Abstract
Prolidase deficiency (PD) is a rare recessive disorder resulting from mutations in the prolidase gene (PEPD); only 17 causative mutant alleles had been so far characterized. Prolidase is a ubiquitous enzyme that hydrolyses dipeptides with C-terminal proline or hydroxyproline residues and indeed, lack of this enzyme activity causes massive urine excretion of undigested iminodipeptides. The clinical manifestations of PD are widely variable, and include intractable skin ulcers, unusual face, different degree of mental retardation, and recurrent infections. No definitive treatment is at present available.
We report an 8-year girl with a typical PD facies, normal intelligence, and recurrent deep ulcerations complicated by infections. She was found to be compound heterozygous for two novel mutations in PEPD, c.1133delACG and c.1301delT, affecting the C-terminal end of the enzyme where the active site is located. Given her life-threatening course, she underwent allogeneic hematopoietic stem cell transplantation (HSCT) from her HLA-identical brother, confirmed heterozygous for the c.1133delACG allele. Successful engraftment was documented by full-donor chimerism. Posttransplant monitoring of erythrocyte prolidase activity showed that the child had converted to a heterozygous pattern. Reduction of excreted urine dipeptides, evaluated by capillary electrophoresis, supported the effectiveness of the treatment. Unfortunately the patient died on day +92 of invasive fungal infection.
Despite the unfavorable outcome, we provide the first evidence that HSCT has the potential to reverse some of the biochemical features of PD patients. The indication to transplant must be balanced against the clinical manifestation of individual patients.
Electronic supplementary material The online version of this article (doi: 10.1007/8904_2011_62) contains supplementary material, which is available to authorized users.
Introduction
Prolidase deficiency (PD; MIM 170100) is a rare disorder with recessive inheritance caused by mutations in the prolidase gene (PEPD, 19cen-q13.11). The prolidase enzyme hydrolyzes dipeptides containing C-terminal proline and hydroxyproline residues, and PD causes massive excretion of urinary iminodipeptides.
The clinical manifestations of PD are widely variable: affected patients show mainly intractable skin ulcers of lower extremities, unusual facial, ocular abnormalities, deafness, splenomegaly, obesity, often with mental retardation, and a history of recurrent infections (Royce and Steinman 2002). The molecular basis of such phenotype is still unknown.
Point mutations causing single amino acid substitutions, premature stop codon or exon splicing, small and large deletions, and a small duplication had so far been reported as causative mutations for PD (Lupi et al. 2008). Due to the limited number of patients investigated, the genotype–phenotype correlations are still poorly understood. Thus, the characterization of new mutant alleles in PD patients, together with their complete clinical description, will be important to better define the pathophysiology of this disease and to help develop appropriate treatments.
No definitive therapy is so far available for PD, although different therapeutic approaches had been attempted, mainly with no or partial rescue of the disease. Oral supplementation with manganese, a cofactor of prolidase, and vitamin C, acting on collagen synthesis, have been used as well as blood transfusions and apheresis, corticosteroid treatment, oral supplementation with antioxidants and topical antibiotics for the skin lesions (Lupi et al. 2008).
Prolidase is a ubiquitous enzyme highly expressed in circulating cells (erythrocytes and leukocytes) which are the preferred target for cell replacement therapy using hematopoietic stem cell transplantation (HSCT). Thus considering the recent advance in the treatment of cancer as well as inborn errors with HSCT, it seemed promising to apply such approach to PD patients (Archuleta et al. 2004; MacMillan et al. 2008; Trounson 2009).
Materials and Methods
Molecular Study
Genomic DNA was extracted from patient, donor and controls peripheral blood by standard techniques. The 14 exons and exons boundary of prolidase gene were amplified by PCR using 0.2 μg of gDNA and the primer pairs and annealing temperature listed in Supplementary Table 1. The PCR conditions were as follow: an initial 3 min denaturation at 94°C, followed by 35 PCR amplification cycles (1 min 94°C, 1 min at the specific annealing temperature and 1 min at 72°C) and a final 10 min extension at 72°C. The amplicons were run on agarose gel, gel purified and directly sequenced. The sequences obtained were compared with the reported PEPD gene sequence (MN_000285.3).
Supplementary Table 1.
