Abstract
Introduction: Niemann–Pick disease type C (NPC) is a lysosomal storage disorder that leads to progressive neurodegeneration. The glucosylceramide synthase blocker miglustat is being used to treat NPC, but monitoring of disease progression and treatment response is difficult. NPC patients have elevated cerebrospinal fluid (CSF) levels of total-tau (T-tau) indicating axonal degeneration, and increased CSF amyloid β (Aβ) indicating abnormal brain amyloid metabolism, but it is unknown if start of miglustat treatment affects these biomarker levels.
Methods: Biomarkers were measured in serial CSF samples from NPC patients who started miglustat between samplings (N=5), were untreated at both samplings (N=5) or received treatment during the whole study (N=6) (median time between samplings 309 days [range 175–644]). CSF was analyzed for Aβ38, Aβ40, Aβ42, α-cleaved soluble APP, β-cleaved soluble APP, T-tau and phospho-tau.
Results: T-tau levels decreased in patients who started miglustat treatment (median 955 [range 338–1,271]ng/L at baseline vs. 382 [187–736]ng/L at follow-up, p=0.043). Untreated patients and continuously treated patients had stable levels (p>0.05). No changes were seen in the other biomarkers.
Conclusion: Reduced CSF T-tau suggests that miglustat treatment might affect axonal degeneration in NPC. However, the results must be interpreted with caution and verified in future studies, since this pilot study was small, treatment was not randomized, and patients starting treatment had higher baseline CSF T-tau than untreated patients.
Introduction
Niemann–Pick type C disease (NPC) is a rare autosomal recessive lysosomal storage disorder with accumulation of cholesterol and glycosphingolipids in late endosomes and lysosomes (Pentchev et al. 1987; Zervas et al. 2001a, b). The incidence is about 1/150,000 live births (Vanier and Millat 2003). The clinical spectrum is broad, with presenting symptoms ranging from lethal fetal ascites to psychiatric disease in adults, but progressive neurodegeneration is the major problem (Imrie et al. 2007; Sevin et al. 2007; Yerushalmi et al. 2002). Most patients have mutations in the NPC1 gene that encodes a membrane protein involved in intracellular cholesterol transport (Ory 2004). Other patients have mutations in the NPC2 gene that encodes a soluble lysosomal cholesterol-binding protein (Naureckiene et al. 2000). These mutations point to a defect in cholesterol transport in NPC, but the lipid accumulation is complex and the primary offending metabolite in the brain is disputed (Lloyd-Evans and Platt 2010).
Substrate reduction therapy is available with the glucosylceramide synthase inhibitor miglustat (N-butyldeoxynojirimycin, Zavesca®, Actelion Inc., Switzerland). Miglustat treatment seems to stabilize the neurological disease in a majority of NPC patients (Patterson et al. 2007, 2010; Pineda et al. 2009; Wraith et al. 2010). However, disease-monitoring may be difficult, and there is a need for improved biomarkers to identify treatment responders, and monitor progression and treatment effect (Galanaud et al. 2009; Platt and Lachmann 2009).
We recently showed that cerebrospinal fluid (CSF) levels of total-tau (T-tau) are elevated in NPC patients (Mattsson et al. 2011). Tau is a microtubule-stabilizing protein abundant in cortical axons, and increased CSF T-tau is considered a marker of axonal degeneration (Hampel et al. 2009). NPC patients also have increased CSF levels of β amyloid (Aβ) peptides (Mattsson et al. 2011). Aβ is formed through processing of the transmembranous amyloid precursor protein (APP) (Andreasson et al. 2007). Aberrant Aβ metabolism is at the core of pathological events in Alzheimer’s disease (AD), where small Aβ oligomers are believed to exert synaptotoxicity and insoluble Aβ fibrils are deposited in extracellular plaques (Querfurth and LaFerla 2010; Zetterberg et al. 2010). In accordance with our recent in vivo patient data, experimental studies have indicated altered amyloid metabolism in NPC (Jin et al. 2004), which is influenced by cholesterol accumulation (Kosicek et al. 2010; Malnar et al. 2010), and might be related to development of pathology (Kodam et al. 2010). The increased CSF Aβ concentration is a unique finding, which has not been described in any other disease. The exact mechanism behind the increase remains unknown, but it might involve altered activity of Aβ-generating enzymes, impaired vesicular trafficking, lysosomal dysfunction, or combinations of these. Further links between NPC and AD are provided by accumulating evidence of lysosomal dysfunction in AD (Lee et al. 2010; Liu et al. 2010; Lorenzen et al. 2010) and AD patients have increased levels of NPC1 in degenerated brain regions (Kagedal et al. 2010).
