Abstract
A 7-day-old girl had an elevated leucine level on newborn screen drawn at 2 days of age and was suspected of having maple syrup urine disease (MSUD). When reported, the patient was immediately admitted to hospital, and started on a modified diet involving high calories with reduced branched chain amino acid (BCAA) formula. Clinical exam was normal at initial assessment. Despite rapid initiation of treatment, the baby became lethargic and somnolent over the next day. Diet was stopped and infusions of 12.5% dextrose and 20% intravenous lipids at 2 g/kg per day were immediately started. Lethargy improved within 3 h of intravenous therapy initiation. Brain magnetic resonance imaging demonstrated diffuse cerebral edema, and symmetric restricted diffusion in bilateral cerebellar white matter, dorsal brainstem, thalami, globi pallidi, posterior limbs of internal capsules, and corona radiata. Plasma leucine was 1.98 mmol/L on admission (normal 0.05–0.17 mmol/L), decreasing to 1.34 mmol/L with diet, however clinical deterioration occurred despite improving leucine levels.
Cerebral edema in MSUD is thought secondary to a combination of increased cerebral BCAA levels, and depleted levels of other essential amino acids, as well as neurotransmitters. Our case illustrates that newborns can develop encephalopathy with cerebral edema despite treatment with special formula initiated while asymptomatic. These findings suggest decompensation may begin early on, so that early introduction of high dextrose infusion and intravenous lipids, in combination with reduced BCAA formula, should be initiated for any patient with a positive newborn screen for MSUD.
Introduction
Maple syrup urine disease (MSUD; OMIM 248600) results from deficiency of the branched chain ketoacid dehydrogenase complex (BCKD) leading to accumulation of branched chain amino acids (BCAA) leucine, isoleucine and valine and their associated alpha-ketoacids (BCKA). In the classic neonatal presentation, infants are typically asymptomatic at birth, but by 4–7 days of age, can develop feeding intolerance, vomiting, and apneas commensurate with encephalopathy. Untreated, there is rapid progression to seizures, coma, and death. MSUD can be difficult to detect in a newborn infant because there may be little, if any, disturbance in typical biochemical markers for metabolic disease such as lactate, ammonia, acidosis, or hypoglycemia. Ketosis can be present. Having clinical suspicion for MSUD in a newborn baby with unexplained encephalopathy remains one of the best methods to direct the necessary diagnostic investigations, initiate early treatment and limit the neurological damage (Strauss et al. 2010).
The pathophysiology of neurologic deterioration in MSUD is not precisely understood; however, the radiologic changes are well characterized. The typical pattern begins with a marked, generalized cerebral edema beginning late in the first week of life (Brismar et al. 1990). This progresses to a more severe, localized edema primarily affecting the deep cerebellar white matter, dorsal brainstem, cerebral peduncles, and dorsal limb of the internal capsule (Brismar et al. 1990). With appropriate treatment, the edema gradually resolves by 6–7 weeks of life, at which point well-demarcated periventricular white matter disease is usually apparent, with some loss of brain substance (Brismar et al. 1990).
Expanded newborn screening has the potential to make a huge impact on early diagnosis and successful treatment of children with MSUD. The value of early diagnosis and initiation of treatment in endemic populations has been demonstrated; however, there is still limited data on the most appropriate intervention in asymptomatic infants with a positive newborn screen for MSUD. In some patients, the newborn screen may be falsely positive and no specific treatment indicated. However, if the screen is true positive, delaying treatment until confirmation by diagnostic tests can lead to progression of the disease and neurological injury. Medical centers specialized in the care of patients with metabolic diseases may have the capability to confirm the findings of the newborn screen by performing quantitative plasma amino acids with results within 24 h or less, but there is little data on whether it is safer to start the patient on treatment while awaiting results of confirmatory tests. In this report, we detail a case that was detected by newborn screening and had dietary intervention started during the presymptomatic stage resulting in falling plasma leucine levels. Our case suggests that in a patient with a positive newborn screen for MSUD starting a high rate dextrose infusion may be preferable to initially treating with diet alone, even if the patient is asymptomatic.