Primer pairs and annealing temperature for the 14 prolidase sequenced exons
| Primers | Primer sequence (5′–3′) | Nt position NM_000285.3 | Ta (°C) | |
|---|---|---|---|---|
| Exon 2 | ex2s | GTAGGTGGAGCTTGGGCAGCTTG | 13974 | 55 |
| ex2as | CAGCAGCACCATCACACACCTGC | 14330 | ||
| Exon 3 | ex3s | TCGGTCTGAGCTGGCTCTCTAG | 15639 | 52 |
| ex3as | CAACCCAGCTCTCTCATCCCAC | 15908 | ||
| Exon 4 | ex4s | GGCAGGCAGGAAGGTGGCCATG | 25820 | 53 |
| ex4as | GTGGCAGTGGAAGGAGAGGAAC | 26018 | ||
| Exon 5 | ex5s | CCACGTCGCCTGTTCTAATGATC | 33432 | 53 |
| ex5as | CGACCTCTGCAGGAGAGGTGGC | 33665 | ||
| Exon 6 | ex6s | CCTGTTCTTACCTGTCCTGAGCC | 36736 | 53 |
| ex6as | ACCTGCTAGTGAAGGTGGGAGTG | 36940 | ||
| Exon 7 | ex7s | GCATCACGTGTGCATGTCTTG | 48713 | 48 |
| ex7as | GGTTCTGGGAATCTGCTTTCTG | 48947 | ||
| Exon 8 | ex8s | CCAGTGCCTCCTGAAAGTCACTG | 62779 | 62 |
| ex8as | CTCTCGCCACACAGCAACACTGC | 62943 | ||
| Exon 9 | ex9s | CATGTCTGTACCACTGCATGCAC | 63730 | 54 |
| ex9as | CACTGCGGCGTCTCATCTGTTAC | 63967 | ||
| Exon 10 | ex10s | GCCTCACAGACTCACACTGAGAG | 113099 | 54 |
| ex10as | GGGCGTGTGAGTGAGCAAGTGTG | 113412 | ||
| Exon 11 | ex11s | AGAGCCTGCTGCAGAAGAATGGGC | 115092 | 57 |
| ex11as | AGAAGGCACCACGCAGGCCGATAG | 115377 | ||
| Exon 12 | ex12s | GTGGGAGACACCAGGTGGCAGTTC | 124859 | 64 |
| ex12as | CCGTTCCCTGCAGGCACCTGCCAC | 125232 | ||
| Exon 13 | ex13s | CACAGCCAGTGTGAGGGCTTCAC | 135319 | 54 |
| ex13as | ACGCCTGCTGCCTCCTAGAGTCC | 135672 | ||
| Exon 14 | ex14s | CCCTCACATGCCCATCTCTCCTG | 138731 | 54 |
| ex14as | CTCCACTGCAGCTGCAGACTCCT | 139050 | ||
| Exon 15 | ex15s | CTGGCACATGAGGCTGCTCTATG | 139362 | 54 |
| ex15as | ATCAAATGCCGAAGCTGGGATCTG | 139658 |
Determination of Prolidase Activity
Blood samples from the patient and healthy age-matched controls were collected in heparinized tubes before and at day 7, 14, 21, 28, 35, and 58 posttransplant. The donor, patient’s brother, blood was also collected before the treatment. Prolidase activity in red blood cells was evaluated. Briefly, whole blood samples were fractionated by centrifugation at 1,200 g for 5–10 min at room temperature. The pelleted red blood cells were resuspended in 2 volumes of phosphate buffer saline (PBS, Sigma), centrifuged again at 1,200 g for 5 min at room temperature and washed 3 times with PBS. The cells were finally resuspended in 4 volumes of water and freeze-thawed twice. The solutions were then dialyzed overnight at 4°C against 50 mM Tris pH8.0, 1 mM MnCl2. The prolidase activity was evaluated according to Myara procedure (Myara et al. 1982), which is based on measurement of proline by Chinard’s reagent (Chinard 1952). Hemoglobin concentration was determined by spectrophotometric measurement (Davis and Sheard 1927). Results were expressed as μmol of proline released in 1 h per g of hemoglobin and presented as percentage with respect the activity of the healthy controls, considered as 100%.
Determination of Dipeptides in Urine
Urine samples from the patient were collected at the same time points than blood samples, before and at day 7, 14, 21, 28, 35, and 58 posttransplant. Urines from the donor patient’s brother and a healthy age/gender-matched control were also collected. The samples were centrifuged (5 min at 1,600 g, at 4°C) and the supernatants were analyzed by capillary electrophoresis (CE).
