Abstract
Mitochondrial acetoacetyl-CoA thiolase (T2) deficiency affects both isoleucine catabolism and ketone body metabolism. The disorder is characterized by intermittent ketoacidotic episodes. We report three Japanese patients. One patient (GK69) experienced two ketoacidotic episodes at the age of 9 months and 3 years, and no further episodes until the age of 25 years. She had two uncomplicated pregnancies. GK69 was a compound heterozygote of the c.431A>C (H144P) and c.1168T>C (S390P) mutations in T2 (ACAT1) gene. She was not suspected of having T2 deficiency during her childhood, but she was diagnosed as T2 deficient at the age of 25 years by enzyme assay using fibroblasts. The other two patients were identical twin siblings who presented their first ketoacidotic crisis simultaneously at the age of 3 years 4 months. One of them (GK77b) died during the first crisis and the other (GK77) survived. Even during severe crises, C5-OH and C5:1 were within normal ranges in their blood acylcarnitine profiles and trace amounts of tiglylglycine and small amounts of 2-methyl-3-hydroxybutyrate were detected in their urinary organic acid profiles. They were H144P homozygotes. This H144P mutation has retained the highest residual T2 activity in the transient expression analysis of mutant cDNA thus far, while the S390P mutation did not retain any residual T2 activity. The “mild” H144P mutation may result in subtle profiles in blood acylcarnitine and urinary organic acid analyses. T2-deficient patients with “mild” mutations have severe ketoacidotic crises but their chemical phenotypes may be subtle even during acute crises.
Introduction
Mitochondrial acetoacetyl-CoA thiolase (T2, gene symbol ACAT1) deficiency (OMIM 203750) is an autosomal recessive inborn error of metabolism that affects the catabolism of isoleucine and ketone bodies. This disorder, first described by Daum et al. (1971), is characterized by intermittent episodes of metabolic ketoacidosis associated with vomiting and unconsciousness often triggered by infections (Fukao et al. 2001). There are no clinical symptoms between episodes. Typical T2 deficiency is easily diagnosed by urinary organic acid analysis, characterized by massive excretion of tiglylglycine, 2-methyl-3-hydroxybutyrate and 2-methylacetoacetate both during ketoacidotic episodes and between episodes (Fukao et al. 2001, 2003). Diagnosis is confirmed by measurement of T2 activity on cultured skin fibroblasts (Robinson et al. 1979; Zhang et al. 2004). T2 deficiency is caused by mutations in the ACAT1 (T2) gene located on chromosome 11q22.3-q23.1 (Fukao et al. 1990; Kano et al. 1991). T2 deficiency is very heterogeneous at the genotype level, with at least 50 different mutations described (Fukao et al. 1995, 1997, 1998, 2001, 2002, 2003, 2007, 2008, 2010a, b; Wakazono et al. 1995; Nakamura et al. 2001; Zhang et al. 2004, 2006; Sakurai et al. 2007).
Some T2-deficient patients with mutations which retain some residual activity do not show typical urinary organic acid profiles (Fukao et al. 2001, 2003). We herein describe three Japanese patients with T2 deficiency whose H144P mutation retains significant residual activity. Their urinary organic acid and blood acylcarnitine profiles were atypical and subtle even during severe ketoacidotic crises.
Materials and Methods
Case Reports
GK69
This Japanese woman (GK69), born in 1984, developed severe metabolic acidosis at the age of 9 months. On admission to a third-level hospital, she was semicomatose, polypneic (48/min), and hypotonic. Laboratory values were: blood glucose 6.8 mmol/L, NH3 92 μmol/L, blood pH 7.225, pCO2 7.2 mmHg, bicarbonate 3 mmol/L, base excess −21.3, Na 153 mEq/L (normal range: 139–146), BUN 28.5 mg/dL (normal range: 10–18), and creatinine 1.1 mg/dL (normal range: 0.18–0.46). Metabolic acidosis was refractory to sodium bicarbonate therapy. Peritoneal dialysis was performed for 2 days. On the second hospital day, polypnea and unconsciousness disappeared and the blood gas data improved. Urinary organic acid analysis showed massive amounts of acetoacetate and 3-hydroxybutyrate with dicarboxylic aciduria. No increases in 2-methyl-3-hydroxybutyrate or tiglylglycine were noted, although this analysis was performed in an outside laboratory and no urine samples were available for reanalysis. At that time, T2 deficiency was excluded from differential diagnosis based on this organic acid data and the tentative diagnosis was succinyl-CoA:3-ketoacid CoA transferase (SCOT) deficiency. However, an enzyme assay for SCOT was not performed. At the age of 3 years, the patient had a similar but milder episode. Subsequently, she had no further ketoacidotic episodes. Growth and development were normal. She had two uncomplicated pregnancies.