Case
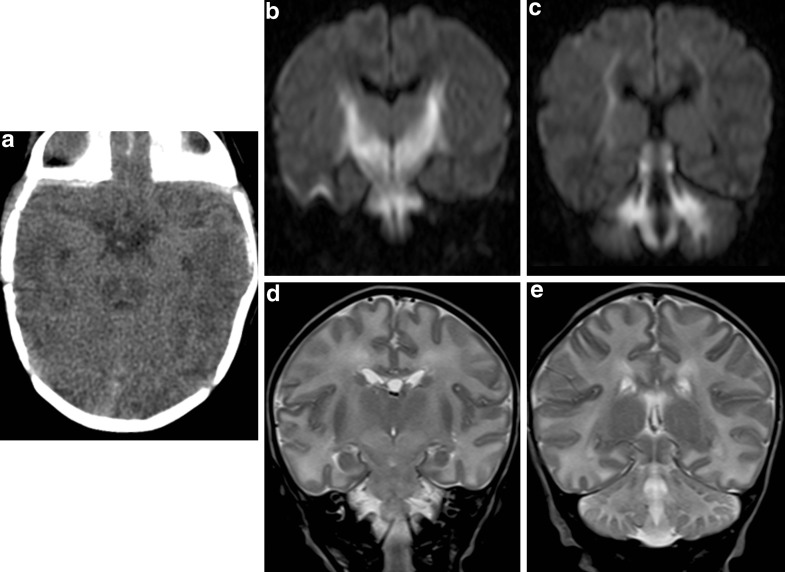
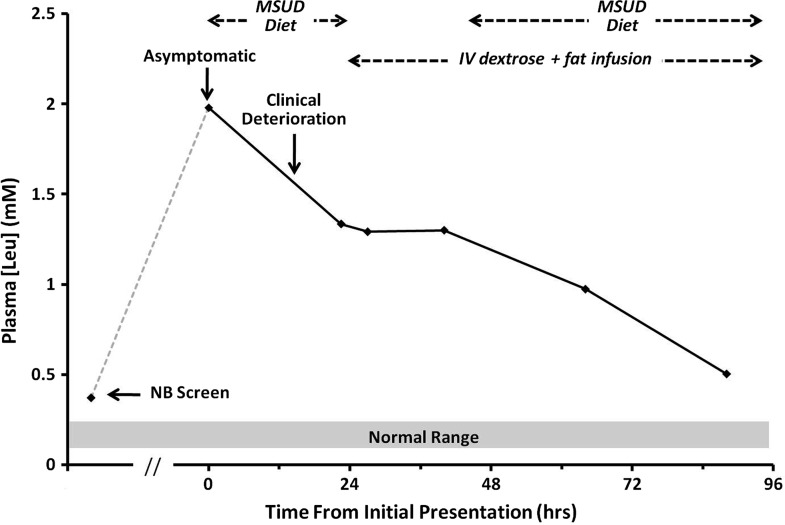
A full term newborn baby girl was reported at 7 days of age to have a positive newborn screen for MSUD (leucine/isoleucine 0.373 mM, screening cut-off 0.250 mM) from a blood spot drawn at 2 days of age. The family was contacted by the Metabolic service and advised to bring the child immediately to hospital, a blood sample for plasma amino acids was drawn, the child admitted to hospital and started on an MSUD formula with zero BCAA. The child was completely asymptomatic and feeding well. The parents were both of Nicaraguan ancestry, nonconsanguineous and there was no family history suspicious for MSUD. Twelve hours after start of diet therapy, the baby was noted to be lethargic and feeding poorly. A glucose infusion of 11 mg/kg per day and 20% intravenous lipids at 2 g/kg per day were immediately started and she did not continue to feed. An emergent cranial computed tomography (CT) was performed, which showed diffuse cerebral swelling (Fig. 1). An urgent MRI within 2 h of her deterioration showed symmetric restricted diffusion in bilateral cerebellar white matter, dorsal brainstem, thalami, globi pallidi, posterior limbs of internal capsules, and corona radiata (Fig. 1). Within 3 h, her lethargy noticeably improved and by 5–6 h had returned to baseline. Plasma BCAAs from the first venous blood sample (prior to diet therapy) showed elevations in leucine, isoleucine, valine, and alloisoleucine and confirmed the diagnosis of MSUD. The overall plasma amino acid panel, as well as urine organic acid profile, was in keeping with a classic presentation of MSUD. Review of her plasma amino acid levels showed that during the time she developed cerebral edema, her plasma leucine levels were decreasing with diet therapy. Plasma BCAA levels were all brought within acceptable levels by the next testing and oral feeding was restarted 24 h following the initial deterioration (Fig. 2). A septic workup was negative showing no alternative explanation for her decompensation. At 6 months follow-up, the baby continues to be developing normally.
