Abstract
The conserved oligomeric Golgi (COG) complex is an eight subunit protein involved in the retrograde transport of Golgi components. It affects the localization of several Golgi glycosyltransferases and hence is involved in N- and O-glycosylation. Genetic defects in this complex belong to the rapidly expanding family of congenital disorders of glycosylation (CDG). Patients have been reported with defects of subunit 1 (CDG1-CDG), subunit 4 (CDG4-CDG), subunit 5 (CDG5-CDG), subunit 6 (CDG6-CDG), subunit 7 (CDG7-CDG), and subunit 8 (CDG8-CDG). This paper is on the second reported patient with COG5-CDG. She showed a mild neurohepatic disease with central as well as peripheral neurological involvement while in the first reported patient (with a different mutation) only mild central neurological involvement was reported.
Introduction
Congenital disorders of glycosylation (CDG) (Freeze 2006; Jaeken and Matthijs 2007; Jaeken et al. 2009) are mostly due to defects in the specific glycosylation machinery but a growing group of CDG is caused by disordered multifunctional proteins e.g., ATP6V0A2-CDG (Guillard et al. 2009) and the COG-CDG (Foulquier 2009; Zeevaert et al. 2008). The cytosolic COG complex is made up of eight subunits and involved in retrograde vesicular Golgi trafficking (reviews in Lees et al. 2010 and in Reynders et al. 2011). Patients have been reported with defects in subunits 1, 4, 5, 6, 7 and 8. The first report on a patient with a defective subunit 5 was by Paesold-Burda et al. (2009). This is the second report on a patient with COG5-CDG.
Patient and Methods
Patient
The patient was the second child of a nonconsanguineous Chinese couple. Intrauterine growth retardation was noted since 20 weeks of gestation with decreased liquor at 30 weeks. She was born at 35 weeks of gestation by Cesarean section with a birth weight of 2.1 kg. Neonatal jaundice was treated with phototherapy. At 1 month, she developed abdominal distension due to dilated bowel, and jaundice. Contrast enema showed a short segment of persistent narrowing in the most distal part of the rectum and anus but rectal biopsy excluded Hirschsprung disease. On liver function testing there was an increase of serum bilirubin [total bilirubin 206 μmol/L (normal: 10–24), unconjugated bilirubin 172 μmol/L (normal 3–17), conjugated bilirubin 13 (normal: 0–10)], alkaline phosphatase [891 U/L (normal: 145–420)], alanine aminotransferase [67 U/L (normal: 5–35)], aspartate aminotransferase [281 U/L (normal: 15–60)] and gamma-glutamyl transpeptidase [149 U/L (normal: 6–22)], with normal albumin. Scintigraphy excluded biliary atresia. Ultrasonography of the abdomen was normal, and the abdominal distension resolved.
Physical examination at 8 months showed microcephaly (39 cm, 2 cm < 3rd percentile). Weight was 6.3 kg (3rd percentile) and height 65.7 cm (10th–25th percentile). There was no dysmorphy. She had global developmental delay (mental age of 3–4 months), hypotonia, fixed flexion contractures of all fingers and mild hepatosplenomegaly. Liver dysfunction persisted except for normalization of bilirubin [alkaline phosphatase 737 U/L (normal: 145–420)], alanine aminotransferase 123 U/L (normal: 5–35), aspartate aminotransferase 271 U/L (normal: 15–60), gamma-glutamyl transpeptidase 41 U/L (normal: 6–22), fasting bile acids 11.4 μM (normal: <7), prothrombin time 13.4 s (normal: 11.3–13.2), activated partial thromboplastin time 40.6 s (normal: 27.6–37.6)). The renal function, blood hemoglobin, and total white cells were normal but there was a mild thrombocytopenia [135 × 109/L (normal: 150–400 × 109)].
A repeat ultrasonography of the abdomen showed liver cirrhosis with regeneration nodules and splenomegaly. There was no significant portal hypertension. A liver biopsy at 12 months confirmed cirrhotic changes. There was no decrease in the number of bile ducts in comparison with the hepatic arterioles, no increased copper or copper-associated protein nor immunohistochemical evidence of alpha-1 antitrypsin deficiency, and the liver cells were negative for hepatitis B surface antigens. No infectious, autoimmune, or metabolic cause of the liver disease was found.
Serum alpha-fetoprotein was increased [at 9 months: 4,178 ng/mL (normal: <50); at 16 months: 72 ng/mL (normal: <20)] with persistent mild hyperlactatemia [2.2/2.3/2.9/3.9 mmol/L (normal: 0.7–2.1)].