Twin Siblings (GK77b and GK77)
GK77b is a twin Japanese boy. He was born at 36 weeks gestation weighing 2,400 g. His parents had no known consanguinity but both were from a small island in Amami islands in Japan. He experienced several febrile illnesses without ketoacidosis. However, at 3 years 4 months of age, after a 3-day history of fever, cough, and vomiting, he developed anorexia, lethargy, and polypnea. He was admitted to a local hospital. His blood glucose level was 2.3 mmol/L. Blood gas analysis was not performed. Hypoglycemia was corrected with intravenous glucose injection of 20 ml of 20% glucose solution followed by continuous infusion of a 2.6% glucose solution. About 30 h after admission, his condition worsened. Blood gas analysis revealed severe metabolic acidosis showed pH 6.88, pCO2 6.1 mmHg, and bicarbonate 1.1 mmol/L. He was transferred to a regional hospital. On arrival at the hospital, he was unconscious with a heart rate of 168/min and respiratory rate of 39/min. Blood laboratory data were: WBC 19,050/μL, CRP 0.2 mg/dL (normal values: <0.15), BUN 36.2 mg/dL (normal range: 10–18) creatinine 0.5 mg/dL (normal range: 0.25–0.49), NH3 33.5 μmol/L, glucose 3.8 mmol/L, pH 7.17, pCO2 20 mmHg, bicarbonate 6.3 mmol/L, base excess −22.4 mmol/L, and total ketone bodies 16.3 mmol/L. He received continuous infusion of 5% glucose solution at 3.4 mg/kg/min and sodium bicarbonate at 0.4–0.47 mEq/kg/h. However, unconsciousness and metabolic acidosis did not improve. On the fifth hospital day, he died before being transferred to a third-level hospital.
GK77 is the twin brother of GK77b. Pyloric stenosis was diagnosed at the age of 1 month and corrected surgically; thereafter, he was well until 3 years 4 months of age. Two days after the onset of his twin brother, he developed frequent repeated vomiting after cough and nasal discharge. Therefore, he was admitted to the regional hospital at the same time as his twin. On admission, he was lethargic. Laboratory findings were: WBC 7,760/μL, CRP 0.5 mg/dL (normal values: <0.15), BUN 20.2 mg/dL (normal range: 10–18), creatinine 0.4 mg/dL (normal range: 0.25–0.49), glucose 3.7 mmol/L, NH3 25 μmol/L, blood pH 7.135, pCO2 19.5 mmHg, bicarbonate 6.3 mmol/L, base excess −22.4 mmol/L, and total ketone bodies 10.1 mmol/L. He received a continuous infusion of 5% glucose solution at 3.4 mg/kg/min and sodium bicarbonate at 0.3 mEq/kg/h. On the third hospital day, his condition worsened and he was transferred to a third-level hospital. On admission, the blood gasses were pH 7.372, pCO2 21.6 mmHg, bicarbonate 12.2 mmol/L, and base excess −11.2 mmol/L. A glucose infusion rate was further increased to 6.5 mg/kg/min with 10% glucose solution. Acidosis normalized with 9 h (pH 7.399, bicarbonate 21.7 mmol/L, base excess −2.6 mmol/L). Two days later, the urinary ketones became negative and he started eating.
GK77 is now 4 years 8 months and has experienced no further ketoacidotic episodes. The family has been advised to avoid fasting and to come to the local hospital if he has a high fever or appetite loss. His growth and development are within normal ranges.
Urinary Organic Acid Analysis and Acylcarnitine Analysis
Urine samples containing 0.2 mg of creatinine were used for our high risk screening of organic acids. As internal standards, 20 mg each of tropate (TA, C9), margarate (MGA, C17), and tetracosane (C24) were added to these samples. Trimethylsilylated samples were analyzed using capillary gas chromatography-mass spectrometry (QP 5050A, Shimadzu Co. Ltd., Kyoto, Japan), as described earlier (Kimura et al. 1999). The values of organic acids were expressed as the peak area (%) relative to IS-1 (margarate) on the mass chromatogram. Quantification of 2-methyl-3-hydroxybutyrate and tiglylglycine in urine samples from GK77b and GK77 was kindly done by Dr. Sass (Freiburg University) (Lehnert 1994). For comparison, quantification was also done in urine samples from T2-deficient patients whose urinary screening profiles had typical T2 deficient ones. We used urine sample in stable condition from GK01 who is a compound heterozygote of A333P and c.149delC (Fukao et al. 1998) and samples in acute and stable conditions from T2-deficient patients from India (GK(Ind)) in our high-risk screening. Blood spot and serum acylcarnitine analysis using tandem mass spectrometry was also done, as described (Kobayashi et al. 2007), and blood spot samples from GK75 and GK79, who are R208X homozygotes (Fukao et al. 2010b) were used as positive controls.