The experiments were performed on a P/ACE MDQ capillary electrophoresis (Beckman, Fullerton, CA, USA) with built-in diode-array detector. The uncoated fused-silica capillaries (50 μm ID, 360 μm OD, effective length 46.8 cm, total length 57 cm) were from Polymicro Technologies (Phoenix, Arizona, USA). Before use, the capillaries were pretreated with 1 M NaOH for 60 min and 50 mM sodium tetraborate buffer pH 9.2 (Merck, Darmstadt, Germany) for 75 min, by applying a pressure of 14.5 psi. Before each run the capillary was rinsed with 0.1 M NaOH for 3 min and background electrolyte (sodium tetraborate buffer pH 9.2) for 3 min at 14.5 psi. Buffer solutions were prepared daily using deionized water filtered through 0.45 μm membrane filters (Millipore, Bedford, MA, USA) and degassed by sonication. The deionized water was produced using a Millipore Direct-Q TM.
The samples were hydrodynamically injected at 1.6 psi for 7 s. The electrophoretic run was carried out by applying a voltage of 25 kV (current 65–70 μA). Capillary cartridge and autosampler were thermostatted at 25°C. The acquisition wavelength was 200 nm.
Standard dipeptides mixture was prepared by mixing four dipeptides (Sigma) in different proportions (final concentrations were 2 mg/ml Gly-Pro, 1 mg/ml Ala-Pro, 1 mg/ml Phe-Pro, 1 mg/ml Leu-Pro). The samples were normalized to the creatinine concentration (Lupi et al. 2005).
Results were expressed (n = 3) as percentage of undigested dipeptides, namely (dipeptide area urine sample/dipeptide area standard mixture)/(creatinine area urine sample/creatinine area healthy age-matched control sample) × 100.
Results
Clinical Features
A 7-year-old female came to our attention because of multiple necrotic skin lesions on the lower limbs and buttocks; the first episode had occurred at the age of 17 months, then recurring at 5, and 6 years, but no diagnosis had been made. There was no significant family history, and past medical history was unremarkable. Physical examination showed dysmorphic features with hypertelorism and splenomegaly, with slight speech delay. A skin biopsy was performed, and showed leukocytoclastic vasculitis involving the venules of dermis and subcutaneous tissues, with extensive parietal fibrinoid necrosis, occlusion of vascular lumen, spongiosis, and ischemic necrosis of the superficial portion of malpighian layer. Clinical finding were suggestive of prolidase deficiency, and the diagnosis was confirmed by enzymatic activity evaluation in erythrocytes (22.41 ± 0.9 μmol/h per g in the patient, 514.14 ± 15.1 μmol/h per g in controls). Initial treatment consisted in low dose systemic steroids, topical preparations with proline and glycine, a proline-free diet, and erythrocytoapheresis. However, treatment had been unsuccessful, and the child still suffered from deep and very painful skin lesions. Infectious complications of the skin lesions occurred repeatedly; in particular, she developed one episode of septicemia that required treatment in the ICU of our children’s hospital. Pseudomonas aeruginosa and Klebsiella pneumoniae were isolated from blood culture; after a life-threatening course, she progressively recovered.
Molecular Characterization
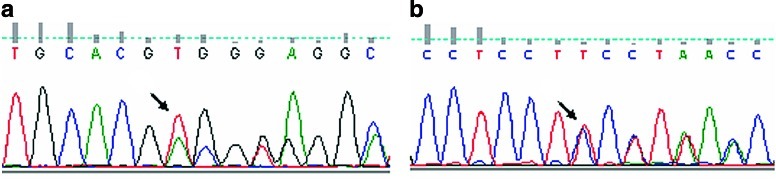
The sequence of all prolidase-coding region and intronic boundaries revealed that the patient was a compound heterozygous for two novel mutations: c.1133delACG, causing the deletion of Asp378 in exon 13, and c.1301delT, causing a frameshift and a premature stop codon in exon 14 (Fig. 1 and Supplementary Fig. 1). Both mutations affect the terminal end of the prolidase, where the active site is known to be located (Lupi et al. 2008; Besio et al. 2009). The parents were carriers, the mother of the mutation in exon 13 (c.1133delACG) and the father of the mutation in exon 14 (c.1301delT); the donor, patient’s brother, was carrier for the maternal c.1133delACG allele (data not shown).