Magnetic resonance imaging (MRI) of the brain at 13 months showed delayed myelination (corresponding to 7–9 months) without other structural abnormalities.
Griffiths Mental Developmental Scale performed at 14 months showed an overall mental age of 9.5 months (9 months for locomotor, 12 months for personal–social, 8.75 months for hearing and speech, 10.5 months for eye and hand coordination, and 7 months for performance domains). At 20 months, there was persistent failure to thrive and generalized hypotonia as well as evidence of peripheral neuropathy including muscle wasting, weakness of both hands and feet (with contractures in the digits), and depressed deep tendon reflexes especially over the lower limbs. There was no cerebellar ataxia or sensory disturbances. In view of the multisystem involvement and hyperlactatemia, a mitochondrial oxidative phosphorylation defect or a congenital disorder of glycosylation was suspected.
She was started on ursodeoxycholic acid at 36 months. At 4 years 5 months, serum transferrin isoelectrofocusing (University Hospital Leuven, Belgium) revealed a type 2 pattern. Additional investigations in the context of a possible glycosylation defect showed normal serum haptoglobin, cholesterol, antithrombin III, factor IX, and factor XI. Echocardiography at 6 years was normal.
Ultrasonography of the abdomen at 9 years showed cirrhotic liver with a normal size without evidence of portal hypertension. The spleen was enlarged to 12 cm. Serial monitoring of liver function showed gradual improvement [alkaline phosphatase 468 U/L (normal: 145–420), aspartate aminotransferase 48 U/L (normal: 15–40), and normalization of all other parameters]. Platelet count went down to 63 × 109/L (normal: 150–400 × 109).
There was catching up of her developmental milestones. She was studying in a mainstream school with mild learning difficulty. Intellectual assessment (Hong Kong Wechsler Intelligence Scale for Children) at 8 years 8 months showed a verbal intelligent quotient of 99 (in normal range) and a performance intelligent quotient of 62 (mild mental retardation range). She showed a mild attention deficit disorder but hearing and visual assessments were normal. Repeat brain MRI at 4 years showed an improvement in myelination corresponding to about 24 months. Brain proton magnetic resonance spectroscopy was normal.
At the age of 9, her microcephaly was nonprogressive and the peripheral neuropathy was stable. Because of the functional limitation, she underwent a corrective surgery for the finger contractures and was considering surgery also for the toes.
Methods
Serum transferrin IEF (Jaeken et al. 1984), serum apolipoprotein C-III IEF (Wopereis et al. 2003), and serum transferrin glycan MALDI TOF analysis (Sturiale et al. 2005) were performed as described.
Mutation analysis of the eight COG subunit genes was performed by direct sequencing.
Results
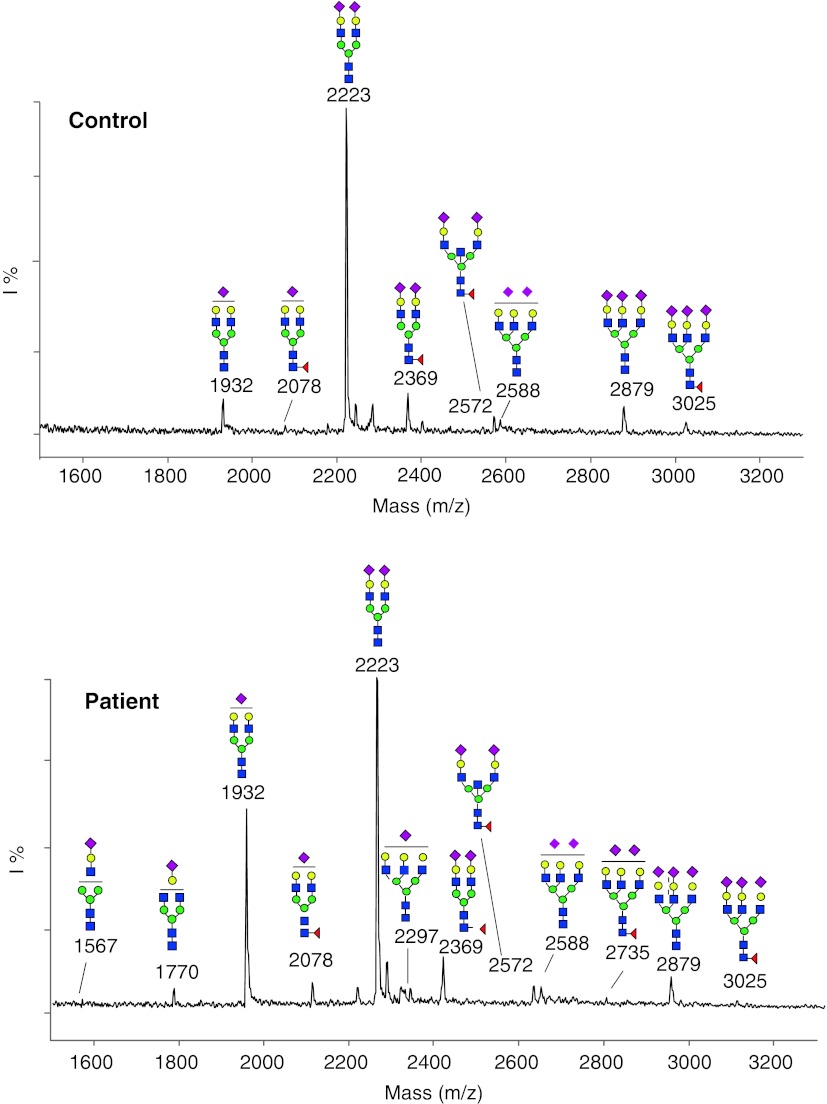
Serum transferrin IEF showed a type 2 pattern and serum apolipoprotein C-III a decrease of the disialo isoform and an increase of the asialo isoform. MALDI TOF analysis of serum transferrin glycans showed mainly evidence of hyposialylation (Fig. 1).