In the previous cross-sectional study (Mattsson et al. 2011), NPC patients treated with miglustat had lower CSF T-tau than untreated patients, suggesting that miglustat therapy may reduce axonal degeneration. In addition, treated patients had lower CSF levels of the Aβ peptide Aβ1-42, and the APP-derived peptides sAPP-α and sAPP-β, suggesting that treatment may alter amyloid metabolism. Longitudinal studies are needed to verify treatment effects on these biomarkers in NPC. A subset of NPC patients were followed with serial CSF collections and we could therefore conduct a small observational longitudinal study on biomarkers in relation to treatment (in this article “treatment” refers to miglustat treatment). Patients who started treatment between two consecutive CSF samplings were compared with patients who remained untreated, and with patients who received treatment both at baseline and at follow-up (“continuous treatment”). We tested the specific hypothesis that miglustat treatment would result in altered CSF biomarker levels, with the most direct effects seen after start of treatment.
Methods
Standard Protocol Approvals and Patient Consent
All subjects or guardians of subjects provided written informed consent, and assent when appropriate. The study was approved by the National Institute of Child Health and Development (NICHD) Institutional Review Board.
Subjects
As previously described, NPC1 patients were enrolled in an ongoing longitudinal observational trial at the National Institutes of Health between August 2006 and April 2009. The study was made known to the NPC community and all patients or guardians of patients who expressed interest in participating were invited. The inclusion criterion was NPC diagnosis, established by biochemical testing or mutation analysis. Forty patients were eligible. One was excluded from CSF collection due to warfarin treatment, which was a contraindication to lumbar puncture. One was under 1 year of age at sampling and excluded from analyses due to strong post-natal effects on CSF biomarkers for Aβ metabolism and axonal degeneration during the first months of life (Mattsson et al. 2010). Sixteen patients underwent serial lumbar punctures. Six of these were already on miglustat treatment at the first CSF sampling and remained on treatment during the study (continuous treatment). Remaining patients either began miglustat therapy between two CSF collections or were untreated throughout the study. Investigators in this observational trial neither provided nor prescribed miglustat. However, the NICHD IRB specifically approved monitoring patients who were prescribed miglustat by other physicians. Miglustat use was off-label (usage without indication approved by the United States Food and Drug Administration). Treatment was therefore primarily determined by insurance coverage but not formally randomized. Disease severity was scored as described previously (Yanjanin et al. 2010). This phenotyping index ascertains neurological signs and symptoms in nine major (ambulation, cognition, eye movement, fine motor, hearing, memory, seizures, speech, and swallowing) and eight minor (auditory brainstem response, behavior, gelastic cataplexy, hyperreflexia, incontinence, narcolepsy, psychiatric and respiratory problems) domains. The total possible score ranges from 0 to 61, with a higher score indicating more severe clinical impairment. Demographic data is available in Table 1.
Table 1.
Demographicsa
| Group | Patient | Age at first symptoms | Age at baseline (y) | Duration (y) | Sex | APOE genotype | Severity at baseline | Severity at follow-up | Time between samplings (d) |
|---|---|---|---|---|---|---|---|---|---|
| Untreated at baseline, treated at follow-up | NPC5 | 1 y | 11.4 | 10.4 | M | E4/E4 | 25 | 26 | 196 |
| NPC19 | 2 y | 3.8 | 1.8 | F | E3/E3 | 3 | 7 | 268 | |
| NPC20 | 2 y | 3.8 | 1.8 | F | E2/E3 | 5 | 9 | 268 | |
| NPC17 | 3 w | 6.1 | 6.1 | M | E3/E3 | 7 | 15 | 456 | |
| NPC23 | 6 m | 7.7 | 7.2 | F | E3/E3 | 1 | 3 | 357 | |
| Sum | 2 y (3 w-2 y) |
6.1b (3.8–11.4) |
6.1b (1.8–10.4) |
2 M/3 F | 5b (1–25) |
9 (3–26) |
268 (196–456) |
||
| Treated at baseline and at follow-up | NPC16 | Neonate | 4.7 | 4.7 | M | E3/E3 | 2 | 3 | 366 |
| NPC2 | 6 m | 7.7 | 7.2 | M | E3/E3 | 5 | 5 | 196 | |
| NPC9 | 2 w | 8.3 | 8.3 | M | ND | 8 | 9 | 434 | |
| NPC11 | 3 m | 5.4 | 5.2 | M | E3/E4 | 8 | 9 | 349 | |
| NPC12 | Neonate | 4.7 | 4.7 | F | E3/E3 | 12 | 13 | 175 | |
| NPC3 | 8 m | 13.5 | 12.8 | F | E3/E3 | 33 | 35 | 196 | |
| Sum | 7 w (0–8 m) |
6.6c (4.7–13.5) |
6.2d (4.7–12.8) |
4 M/2 F | 8e (2–33) |
9f (3–35) |
273 (196–434) |
||
| Untreated at baseline and at follow-up | NPC6 | 1.5 y | 16.8 | 15.3 | M | ND | 18 | 25 | 189 |
| NPC15 | 39 y | 51.3 | 12.3 | F | E3/E3 | 24 | 24 | 183 | |
| NPC24 | 5 y | 21.5 | 16.5 | F | E3/E3 | 35 | 40 | 357 | |
| NPC13 | 8 y | 32.1 | 24.1 | M | E3/E3 | 39 | 43 | 644 | |
| NPC4 | Neonate | 5.5 | 5.5 | M | E3/E3 | 12 | 14 | 427 | |
| Sum | 5 y (0–39 y) |
21.5 (5.5–52.3) |
12.3 (5.5–24.1) |
3 M/2 F | 24 (12–39) |
25 (14–43) |
357 (183–644) |
aData for sum are median(range); bp = 0.047 vs. untreated; cp = 0.030 vs. untreated; dp = 0.052 vs. untreated;ep = 0.035 vs. untreated; fp = 0.028 vs. untreated
Variables
The endpoints of the study were differences in longitudinal CSF biomarker changes between groups. The main predictor was start of miglustat treatment. We expected to find the strongest biomarker responses directly after start of treatment, since biomarker levels may change and stabilize after start of treatment, reflecting the effect of treatment on the disease process. This may attenuate further biomarker changes.