Fig. 1.
Noncontrast CT of the brain (a) shows diffuse cerebral edema. The ambient cistern is effaced. Focal hypodense areas are seen bilaterally in the posterior aspect of the midbrain. Coronal diffusion-weighted images (b and c, DWI b factor = 1,000 s/mm2) show hyperintense signal in white matter of cerebellum, dorsal brainstem, thalami, globi pallidi, internal capsules, and the corona radiata. These changes are hypointense on apparent diffusion coefficient (ADC) map (not shown), which is consistent with restricted diffusion. On T2-weighted images (d and e), these areas show increased signal, although the changes can be difficult to recognize without comparison to normal subjects of the same age
Fig. 2.
Serum leucine measurements during the patient’s initial presentation. New born screen was done at 2 days of life. The patient presented at 7 days of life, while asymptomatic, and treatment was initiated immediately. Despite improvement in leucine concentration, the patient experienced a clinical deterioration 12 h later
Discussion
Cerebral edema in MSUD is thought secondary to a combination of increased cerebral BCAA/BCKA levels, and depleted cerebral levels of other essential amino acids, as well as neurotransmitter depletion (Strauss et al. 2010). With respect to the latter, mouse model experiments have shown lower CSF levels of glutamate, dopamine, and GABA, as well as lower plasma levels of tryptophan, the precursor to serotonin (Zinnanti et al. 2009). The BCKAs appear to exert effects, at least partially through disruption of pyruvate metabolism (Patel et al. 1973) and respiratory chain function (Sgaravatti et al. 2003). Nevertheless, the exact mechanism by which edema is generated remains unclear, thus designing and evaluating an effective initial management approach for a presymptomatic newborn infant with a positive newborn screen for MSUD is difficult. Our experience with this case suggests to us that dietary management alone, even if plasma leucine levels are falling, may not be sufficient to prevent clinical decompensation. Dextrose infusion may slow conversion of BCAAs to the more toxic BCKAs, a hypothesis supported by the observed clinical improvement with dextrose infusion, over a period when serum BCAA levels had actually plateaued.
We feel this case illustrates that the following actions should be considered for reported positive newborn screens for MSUD:
Plasma leucine levels should be obtained immediately in infants with a positive newborn screen for MSUD.
Treatment with a high infusion rate of dextrose and intravenous lipids, in addition to BCAA-free formula, should be initiated immediately. If the child is not in a tertiary center, IV therapy should be started immediately, and maintained during transport to a facility with appropriate dietary and molecular-testing capabilities. When leucine levels can be confirmed to have returned to the normal range, a transition to oral therapy with appropriate low BCAA diet can be made.
More data is needed on whether neuroimaging should be performed to determine whether cerebral edema is a common finding that can be prevented by early treatment or whether the results can guide therapy. If the clinical examination is not reassuring, neuroimaging can help identify evidence of cerebral edema. If an urgent MRI scan is not possible, a brain CT can be performed to evaluate the extent of cerebral swelling. For neonates with positive screen tests for MSUD, an MRI of the brain can assess the extent of brain swelling, the presence of restricted diffusion classically in the myelinated white matter, as well as the level of BCAA on proton spectroscopy (Cavalleri et al. 2002; Jan et al. 2003; Sakai et al. 2005). As most of the neonates with MSUD at presentation are lethargic, an MRI without sedation can be attempted. Close cooperation with the neuroimaging service to avoid any additional sedative or anesthesia in a child already with mild features of encephalopathy would be prudent.