Fig. 1.
MALDI TOF analysis of the acidic N-glycans from serum transferrin shows a remarkable underglycosylation mainly due to the lack of a terminal sialic acid (increased peaks at m/z 1932, m/z 2078, m/z 2588, m/z 2735) and, to a much lesser extent, also due to the presence of truncated biantennary glycoforms at m/z 1770 (lacking a terminal sialic acid and a galactose unit) and at m/z 1567 (trace amount of a species lacking a terminal trisaccharide composed of a sialic acid, a galactose and an N-acetylglucosamine residue). Blue squaresN-acetylglucosamine; green circles mannose; yellow circles galactose; red diamonds sialic acid
Mutation analysis of COG5 showed two novel mutations: c.556–560delAGTAAinsCT (maternal) and c.1856T > C (p.I619T) (paternal).
Discussion
Genetic defects in subunits of the COG complex belong to a peculiar subgroup of the CDG family since these disorders affect proteins that are not specifically involved in the glycosylation machinery. Patients have been reported with defects in subunit 1 (COG1-CDG; three patients from different families), in subunit 4 (COG4-CDG; one patient), in subunit 5 (COG5-CDG; one patient), in subunit 6 (COG6-CDG; two siblings), in subunit 7 (COG7-CDG; 7 patients from 5 families), and in subunit 8 (COG8-CDG; 2 unrelated patients) (reviews in Foulquier 2009 and in Zeevaert et al. 2008; Lübbehusen et al. 2010; Paesold-Burda et al. 2009; Reynders et al. 2009). Their clinical presentations are variable (mild as well as severe) combinations mainly of neurological, morphological, and hepatic abnormalities. COG7-CDG is actually the largest group and, interestingly, six of the seven reported patients showed the same homozygous mutation and a fairly homogenous phenotype. They were from Moroccan or Tunesian descent and their disease was characterized by prenatal growth retardation, prenatal microcephaly with postnatal progression, feeding problems, severe psychomotor retardation, epilepsy, dysmorphy of face and hands (retro/micrognathy, small mouth, short neck, wrinkled skin, overlapping fingers), episodes of hyperthermia, and liver dysfunction. The number of patients with the other COG-CDG is too small to associate them with a consistent phenotype except for the fact that two (unrelated) patients with the same homozygous, intronic mutation in COG subunit 1 showed a cerebrocostomandibular-like syndrome (Zeevaert et al. 2009).
The one reported patient with COG5-CDG showed a mild neurological phenotype with psychomotor retardation and delayed motor and language development, and hyposialylation of N- and O-glycans. Mutation analysis showed a homozygous intronic substitution (c.1669–15T > C) leading to exon skipping and severely reduced expression of the COG5 protein. The present patient also shows a mild phenotype but her neurological involvement is central as well as peripheral and there is also a mild, stable hepatic phenotype. She has two mutations, different from the one found in the other reported patient.