CSF Sampling
All CSF samples were collected in the morning by lumbar puncture in the L4/L5 interspace, after an age-appropriate overnight fast. The lumbar puncture was done under anesthesia and concurrent with MRI. CSF was collected in a polystyrene tube, and immediately transported to a local laboratory where it was aliquoted into polypropylene tubes. Samples were frozen on dry ice and stored at −80°C prior to assay. Samples were coded prior to sending to the Clinical Neurochemistry Laboratory in Mölndal, Sweden.
CSF Biomarkers of Amyloid Metabolism and Neuronal Cell Damage
CSF levels of Aβ1–42, the axonal damage marker T-tau and tau phosphorylated at threonine 181 (P-tau) were determined using xMAP technology, as previously described (Olsson et al. 2005). CSF sAPP-α and sAPP-β levels were determined using the MSD® sAPPα/sAPPβ Multiplex Assay as described by the manufacturer (Meso Scale Discovery, Gaithersburg, MD, USA). This assay employs the 6E10 antibody to capture sAPP-α and a neoepitope-specific antibody to capture sAPP-β. Both isoforms are detected by SULFO-TAG™-labeled anti-APP antibody p2–1. CSF Aβx-38, Aβx–40 and Aβx–42 were measured using the MSD® Human/Rodent (4G8) Abeta Triplex Assay as described by the manufacturer. This assay employs C-terminal specific antibodies to specifically capture Aβx–38, Aβx–40 and Aβx–42. All isoforms are detected by SULFO-TAG™-labeled 4G8 detection antibody. Intra-assay coefficients of variation (CVs) were <5% for all analyses, except for Aβ38 (11.7%), sAPP-β (10.9%) and one kit of P-tau (5.13%). Aβ42 measured by MSD correlated to Aβ1–42 measured by Luminex in the total study population (R = 0.93, p < 0.001) and Aβ1–42 and Aβx–42 behaved similar in all statistical analyses. If not stated otherwise, results below are for Aβx–42. All biochemical analyses were performed at the Clinical Neurochemistry Laboratory in Mölndal, Sweden, by experienced and certified laboratory technicians who were blinded to diagnoses and clinical data. Two internal control samples (aliquots of pooled CSF) were run on each plate, and strict acceptance criteria were used for approval of each assay.
Statistics
Statistical calculations were performed using PASW 18.0 (SPSS Inc., Chicago, USA). As the distribution of quantitative measures was significantly skewed, statistical tests involving these variables were conducted using the nonparametric Mann–Whitney U test for pair-wise comparisons between groups. Wilcoxon Signed Ranks test was used for pair-wise comparisons of two related samples of quantitative data. The Spearman correlation coefficient was used for analyses of correlation between variables. Quantitative variables are presented as median (range). To investigate potential confounding factors, we examined correlations between biomarkers and age and disease duration. Subgroup analyses were done on patients without treatment, patients starting treatment, and patients on continuous treatment. The significance level threshold was set to p < 0.05.