Recent case reports have shown that follow up MRI with DWI may be of predictive value for the efficacy of treatment. In the reported cases, the restricted diffusion predominantly in the areas of myelinated white matter was reversible after treatment, whereas the methyl resonance of BCAAs shown on proton MR spectroscopy persisted even after the acute metabolic crisis had been controlled and the plasma leucine level had reached normal (Sener 2007; Ferraz-Filho et al. 2009).
Synopsis
Cerebral edema and clinical deterioration can occur in the initial presentation of MSUD despite improving serum leucine levels, thus initial therapy for positive newborn screens should include intravenous dextrose and fat infusions, in combination with BCAA-free formula.
Disclosures
None of the authors have any interests or relationships, financial or otherwise, that would be influenced by the findings reported in this paper.
Footnotes
Competing interests: None declared.
References
- Brismar J, Aqeel A, Brismar G, et al. Maple syrup urine disease: findings on CT and MR scans of the brain in 10 infants. AJNR Am J Neuroradiol. 1990;11:1219–1228. [PMC free article] [PubMed] [Google Scholar]
- Cavalleri F, Berardi A, Burlina AB, et al. Diffusion-weighted MRI of maple syrup urine disease encephalopathy. Neuroradiology. 2002;44:499–502. doi: 10.1007/s00234-002-0771-5. [DOI] [PubMed] [Google Scholar]
- Ferraz-Filho JR, Floriano VH, Quirici MB, et al. Contribution of the diffusion-weighted MRI in the diagnosis and follow-up of encephalopathy caused by maple syrup urine disease in a full-term newborn. Arq Neuropsiquiatr. 2009;67:719–723. doi: 10.1590/S0004-282X2009000400033. [DOI] [PubMed] [Google Scholar]
- Jan W, Zimmerman RA, Wang ZJ, et al. MR diffusion imaging and MR spectroscopy of maple syrup urine disease during acute metabolic decompensation. Neuroradiology. 2003;45:393–399. doi: 10.1007/s00234-003-1035-8. [DOI] [PubMed] [Google Scholar]
- Patel MS, Auerbach VH, Grover WD, et al. Effect of the branched-chain alpha-keto acids on pyruvate metabolism by homogenates of human brain. J Neurochem. 1973;20:1793–1796. doi: 10.1111/j.1471-4159.1973.tb00298.x. [DOI] [PubMed] [Google Scholar]
- Sakai M, Inoue Y, Oba H, et al. Age dependence of diffusion-weighted magnetic resonance imaging findings in maple syrup urine disease encephalopathy. J Comput Assist Tomogr. 2005;29:524–527. doi: 10.1097/01.rct.0000164667.65648.72. [DOI] [PubMed] [Google Scholar]
- Sener RN. Maple syrup urine disease: diffusion MRI, and proton MR spectroscopy findings. Comput Med Imaging Graph. 2007;31:106–110. doi: 10.1016/j.compmedimag.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Sgaravatti AM, Rosa RB, Schuck PF, et al. Inhibition of brain energy metabolism by the alpha-keto acids accumulating in maple syrup urine disease. Biochim Biophys Acta. 2003;1639:232–238. doi: 10.1016/j.bbadis.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Strauss KA, Wardley B, Robinson D, et al. Classical maple syrup urine disease and brain development: principles of management and formula design. Mol Genet Metab. 2010;99:333–345. doi: 10.1016/j.ymgme.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinnanti WJ, Lazovic J, Griffin K, et al. Dual mechanism of brain injury and novel treatment strategy in maple syrup urine disease. Brain. 2009;132:903–918. doi: 10.1093/brain/awp024. [DOI] [PMC free article] [PubMed] [Google Scholar]