Fig. 1.
Determination of the two novel PEPD mutations in patient gDNA. (a) 1131delACG, causing the deletion of Asp378 in exon 13. (b) 1301delT, causing a frameshift in exon 14 and the creation of a premature stop codon. The arrows indicate the position of the mutations
Supplementary Fig. 1.
Effect of WT (a), 1133delACG (b) and 1301delT alleles (c) on the prolidase protein sequence. In (b) the position of the deleted 378D due to the 1133delACG is marked with a vertical line. In (c) the abnormal sequence following aminoacid 434 due to the 1301delT is underlined and the premature stop codon reported
Hematopoietic Stem Cell Transplantation
Given her clinical course, characterized by not only significant morbidity but also by one life-threatening infectious complication, the hypothesis of performing an allogeneic HSCT was considered. Based on the HLA typing, her brother turned to be HLA-identical. He was then studied under the metabolic point of view, and confirmed to be heterozygous for PD. Thus, after a thorough discussion with the parents, indication to HSCT was defined. The diagnostic pretransplant work-up showed that her liver and pulmonary function were normal. After a conditioning regimen with Busulfan 16/mg per kg + cyclofosfamide 200 mg/kg, we infused bone marrow HSC with 3.52 millions CD34+/donor kg. Defibrotide 10 mg/Kg was given for veno-occlusive disease (VOD) prophylaxis from day +1; Graft versus host disease prophylaxis consisted of methotrexate 10 mg/sqm i.v. on day +3, +6, +11, and cyclosporin 3 mg/Kg from day −1. Prophylaxis of infection consisted of: fluconazole 6 mg/kg per day i.v., and ayclovir 10 mg/kg per die i.v. q 8 h since day +1, and cefepime 100 mg/kg per day i.v. since day +4. The neutrophil take was recorded on day +16, while no platelet take was obtained; full-donor chimerism was repeatedly documented. On day +16 she developed spiking AST/ALT levels, up to 10,460/4,507 IU/L on +19, with jaundice, hepatomegaly and weight gain. Liver biopsy confirmed the histological diagnosis of VOD. The child died on +92 of invasive by Geotrichum Capitatum infection. A list of PRBC transfusion as well as time of urine and blood collection is listed in Table 1.
Table 1.
Time of red blood cell transfusion, prolidase activity evaluation and urine dipeptide excretion measurement from the transplantation
| Transfusiona | Transfusion volume (Units) | Activity measurementa | Dipeptide analysisa |
|---|---|---|---|
| 7 | 1 | 7 | 7 |
| 12 | 1.5 | ||
| 14 | 14 | ||
| 18 | 1 | ||
| 21 | 1 | 21 | 21 |
| 22 | 1 | ||
| 28 | 28 | ||