Results
Group Characteristics
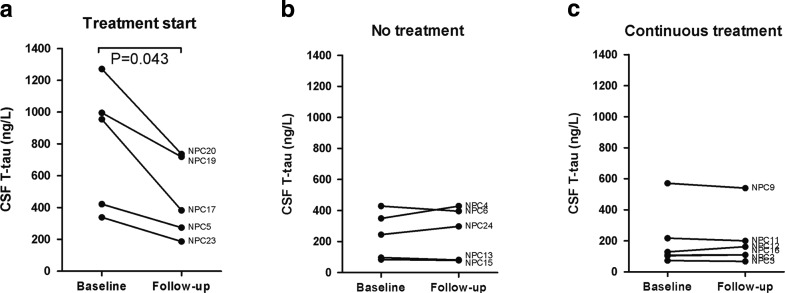
Treatment was not formally randomized in this observational trial, and unfortunately the groups of untreated patients, patients starting treatment and patients on continuous treatment were not optimally matched. The untreated group had latest onset of neurological disease, were older at CSF sampling, and had longer disease duration and higher baseline disease severity score than the other two groups (Table 1). Patients starting treatment had higher baseline CSF T-tau levels than the other patients (Fig. 1).
Fig. 1.
CSF T-tau and miglustat treatment. Follow-up sample was the next collected consecutive sample after basal sample. At baseline, patients starting treatment (a) had higher T-tau than patients without treatment (b) (p = 0.047) and patients with continuous treatment (c) (p = 0.018), but there was no significant difference between patients without treatment and patients on continuous treatment
Longitudinal Change in T-Tau
Baseline data have previously been reported for a larger set of NPC patients, including the patients presented here (Mattsson et al. 2011). In that study, patients on miglustat had lower CSF T-tau than patients without miglustat treatment. Five patients in this longitudinal study started miglustat therapy within 1 week after baseline, and decreased in T-tau levels between samplings (955 [338–1,271] vs. 382 [187–736] ng/L, p = 0.043, Fig. 1a). The decrease in T-Tau appears to be associated with initiation of miglustat therapy, since T-tau levels were stable in the untreated patients (245 [84–429] vs. 298 [79–429] ng/L, p = 0.69, Fig. 1b) and the patients on continuous treatment (119 [73–571] vs. 137 [68–540] ng/L, p = 0.83, Fig. 1c). In these 16 patients, there was no significant difference in T-tau levels between treated and untreated patients at the follow-up sampling (p = N.S). Compared to previously published control data (CSF T-tau median 79 ng/L, range 23–186 ng/L) (Mattsson et al. 2011), all groups in this study had higher CSF T-tau at baseline (p = 0.014 for untreated patients; p = 0.001 for patients starting treatment; p = 0.026 for patients on continuous treatment) and at follow-up (p = 0.042 for untreated patients; p = 0.001 for patients starting treatment; p = 0.029 for patients on continuous treatment). This suggests that miglustat treatment did not fully stop the axonal neurodegeneration in these patients.
P-Tau
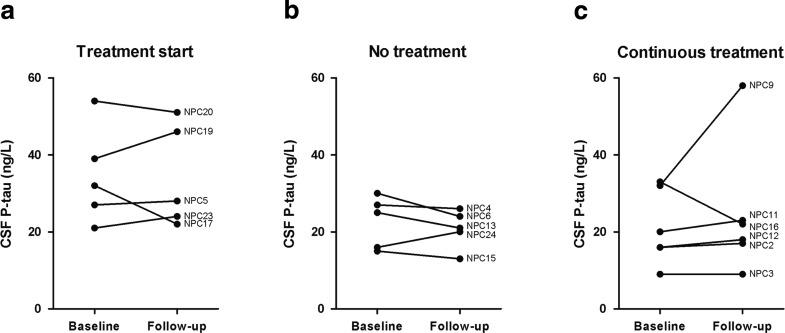
P-tau levels did not change significantly over time in any of the three groups (Fig. 2). This is consistent with the previous study, where there were no differences between patients and controls, or between treated and untreated patients.
Fig. 2.
CSF P-tau and miglustat treatment. Follow-up sample was the next collected consecutive sample after basal sample
Amyloid Markers
All Aβ and sAPP markers correlated between baseline and follow-up measurements, indicating low analytical and biological variability for these measurements (Fig. 3). There were no changes over time in Aβ or sAPP measurements in any of the groups (p > 0.05)
Fig. 3.
