibel T, Balow JE, Brady RO. Enzyme replacement therapy in Fabry disease: a randomized controlled trial. JAMA. 2001;285(21):2743–2749. doi: 10.1001/jama.285.21.2743. [DOI] [PubMed] [Google Scholar]
- Schiffmann R, Ries M, Timmons M, Flaherty JT, Brady RO. Long-term therapy with agalsidase alfa for Fabry disease: safety and effects on renal function in a home infusion setting. Nephrol Dial Transplant. 2006;21(2):345–354. doi: 10.1093/ndt/gfi152. [DOI] [PubMed] [Google Scholar]
- Schiffmann R, Askari H, Timmons M, Robinson C, Benko W, Brady RO, Ries M. Weekly enzyme replacement therapy may slow decline of renal function in patients with Fabry disease who are on long-term biweekly dosing. J Am Soc Nephrol. 2007;18(5):1576–1583. doi: 10.1681/ASN.2006111263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togawa T, Kodama T, Suzuki T, Sugawara K, Tsukimura T, Ohashi T, Ishige N, Suzuki K, Kitagawa T, Sakuraba H. Plasma globotriaosylsphingosine as a biomarker of Fabry disease. Mol Genet Metab. 2010;100(3):257–261. doi: 10.1016/j.ymgme.2010.03.020. [DOI] [PubMed] [Google Scholar]
- Torra R, Algaba F, Ars E, Santin S, Fernandez-Llama P, Ballarin J. Preservation of renal function in a patient with Fabry nephropathy on enzyme replacement therapy. Clin Nephrol. 2008;69(6):445–449. doi: 10.5414/cnp69445. [DOI] [PubMed] [Google Scholar]
- van Breemen MJ, Rombach SM, Dekker N, Poorthuis BJ, Linthorst GE, Zwinderman AH, Breunig F, Wanner C, Aerts JM, Hollak CE. Reduction of elevated plasma globotriaosylsphingosine in patients with classic Fabry disease following enzyme replacement therapy. Biochim Biophys Acta. 2011;1812(1):70–76. doi: 10.1016/j.bbadis.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Vedder AC, Linthorst GE, Houge G, Groener JE, Ormel EE, Bouma BJ, Aerts JM, Hirth A, Hollak CE. Treatment of Fabry disease: outcome of a comparative trial with agalsidase alfa or beta at a dose of 0.2 mg/kg. PLoS One. 2007;2(7):e598. doi: 10.1371/journal.pone.0000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedder AC, Breunig F, Donker-Koopman WE, Mills K, Young E, Winchester B, Ten Berge IJ, Groener JE, Aerts JM, Wanner C, Hollak CE. Treatment of Fabry disease with different dosing regimens of agalsidase: effects on antibody formation and GL-3. Mol Genet Metab. 2008;94(3):319–325. doi: 10.1016/j.ymgme.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Watt T, Burlina AP, Cazzorla C, Schonfeld D, Banikazemi M, Hopkin RJ, Martins AM, Sims K, Beitner-Johnson D, O'Brien F, Feldt-Rasmussen U. Agalsidase beta treatment is associated with improved quality of life in patients with Fabry disease: findings from the Fabry Registry. Genet Med. 2010;12(11):703–712. doi: 10.1097/GIM.0b013e3181f13a4a. [DOI] [PubMed] [Google Scholar]
- Whybra C, Kampmann C, Krummenauer F, Ries M, Mengel E, Miebach E, Baehner F, Kim K, Bajbouj M, Schwarting A, Gal A, Beck M. The Mainz Severity Score Index: a new instrument for quantifying the Anderson-Fabry disease phenotype, and the response of patients to enzyme replacement therapy. Clin Genet. 2004;65(4):299–307. doi: 10.1111/j.1399-0004.2004.00219.x. [DOI] [PubMed] [Google Scholar]
- Whybra C, Miebach E, Mengel E, Gal A, Baron K, Beck M, Kampmann C. A 4-year study of the efficacy and tolerability of enzyme replacement therapy with agalsidase alfa in 36 women with Fabry disease. Genet Med. 2009;11(6):441–449. doi: 10.1097/GIM.0b013e3181a23bec. [DOI] [PubMed] [Google Scholar]
- Wilcox WR, Banikazemi M, Guffon N, Waldek S, Lee P, Linthorst GE, Desnick RJ, Germain DP. Long-term safety and efficacy of enzyme replacement therapy for Fabry disease. Am J Hum Genet. 2004;75(1):65–74. doi: 10.1086/422366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young E, Mills K, Morris P, Vellodi A, Lee P, Waldek S, Winchester B. Is globotriaosylceramide a useful biomarker in Fabry disease? Acta Paediatr Suppl. 2005;94(447):51–54. doi: 10.1080/08035320510028111. [DOI] [PubMed] [Google Scholar]