| 35 | 35 | ||
| 37 | 1 | ||
| 51 | 0.8 | ||
| 58 | 58 |
aDays from HSCT considered as d1
Enzyme Study and Urine Dipeptide Analysis
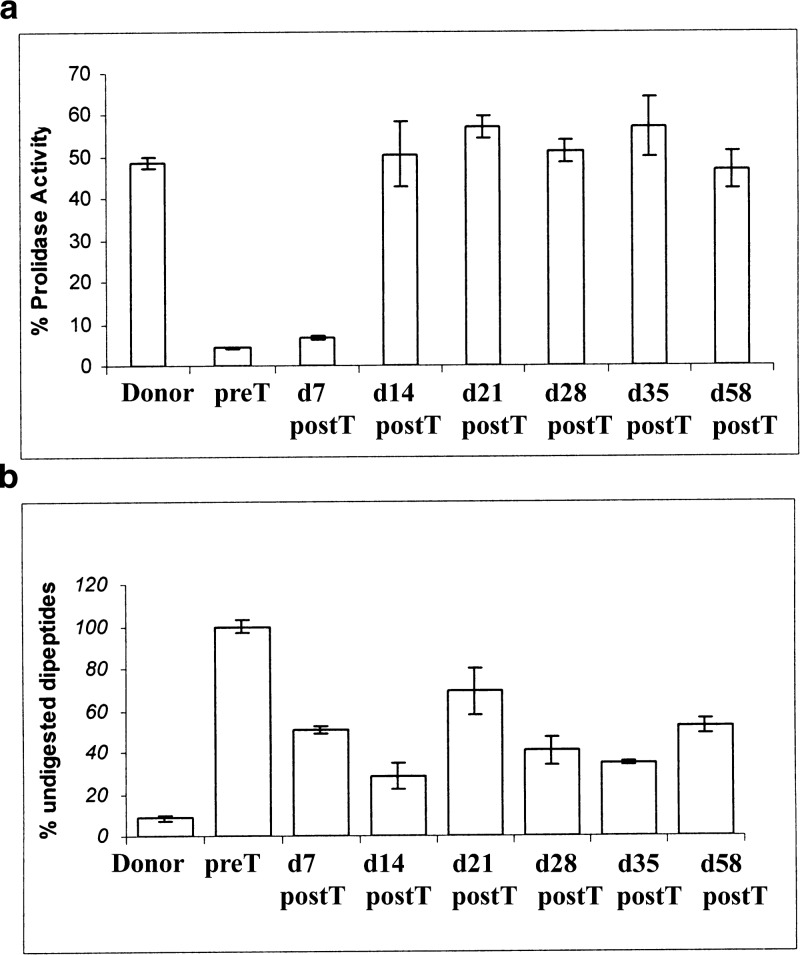
The patient’s prolidase activity prior to transplantation was 4.36 ± 0.22% compared to age-matched controls. The sibling donor showed a prolidase activity ~50% of the control, confirming his carrier status for the disease also at the biochemical level. The patient’s activity at d7 posttransplant was unchanged, whereas an increase to 50.51 ± 7.74% was detected 2 weeks after the transplant, to remain stable over the following 8 weeks (Fig. 2a).
Fig. 2.
Prolidase activity measurement and urinary iminodipeptides analysis. (a) Prolidase activity of the donor brother’s patient and of the patient pretransplant and 7, 14, 21, 28, 35 and 58 days posttransplant are expressed as percentage with respect to age-matched control. (b) Percentage of donor and patient’s undigested peptides, pretransplant and 7, 14, 21, 28, 35 and 58 days posttransplant, as quantified by capillary electrophoresis. Controls did not reveal any measurable urinary dipeptides
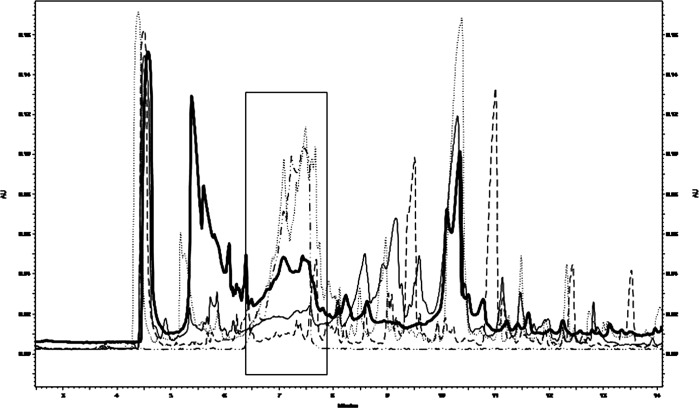
Pretransplant dipeptides measured by CE was assumed to be 100% with respect to an age-matched control (0%), which, as expected, did not reveal any peak in the imidodipeptides chromatographic region (Fig. 3). The urine from the donor showed a modest percentage of undigested peptides of 8.46% ± 1.20 (Fig. 2b). As shown in Fig. 2b, the patient’s percentage of undigested dipeptides declined after transplant down to 34.66 ± 1.19 at d35. The increment to 52.58 ± 3.51 at d58 was not due to exogenous administration of glutamine, which was ruled out; whether it may depend on liver alteration, remains unclear.
Fig. 3.
Representative overlapped CE electropherograms of urine samples; the frame represents the time window where dipeptides migrate. From bottom to top: healthy age/gender-matched control, donor patient’s brother urine, posttransplant patient’s urine (corresponding to the second collection posttransplant), standard dipeptide mixture, pretransplant patient’s urine (dotted line)
Discussion
Up to now only 17 PEPD pathogenic mutations had been identified in patients with PD; thus, the genotype–phenotype correlations remain largely unclear (Lupi et al. 2008). The wide range of clinical severity and age of onset in patients with the same molecular defects, leaves open the possibility that modifier genes could play a role in PD phenotype and outcome; thus, reporting new mutations remains relevant.