Correlation of CSF amyloid markers at baseline and follow-up. The Spearman correlation coefficient was used for analyses of correlations. Follow-up sample was the next collected consecutive sample after basal sample. Patients starting miglustat treatment (circles), patients on continuous treatment (triangles), and untreated patients (boxes). As previously reported, NPC patients treated with miglustat had lower CSF Aβ1-42, sAPP-α and sAPP-β, (Mattsson et al. 2011), but no effects of treatment start could be seen in this study
Discussion
CSF T-tau levels decreased after start of miglustat treatment, while untreated and continuously treated patients had stable levels. This might represent a dynamic decrease in axonal degeneration following start of treatment (Hampel et al. 2009). The finding was in accordance with our hypothesis, which was based on a previous cross-sectional study where patients on miglustat had lower T-tau than untreated patients (Mattsson et al. 2011). Miglustat inhibits glucosylceramide synthase, which is believed to reduce the brain load of GM2 and GM3 gangliosides in NPC. Since miglustat treatment affects cognitive symptoms, interference with the pathological lipid metabolism could reduce axonal degeneration, and thus decrease T-tau levels (Patterson et al. 2007). There are at least three possible explanations as to why T-tau decreased in patients starting treatment but not in continuously treated patients. First, T-tau is believed to correlate to the current rate of axonal loss, but not to the total sum of neurons that have been lost during the course of the disease. Levels may therefore be stable despite clinical progression, such as in AD (Blennow et al. 2007; Zetterberg et al. 2007). If a treatment reduces neurodegeneration, T-tau will be expected to decrease corresponding to the treatment-induced lower rate of axonal loss, but not further. Second, if there would be a continuous decrease in T-tau, extended follow-up times might be needed to detect further changes after an initial drop. Third, T-tau levels in continuously treated patients were closer to levels in controls, which intrinsically makes further decrease less likely [control levels published in (Mattsson et al. 2011)].
This study exemplifies how small pilot studies may give information about biochemical drug effects in vivo in humans. Since miglustat may stabilize neurological symptoms in NPC (Patterson et al. 2010; Wraith et al. 2010), these data are encouraging and support the use of T-tau to detect effects on axonal degeneration in clinical trials for neurodegenerative diseases. The results may be interpreted in relation to prior observations on longitudinal T-tau measurements in other brain diseases. In the AN1792 trial reduced levels of T-tau were seen in AD patients immunized against Aβ and interpreted as a possible reduction of cellular degeneration (Gilman et al. 2005). Patients with stroke initially have increased T-tau levels, which normalize after a few months (Hesse et al. 2001). In amateur boxers, head trauma leads to increased T-tau levels, which normalize after a few months of rest (Zetterberg et al. 2006). In patients treated with electroconvulsive therapy, T-tau levels are stable after up to six treatment sessions, indicating that such treatment does not induce neuronal damage (Zachrisson et al. 2000).
NPC and Amyloid Metabolism
Treatment did not affect amyloid markers, suggesting that miglustat did not significantly alter brain amyloid metabolism during the study. Amyloid metabolism is disturbed in NPC, but it is unknown if this plays a role in neurodegeneration (Jin et al. 2004). Larger studies are needed to explore effects of treatment on NPC amyloid metabolism. Such studies might elucidate the links between amyloid metabolism, lipid homeostasis and vesicular trafficking, which are receiving increasing attention in different neurodegenerative diseases, including AD (Grimm et al. 2007; Hirsch-Reinshagen et al. 2009; Lloyd-Evans and Platt 2010). The longitudinal stability of Aβ biomarkers suggests low analytical and biological variability of these parameters in NPC, opening for Aβ measurements to study biochemical effects from amyloid-targeting drugs in NPC. However, since Aβ levels are related to disease severity (Mattsson et al. 2011), these parameters might change over longer time periods.
Limitations of the Study
This is the first longitudinal study of these biomarkers in NPC patients, generating data on in vivo human properties that are unobtainable from animal or cell studies. The major limitation of the study is its design as an observational trial without formal randomization of treatment, and the resulting poor group matching. Patients starting treatment were younger at disease onset and time of sampling, and had higher T-tau at baseline than the other patients, which introduces the risk that T-tau might decrease more in younger patients independently of treatment. However, this hypothetical effect was contradicted by the stable T-tau levels in three of the five youngest patients, who were untreated or continuously treated (NPC4, NPC12, NPC16, Fig. 1). Another possibility is that the decrease in T-tau was influenced by regression to the mean, but this would not explain the decrease in T-tau after start of treatment in patients with baseline T-tau well in the range of the other groups (NPC5, 35% decrease from baseline; NPC23, 45% decrease from baseline). In patients not starting treatment, the largest relative T-tau decrease was 15% (NPC13) and the largest absolute decrease was from 429 to 396 ng/L (NPC6), which was markedly less than for any of the patients starting treatment (Fig. 1). Finally, the stability of amyloid markers and P-tau increases the likelihood that the change in T-tau represents a genuine effect in the nervous system, rather than a measurement artifact.
Future Prospects of Biomarkers in NPC
The efficiency of miglustat therapy on neurological symptoms varies between patients, and it has been suggested that treatment might be more successful in less-advanced disease stages, and in patients with early-infantile onset (Pineda et al. 2009; Wraith et al. 2009). Since miglustat therapy has side-effects and is economically costly, biomarkers identifying patients most likely to benefit from treatment would be valuable. Such biomarkers for patient stratification could be discovered using post hoc analyses in treatment studies collecting biological samples at baseline and follow-up.