We propose to perform mutation analysis for the COG subunit genes in patients with a type 2 serum transferrin IEF pattern and aspecific serum transferrin glycan abnormalities on MALDI TOF (hyposialylation and/or hypogalactosylation) unless there is a typical clinical cutis laxa type II syndrome (ATP6V0A2-CDG).
Abbreviations
- CDG
Congenital disorder(s) of glycosylation
- COG
Conserved oligomeric GoLgi complex
- IEF
Isoelectrofocusing
Footnotes
Competing interests: None declared.
References
- Foulquier F. COG defects, birth and rise! Biochim Biophys Acta. 2009;1792:896–902. doi: 10.1016/j.bbadis.2008.10.020. [DOI] [PubMed] [Google Scholar]
- Freeze HH. Genetic defects in the human glycome. Nat Rev Genet. 2006;7:537–551. doi: 10.1038/nrg1894. [DOI] [PubMed] [Google Scholar]
- Guillard M, Dimopovlov A, Fischer B et al (2009) Vacuolar H+−AT Pase meets glycosylation in patients with cutis laxa. Biochim Biophys Acta 1792: 903–914 [DOI] [PubMed]
- Jaeken J, van Eijk HG, van der Heul C, et al. Sialic acid-deficient serum and cerebrospinal fluid transferrin in a newly recognized syndrome. Clin Chim Acta. 1984;144:245–247. doi: 10.1016/0009-8981(84)90059-7. [DOI] [PubMed] [Google Scholar]
- Jacken J, Matthys G (2007) Congenital disorders of glycosylation: a rapidly expanding disease family. Ann Rev Genomics Atom Genet 8:261–278 [DOI] [PubMed]
- Jaeken J, Hennet T, Matthijs G, Freeze HH. CDG nomenclature: time for a change! Biochim Biophys Acta. 2009;1792:825–826. doi: 10.1016/j.bbadis.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees AJ, Yip CK, Walz T, Hughson FM. Molecular organization of the COG vesicle tethering complex. Nat Struct Mol Biol. 2010;17:1292–1297. doi: 10.1038/nsmb.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lübbehusen J, Thiel C, Rind N, et al. Fatal outcome due to deficiency of subunit 6 of the conserved oligomeric Golgi complex leading to a new type of congenital disorders of glycosylation. Hum Mol Genet. 2010;19:3623–3633. doi: 10.1093/hmg/ddq278. [DOI] [PubMed] [Google Scholar]
- Paesold-Burda P, Maag C, Troxler H, et al. Deficiency in COG5 causes a moderate form of congenital disorders of glycosylation. Hum Mol Genet. 2009;18:4350–4356. doi: 10.1093/hmg/ddp389. [DOI] [PubMed] [Google Scholar]
- Reynders E, Foulquier F, Leão TE, et al. Golgi function and dysfunction in the first COG4-deficient CDG type II patient. Hum Mol Genet. 2009;18:3244–3256. doi: 10.1093/hmg/ddp262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynders E, Foulquier F, Annaert W, Matthijs G. How Golgi glycosylation meets and needs trafficking: the case of the COG complex. Glycobiology. 2011;21:853–863. doi: 10.1093/glycob/cwq179. [DOI] [PubMed] [Google Scholar]
- Sturiale L, Barone R, Fiumara A, et al. Hypoglycosylation with increased fucosylation and branching of serum transferrin N-glycans in untreated galactosemia. Glycobiology. 2005;15:1268–1276. doi: 10.1093/glycob/cwj021. [DOI] [PubMed] [Google Scholar]
- Wopereis S, Grünewald S, Morava E, et al. Apolipoprotein C-III isofocusing in the diagnosis of genetic defects in O-glycan biosynthesis. Clin Chem. 2003;49:1839–1845. doi: 10.1373/clinchem.2003.022541. [DOI] [PubMed] [Google Scholar]
- Zeevaert R, Foulquier F, Jaeken J, Matthijs G. Deficiencies in subunits of the conserved oligomeric Golgi (COG) complex define a novel group of congenital disorders of glycosylation. Mol Genet Metab. 2008;93:261–278. doi: 10.1016/j.ymgme.2007.08.118. [DOI] [PubMed] [Google Scholar]
- Zeevaert R, Foulquier F, Dimitrov B, et al. Cerebrocostomandibular-like syndrome and a mutation in the conserved oligomeric Golgi complex subunit 1. Hum Mol Genet. 2009;18:517–524. doi: 10.1093/hmg/ddn379. [DOI] [PubMed] [Google Scholar]