Here, we describe the third PEPD allele carrying a three base pairs deletion causing the lack of a single amino acid. Both mutations, c.691delTAC, resulting in p.231delTyr (Lupi et al. 2004), and c.1354delGAG, resulting in p.452delGlu (Ledoux et al. 1994), had been previously reported in patients with PD. The deletion of Tyr231 was hypothesized to be responsible for the disruption of the prolidase structure, whereas the deletion of Glu452 impaired the Mn2+ binding at the enzyme active site. In our patient, one of the PEPD allele carried c.1133delACG, determining the absence of the Asp378 that, although not directly involved in the catalytic active metal-binding site, is located in that region; furthermore, the lack of a negative-charged residue could probably alter its structure (Besio et al. 2009).
The second mutation documented in our patient, c.1301delT, is a novel one. It is responsible for a frameshift in exon 14 and the consequent creation of a premature stop codon altering the prolidase sequence from residue 434. This caused, in the mutant allele, the absence of two relevant residues, Glu412 and Glu452, known to be involved in Mn2+ binding.
To improve the quality of life of patients with PD, and to prevent fatal complications, several therapeutic approaches have been applied. Interestingly, the use of systemic therapy induced a partial phenotype amelioration. Berardesca et al. reported a 15-year-old boy with PD and defective erythrocyte prolidase activity, which, after blood transfusions, increased to 15.7% of donor activity, then declining to 12% and 3.4% of normal activity after 8 and 45 days, respectively (Berardesca et al. 1992). Apheresis exchanges were repeated monthly for four consecutive months, in parallel, on two patients, replacing prolidase-deficient red blood cells with normal filtered cells. This allowed the constant presence of active prolidase inside cells leading to a continuous, although partial, degradation of imidodipeptides, with a concomitant improvement of skin ulceration (Lupi et al. 2002). The efficacy of such approaches is still unclear. Since prolidase is a cytosolic enzyme, the effectiveness of blood transfusion could be either determined by enzyme release due to cell lysis or by normal red blood cell undigested dipeptides uptake through simple diffusion or low affinity carrier and their subsequent intracellular hydrolysis (Lochs et al. 1990; Odoom et al. 1990).
Prolidase is an ubiquitously expressed enzyme, and HSCT is expected to replace the host hematopoietic cells with donor-derived ones. This might end up in rescuing enzyme activity in only a few cell types, not in other tissues. Yet, the high prolidase expression in blood cells, and the reported evidence that repeated blood transfusions contributed to reduce the clinical manifestations in patients with severe PD, suggest that the systemic presence of this enzyme could contribute to dispose dipeptide accumulation, contributing to the disease phenotype (Forlino et al. 2002). These observations pointed to HSCT as a potentially valuable therapeutic approach.
In the present case, the parents of a child with a long-lasting history of skin ulcers and infectious complications, which turned to be life-threatening in at least one event, asked us to apply any novel therapeutic approach, in the search for a cure for PD. The availability of an HLA-identical sibling raised the discussion on the possible indication to HSCT, a long-time recognized therapeutic option for selected inborn errors in which the source of the defective molecule can be replaced by stem-cell derived cells (Prasad and Kurtzberg 2008; Massberg and von Andrian 2009).
Successful engraftment of the transplant was documented by full-donor chimerism. In parallel, posttransplant monitoring of blood prolidase activity showed that, in keeping with the full-donor chimerism, the child had converted to a heterozygous pattern.
We are aware that RBC independence posttransplantation is extremely variable and that by evaluating prolidase activity from red blood cells we could have simply measured the donor cell activity, but the very low prolidase activity at d7 posttransplant allowed us to exclude this possibility. During the follow-up the patient was also transfused with PRBC (see Table 1), but her enzymatic activity remained stable, independently from the time between each transfusion and the activity measurement. This strongly suggest that the prolidase activity detected was mainly due to donor cells engrafted in the patient, although we cannot exclude a partial contribution by the transfused erythrocytes. It is important to remember that prolidase activity in PD patients after PRBC transfusion was previously reported to decline already after 5 days (Lupi et al. 2002); thus the stable prolidase activity documented on days +28 and +35 posttransplant, respectively 7 and 14 days following any transfusion, supports our conclusion.
CE analysis of the patient’s urines also showed a reduction of iminodipeptides peaks. Whether incomplete elimination in the urine of the secreted iminodipeptides may depend on the patient’s multiorgan failure remains to be assessed.