Efficacy biomarkers for NPC treatment would be valuable both in clinical practice and in research. Such biomarkers may be either primary or secondary. Primary treatment biomarkers monitor the main targets of a drug, while secondary biomarkers monitor downstream effects. We propose that T-tau might be a secondary biomarker for axonal degeneration in miglustat treatment of NPC. To verify this, the biomarker should be investigated in relation to other measures of axonal degeneration in larger controlled clinical trials. T-tau should also be examined in relation to clinical outcome. We refrained from such analysis here, due to the small number of participants and the nonrandomized treatment.
The longitudinal response of T-tau has implications for its usability in clinical practice and in research. The decreased levels in patients starting treatment suggest that T-tau may identify short-term changes after start of treatment, but the stable levels in patients on continuous treatment raise concerns about the biomarker’s usability for long-term follow-up. Larger studies are needed to clarify this. T-tau might be useful to identify responses to start of treatment or change of dose, but not disease progression, at least over the follow-up time in this study.
Conclusions
CSF biomarkers of axonal degeneration and amyloid metabolism might be useful in clinical settings, clinical research and for drug development in NPC. Although this small pilot study must be interpreted with caution, and validated in larger studies, the results add support to the use of CSF T-tau as a biomarker for treatment effects on axonal degeneration.
Acknowledgments
We thank Åsa Källén, Monica Christiansson, Sara Hullberg and Dzemila Secic for excellent technical assistance. We are grateful to Mrs. Chris Hempel for her support of this study. We wish to thank the caretakers and patients for their participation.
Synopsis
Cerebrospinal fluid levels of the axonal degeneration marker tau may decrease after start of miglustat treatment in Niemann–Pick type C.
Disclosures
This study was funded with grants from the Swedish Research Council (projects 14002, 2006–6227, 2006–2740 and 2006–3505), the Alzheimer’s Association (NIRG-08-90356), cNEUPRO, the Royal Swedish Academy of Sciences, Sahlgrenska University Hospital, the Söderberg Foundation, the Lundbeck Foundation, the Gothenburg Medical Society, the Swedish Medical Society, Swedish Brain Power, Stiftelsen Gamla Tjänarinnor, Gun och Bertil Stohnes stiftelse, Åhlén-stiftelsen, Alzheimer Foundation, Sweden, The Dementia Association, Sweden, a Bench to Bedside grant from the NIH Office of Rare Diseases, Therapeutics for Rare and Neglected Diseases program, and the intramural research program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Dr Henrik Zetterberg has participated in an advisory board for GlaxoSmithKline.
Nicole M Yanjanin’s position has been supported by the Ara Parseghian Medical Research Foundation and Dana’s Angels Research Trust.
Dr Kaj Blennow has participated in an advisory board for Innogenetics.
Dr Niklas Mattsson has participated in an advisory board for Actelion Inc.
Dr Simona Bianconi reports no disclosures.
Dr Jan-Eric Månsson reports no disclosures.
Rao Fu reports no disclosures.
Dr Forbes D. Porter reports no disclosures.
Author Contributions
NM, KB, HZ and FP designed the study. SB, NY and FP established the clinical protocol, managed patients and collected samples. RF performed genotyping. NM analyzed the data and performed the statistical analysis. All authors participated in the interpretation of the data. NM drafted the manuscript and all other authors revised the manuscript.
Footnotes
Competing interests: None declared.
Contributor Information
Henrik Zetterberg, Email: Henrik.zetterberg@clinchem.gu.se.
Simona Bianconi, Email: biancons@mail.nih.gov.
Nicole M. Yanjanin, Email: nyanjanin@mail.nih.gov
Rao Fu, Email: fur@mail.nih.gov.
Jan-Eric Månsson, Email: jan-eric.mansson@vgregion.se.
Forbes D. Porter, Email: fdporter@mail.nih.gov
Kaj Blennow, Email: kaj.blennow@neuro.gu.se.