The main limitation of the present study was the relatively short follow-up that made us impossible to clearly determine the clinical effects of HSCT in the patient, thus forcing us to consider only the surrogate, biochemical parameters.
With the aim of preventing transplant rejection, an event that may be more frequent in patients with some inborn errors such as mucopolysaccaridosis (Boelens et al. 2009), we decided to apply a myeloablative conditioning regimen. VOD is one possible complication of HSCT. To address this issue, the child received specific prophylaxis, which yet turned to be insufficient to prevent the complication. Whether or not underlying PD may have been responsible for any predisposing condition to liver VOD, remains questionable at present.
In conclusion, despite the unfavorable outcome in this case, we provide the first evidence that PD may be at least partially reversed by HSCT. The indication to transplant must be balanced against the clinical manifestation of individual patients.
Acknowledgments
This work was supported by MIUR 2008 (2008XA48SC), by Fondazione Cariplo 2007 to A.F. and by Progetto Regione Lombardia (cod. SAL/45) “Dalla scienza dei materiali alla medicina molecolare” to A.F., A.R., and E.D.L.
Abbreviations
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- HLA
Human leukocyte antigen
- HSC
Hematopoietic stem cells
- HSCT
Hematopoietic stem cell transplantation
- PRBC
Packed red blood cells
Short Summary
Two novel causative mutations were documented in a patient with Prolidase Deficiency (PD), a rare autosomal recessive disease of connective tissue for which only 17 mutant causative alleles had been reported. No therapies with curative potential are available for PD patients; this is the first report of HSCT with at least partial rescue of this disease. Reversal of enzyme activity and urine dipeptides excretion suggested that PD could be added to the disorders treatable by transplantation.
Accession Codes: OMIM: 170100 (Prolidase Deficiency); E.C.: 3.4.13.9 (Prolidase); HUGO: PEPD (Proliase); GeneBank: NM_000285.3 (Prolidase mRNA)
Contributions of Individual Authors
Rolando Cimaz made initial diagnosis and designed therapeutic approach
Maurizio Aricò Désirée Caselli: took care of the evaluation of the feasibility and then of performing HSCT transplantation
Silvia Riva Marco Spada: provided expert clinical care during the phase of severe liver failure
Luca Cantarini: performed the molecular characterization of the patient and her family
Antonella Forlino Roberta Besio, Antonio Rossi performed the molecular and biochemical characterization of the patient and determined prolidase activity at the different pre and posttransplantation time
Ersilia De Lorenzi Raffaella Colombo: performed the capillary electrophoresis experiments to evaluate urine dipeptide excretion following transplantation
Maurizio Aricò and Antonella Forlino: planned the study analyzed the data and wrote the manuscript
The guarantor for the present article is Maurizio Aricò.
All authors declare that the answers to all questions on the JMDI competing interest questionnaire are No and therefore they have nothing to declare.
Funding
This work was supported by MIUR 2008 (2008XA48SC) by Fondazione Cariplo 2007 to A.F. and by Progetto Regione Lombardia (cod. SAL/45) “Dalla scienza dei materiali alla medicina molecolare” to A.F., A.R. and E.D.L.
The authors confirm independence from the sponsors the content of the article has not been influenced by the sponsor. Informed consent for all the laboratory and genetic studies, and for treatment including HSCT was obtained from both parents.
Footnotes
Competing interests: None declared.
Contributor Information
Antonella Forlino, Email: aforlino@unipv.it.
Maurizio Aricò, Email: m.arico@meyer.it.