References
- Andreasson U, Portelius E, Andersson ME, Blennow K, Zetterberg H. Aspects of beta-amyloid as a biomarker for Alzheimer’s disease. Biomarkers Med. 2007;1:59–78. doi: 10.2217/17520363.1.1.59. [DOI] [PubMed] [Google Scholar]
- Blennow K, Zetterberg H, Minthon L, et al. Longitudinal stability of CSF biomarkers in Alzheimer’s disease. Neurosci Lett. 2007;419:18–22. doi: 10.1016/j.neulet.2007.03.064. [DOI] [PubMed] [Google Scholar]
- Galanaud D, Tourbah A, Lehericy S, et al. 24 month-treatment with miglustat of three patients with Niemann-Pick disease type C: follow up using brain spectroscopy. Mol Genet Metab. 2009;96:55–8. doi: 10.1016/j.ymgme.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Gilman S, Koller M, Black RS, et al. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64:1553–62. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- Grimm MO, Grimm HS, Hartmann T. Amyloid beta as a regulator of lipid homeostasis. Trends Mol Med. 2007;13:337–44. doi: 10.1016/j.molmed.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Hampel H, Blennow K, Shaw LM, Hoessler YC, Zetterberg H, Trojanowski JQ. Total and phosphorylated tau protein as biological markers of Alzheimer’s disease. Exp Gerontol. 2009;45:30–40. doi: 10.1016/j.exger.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse C, Rosengren L, Andreasen N, et al. Transient increase in total tau but not phospho-tau in human cerebrospinal fluid after acute stroke. Neurosci Lett. 2001;297:187–90. doi: 10.1016/S0304-3940(00)01697-9. [DOI] [PubMed] [Google Scholar]
- Hirsch-Reinshagen V, Burgess BL, Wellington CL. Why lipids are important for Alzheimer disease? Mol Cell Biochem. 2009;326:121–9. doi: 10.1007/s11010-008-0012-2. [DOI] [PubMed] [Google Scholar]
- Imrie J, Dasgupta S, Besley GT, et al. The natural history of Niemann-Pick disease type C in the UK. J Inherit Metab Dis. 2007;30:51–9. doi: 10.1007/s10545-006-0384-7. [DOI] [PubMed] [Google Scholar]
- Jin LW, Shie FS, Maezawa I, Vincent I, Bird T. Intracellular accumulation of amyloidogenic fragments of amyloid-beta precursor protein in neurons with Niemann-Pick type C defects is associated with endosomal abnormalities. Am J Pathol. 2004;164:975–85. doi: 10.1016/S0002-9440(10)63185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagedal K, Kim WS, Appelqvist H, et al. Increased expression of the lysosomal cholesterol transporter NPC1 in Alzheimer’s disease. Biochim Biophys Acta. 2010;1801:831–8. doi: 10.1016/j.bbalip.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Kodam A, Maulik M, Peake K, et al. Altered levels and distribution of amyloid precursor protein and its processing enzymes in Niemann-Pick type C1-deficient mouse brains. Glia. 2010;58:1267–81. doi: 10.1002/glia.21001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosicek M, Malnar M, Goate A, Hecimovic S. Cholesterol accumulation in Niemann Pick type C (NPC) model cells causes a shift in APP localization to lipid rafts. Biochem Biophys Res Commun. 2010;393:404–9. doi: 10.1016/j.bbrc.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Yu WH, Kumar A, et al. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell. 2010;141:1146–58. doi: 10.1016/j.cell.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RQ, Zhou QH, Ji SR, et al. Membrane localization of beta-amyloid 1–42 in lysosomes: a possible mechanism for lysosome labilization. J Biol Chem. 2010;285:19986–96. doi: 10.1074/jbc.M109.036798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Evans E, Platt FM. Lipids on trial: the search for the offending metabolite in Niemann-Pick type C disease. Traffic. 2010;11:419–28. doi: 10.1111/j.1600-0854.2010.01032.x. [DOI] [PubMed] [Google Scholar]
- Lorenzen A, Samosh J, Vandewark K, et al. Rapid and direct transport of cell surface APP to the lysosome defines a novel selective pathway. Mol Brain. 2010;3:11. doi: 10.1186/1756-6606-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malnar M, Kosicek M, Mitterreiter S, et al. Niemann-Pick type C cells show cholesterol dependent decrease of APP expression at the cell surface and its increased processing through the beta-secretase pathway. Biochim Biophys Acta. 2010;1802:682–91. doi: 10.1016/j.bbadis.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson N, Savman K, Osterlundh G, Blennow K, Zetterberg H. Converging molecular pathways in human neural development and degeneration. Neurosci Res. 2010;66:330–2. doi: 10.1016/j.neures.2009.11.012. [DOI] [PubMed] [Google Scholar]
- Mattsson N, Zetterberg H, Bianconi S, et al. Gamma-secretase-dependent amyloid-beta is increased in Niemann-Pick type C: a cross-sectional study. Neurology. 2011;76:366–72. doi: 10.1212/WNL.0b013e318208f4ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naureckiene S, Sleat DE, Lackland H, et al. Identification of HE1 as the second gene of Niemann-Pick C disease. Science. 2000;290:2298–301. doi: 10.1126/science.290.5500.2298. [DOI] [PubMed] [Google Scholar]
- Olsson A, Vanderstichele H, Andreasen N, et al. Simultaneous measurement of beta-amyloid(1–42), total tau, and phosphorylated tau (Thr181) in cerebrospinal fluid by the xMAP technology. Clin Chem. 2005;51:336–45. doi: 10.1373/clinchem.2004.039347. [DOI] [PubMed] [Google Scholar]
- Ory DS. The niemann-pick disease genes; regulators of cellular cholesterol homeostasis. Trends Cardiovasc Med. 2004;14:66–72. doi: 10.1016/j.tcm.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Patterson MC, Vecchio D, Prady H, Abel L, Wraith JE. Miglustat for treatment of Niemann-Pick C disease: a randomised controlled study. Lancet Neurol. 2007;6:765–72. doi: 10.1016/S1474-4422(07)70194-1. [DOI] [PubMed] [Google Scholar]
- Patterson MC, Vecchio D, Jacklin E, et al. Long-term miglustat therapy in children with Niemann-Pick disease type C. J Child Neurol. 2010;25:300–5. doi: 10.1177/0883073809344222. [DOI] [PubMed] [Google Scholar]
- Pentchev PG, Comly ME, Kruth HS, et al. Group C Niemann-Pick disease: faulty regulation of low-density lipoprotein uptake and cholesterol storage in cultured fibroblasts. FASEB J. 1987;1:40–5. doi: 10.1096/fasebj.1.1.3609608. [DOI] [PubMed] [Google Scholar]
- Pineda M, Wraith JE, Mengel E, et al. Miglustat in patients with Niemann-Pick disease Type C (NP-C): a multicenter observational retrospective cohort study. Mol Genet Metab. 2009;98:243–9. doi: 10.1016/j.ymgme.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Platt FM, Lachmann RH. A new surrogate marker for CNS pathology in Niemann-Pick disease type C? Mol Genet Metab. 2009;96:53–4. doi: 10.1016/j.ymgme.2008.10.015. [DOI] [PubMed] [Google Scholar]
- Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362:329–44. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- Sevin M, Lesca G, Baumann N, et al. The adult form of Niemann-Pick disease type C. Brain. 2007;130:120–33. doi: 10.1093/brain/awl260. [DOI] [PubMed] [Google Scholar]
- Vanier MT, Millat G. Niemann-Pick disease type C. Clin Genet. 2003;64:269–81. doi: 10.1034/j.1399-0004.2003.00147.x. [DOI] [PubMed] [Google Scholar]
- Wraith JE, Baumgartner MR, Bembi B, et al. Recommendations on the diagnosis and management of Niemann-Pick disease type C. Mol Genet Metab. 2009;98:152–65. doi: 10.1016/j.ymgme.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Wraith JE, Vecchio D, Jacklin E, et al. Miglustat in adult and juvenile patients with Niemann-Pick disease type C: long-term data from a clinical trial. Mol Genet Metab. 2010;99:351–7. doi: 10.1016/j.ymgme.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Yanjanin NM, Velez JI, Gropman A, et al. Linear clinical progression, independent of age of onset, in Niemann-Pick disease, type C. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:132–40. doi: 10.1002/ajmg.b.30969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerushalmi B, Sokol RJ, Narkewicz MR, Smith D, Ashmead JW, Wenger DA. Niemann-pick disease type C in neonatal cholestasis at a North American Center. J Pediatr Gastroenterol Nutr. 2002;35:44–50. doi: 10.1097/00005176-200207000-00011. [DOI] [PubMed] [Google Scholar]
- Zachrisson OC, Balldin J, Ekman R, et al. No evident neuronal damage after electroconvulsive therapy. Psychiatry Res. 2000;96:157–65. doi: 10.1016/S0165-1781(00)00202-X. [DOI] [PubMed] [Google Scholar]
- Zervas M, Dobrenis K, Walkley SU. Neurons in Niemann-Pick disease type C accumulate gangliosides as well as unesterified cholesterol and undergo dendritic and axonal alterations. J Neuropathol Exp Neurol. 2001;60:49–64. doi: 10.1093/jnen/60.1.49. [DOI] [PubMed] [Google Scholar]
- Zervas M, Somers KL, Thrall MA, Walkley SU. Critical role for glycosphingolipids in Niemann-Pick disease type C. Curr Biol. 2001;11:1283–7. doi: 10.1016/S0960-9822(01)00396-7. [DOI] [PubMed] [Google Scholar]
- Zetterberg H, Hietala MA, Jonsson M, et al. Neurochemical aftermath of amateur boxing. Arch Neurol. 2006;63:1277–80. doi: 10.1001/archneur.63.9.1277. [DOI] [PubMed] [Google Scholar]
- Zetterberg H, Pedersen M, Lind K, et al. Intra-individual stability of CSF biomarkers for Alzheimer’s disease over two years. J Alzheimers Dis. 2007;12:255–60. doi: 10.3233/jad-2007-12307. [DOI] [PubMed] [Google Scholar]
- Zetterberg H, Blennow K, Hanse E. Amyloid beta and APP as biomarkers for Alzheimer’s disease. Exp Gerontol. 2010;45:23–9. doi: 10.1016/j.exger.2009.08.002. [DOI] [PubMed] [Google Scholar]