References
- Archuleta TD, Devetten MP, Armitage JO. Hematopoietic stem cell transplantation in hematologic malignancy. Panminerva Med. 2004;46(1):61–74. [PubMed] [Google Scholar]
- Berardesca E, Fideli D, Bellosta M, Dyne KM, Zanaboni G, Cetta G. Blood transfusions in the therapy of a case of prolidase deficiency. Br J Dermatol. 1992;126(2):193–195. doi: 10.1111/j.1365-2133.1992.tb07820.x. [DOI] [PubMed] [Google Scholar]
- Besio R, Alleva S, Forlino A, et al. Identifying the structure of the active sites of human recombinant prolidase. Eur Biophys J. 2009;5:5. doi: 10.1007/s00249-009-0459-4. [DOI] [PubMed] [Google Scholar]
- Boelens JJ, Rocha V, Aldenhoven M, et al. on behalf of EUROCORD, Inborn Error Working Party of EBMT and Duke University (2009). Risk Factor Analysis of Outcomes after Unrelated Cord Blood Transplantation in Patients with Hurler Syndrome. Biol Blood Marrow Transplant; 15:618–625 [DOI] [PubMed]
- Chinard FP. Photometric estimation of proline and ornithine. J Biol Chem. 1952;199(1):91–95. [PubMed] [Google Scholar]
- Davis GE, Sheard C. The spectrophotometric determination of hemoglobin. Arch Intern Med. 1927;40:226–236. doi: 10.1001/archinte.1927.00130080100009. [DOI] [Google Scholar]
- Forlino A, Lupi A, Vaghi P, et al. Mutation analysis of five new patients affected by prolidase deficiency: the lack of enzyme activity causes necrosis-like cell death in cultured fibroblasts. Hum Genet. 2002;111(4–5):314–322. doi: 10.1007/s00439-002-0792-5. [DOI] [PubMed] [Google Scholar]
- Ledoux P, Scriver C, Hechtman P. Four novel PEPD alleles causing prolidase deficiency. Am J Hum Genet. 1994;54(6):1014–1021. [PMC free article] [PubMed] [Google Scholar]
- Lochs H, Morse EL, Adibi SA. Uptake and metabolism of dipeptides by human red blood cells. Biochem J. 1990;271(1):133–137. doi: 10.1042/bj2710133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupi A, Casado B, Soli M, et al. Therapeutic apheresis exchange in two patients with prolidase deficiency. Br J Dermatol. 2002;147(6):1237–1240. doi: 10.1046/j.1365-2133.2002.04998.x. [DOI] [PubMed] [Google Scholar]
- Lupi A, De Riso A, Torre SD, et al. Characterization of a new PEPD allele causing prolidase deficiency in two unrelated patients: natural-occurrent mutations as a tool to investigate structure-function relationship. J Hum Genet. 2004;49(9):500–506. doi: 10.1007/s10038-004-0180-1. [DOI] [PubMed] [Google Scholar]
- Lupi A, Rossi A, Vaghi P, Gallanti A, Cetta G, Forlino A. N-benzyloxycarbonyl-L-proline: an in vitro and in vivo inhibitor of prolidase. Biochim Biophys Acta. 2005;1744(2):157–163. doi: 10.1016/j.bbamcr.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Lupi A, Tenni R, Rossi A, Cetta G, Forlino A. Human prolidase and prolidase deficiency: an overview on the characterization of the enzyme involved in proline recycling and on the effects of its mutations. Amino Acids. 2008;35(4):739–752. doi: 10.1007/s00726-008-0055-4. [DOI] [PubMed] [Google Scholar]
- MacMillan ML, Davies SM, Nelson GO, et al. Twenty years of unrelated donor bone marrow transplantation for pediatric acute leukemia facilitated by the national marrow donor program. Biol Blood Marrow Transplant. 2008;14(9 Suppl):16–22. doi: 10.1016/j.bbmt.2008.05.019. [DOI] [PubMed] [Google Scholar]
- Massberg S, von Andrian UH. Novel trafficking routes for hematopoietic stem and progenitor cells. Ann N Y Acad Sci. 2009;1176:87–93. doi: 10.1111/j.1749-6632.2009.04609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myara I, Charpentier C, Lemonnier A. Optimal conditions for prolidase assay by proline colorimetric determination: application to iminodipeptiduria. Clin Chim Acta. 1982;125(2):193–205. doi: 10.1016/0009-8981(82)90196-6. [DOI] [PubMed] [Google Scholar]
- Odoom JE, Campbell ID, Ellory JC, King GF. Characterization of peptide fluxes into human erythrocytes. A proton-n.m.r. study. Biochem J. 1990;267(1):141–147. doi: 10.1042/bj2670141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad VK, Kurtzberg J. Emerging trends in transplantation of inherited metabolic diseases. Bone Marrow Transplant. 2008;41(2):99–108. doi: 10.1038/sj.bmt.1705970. [DOI] [PubMed] [Google Scholar]
- Royce PM, Steinman B. Prolidase deficiency. In: Royce PM, Steinman B, editors. Connective tissue and its heritable disorders. New York: Wiley-Liss; 2002. [Google Scholar]
- Trounson A. New perspectives in human stem cell therapeutic research. BMC Med. 2009;7(29):29. doi: 10.1186/1741-7015-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.