Enzyme Assay and Immunoblot Analysis Using Fibroblasts
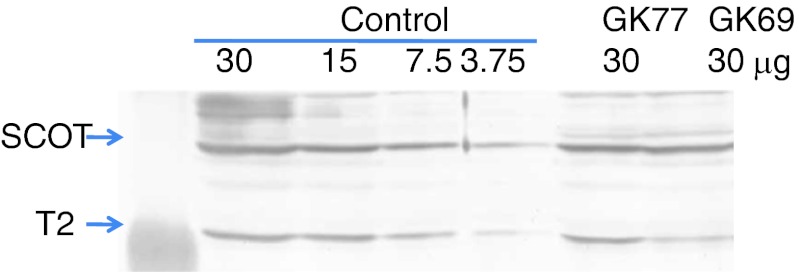
Control and patients’ fibroblasts were cultured in Eagle’s minimum essential medium containing 10% fetal calf serum. Acetoacetyl-CoA thiolase activity was assayed, as described (Robinson et al. 1979; Zhang et al. 2004). We assayed acetoacetyl-CoA thiolase activity in the presence and absence of potassium-ion, since T2 is the only thiolase which is activated by the ion. Immunoblot analysis was done, as described (Fukao et al. 1997). In the cases of the controls, twofold serial dilution samples from 30 to 3.75 μg were electrophoresed together with samples (30 μg) of GK68 and GK77 to determine the amount of T2 protein in the patients’ fibroblasts relative to that in the control fibroblasts.
Mutation Analysis
This study was approved by the Ethical Committee of the Graduate School of Medicine, Gifu University. Genomic DNA was extracted from fibroblasts using a SepaGene kit (Sanko Junyaku, Tokyo, Japan). Mutation screening was performed by PCR and direct sequencing of genomic fragments that included each exon and its surrounding intron sequences (Fukao et al. 1998). For GK77b and the parents, exon 5 was amplified from a dried blood spot 1.25 mm in diameter, which was used for tandem mass spectrometry, using Amplidirect Plus (Shimadzu Biotech, Tsukuba, Japan).
Restriction Enzyme Assay to Detect c.431A>C (H144P)
The c.431A>C (H144P) mutation creates a new BmgT120 I site (GGACC). DNAs from 110 Japanese controls were examined using a restriction enzyme assay, as follows.
A fragment (314 bp), including exon 5 and its surrounding introns, was amplified using the following primers:
In4 as (in intron, −69 to −48)5′-CATGCTCTATTAAGTTCTGCAG-3′
In5 as (in intron, +137 to +119) 5′-ATCCAGACACTCTTGAGCA-3′
An aliquot of the resulting amplicon was digested with BmgT120 I, then resolved on a 5% polyacrylamide gel. The c.431A fragment (wild-type) is 314-bp long and the c.421C fragment is cut into 162-bp and 152-bp fragments.
Transient Expression Analysis of Mutant cDNAs
Transient expression of T2 cDNAs was performed using a pCAGGS eukaryote expression vector (Niwa et al. 1991), as described (Sakurai et al. 2007). After transfection, cells were cultured at 37°C or 40°C for 48 h, then harvested and kept at −80°C until use. Cells were freeze-thawed and sonicated in 50 mM sodium phosphate (pH 8.0) and 0.1% Triton X-100. After centrifugation at 10,000 × g for 10 min, the supernatant was used in an enzyme assay for acetoacetyl-CoA thiolase activity and for immunoblot analysis.
Results and Discussion
Confirmation of the Diagnosis
GK69’s fibroblasts were assayed for SCOT activity to confirm the diagnosis in 2008, when GK69 was 24 years old. As shown in Table 1, she was diagnosed as having T2 deficiency but not as having SCOT deficiency.
Table 1.
Acetoacetyl-CoA thiolase activities in the absence and presence of potassium ions
| Fibroblasts | Acetoacetyl-CoA thiolase activity | SCOT activity | ||
|---|---|---|---|---|
| −K+ | +K+ | +K+/−K+ | ||
| Controls (n = 5) | 5.0 ± 0.7 | 10.8 ± 0.9 | 2.2 ± 0.3 | 6.7 ± 2.1 |
| GK69 | 3.6 ± 0.5 | 4.1 ± 0.9 | 1.2 ± 0.1 | 4.7 ± 1.4 |
| GK77 | 4.2 ± 0.3 | 5.8 ± 1.5 | 1.4 ± 0.3 | 3.9 ± 0.5 |
| T2D | 4.5 ± 1.4 | 4.7 ± 1.6 | 1.0 ± 0.1 | 5.6 ± 0.5 |
Enzyme activity is expressed as nmol/min/mg of protein. In cases of patients, enzyme assay was done three times and shows average ± SD. T2D, A disease control
SCOT deficiency was first suspected in GK77 and GK77b, based on the following facts (1) Two of the four SCOT deficient Japanese families were from the Amami islands, the population of which is about 120,000. They were T435N homozygotes (Fukao et al. 2004). (2) The acylcarnitine profiles and urinary organic acid analysis during acute ketoacidotic crisis in both patients had no typical profile for T2 deficiency, as discussed below. As shown in Table 1, GK69’s and GK77’s fibroblasts had normal SCOT activity and a higher ratio (1.3) of acetoacetyl-CoA thiolase activity in the presence to the absence of potassium ions than typical T2-deficient fibroblasts (the ratio was around 1.0). Immunoblot analysis also showed a clearly detectable amount of T2 protein in GK77’s fibroblasts, and a lower amount in GK69’s fibroblasts. Densitometric analysis showed that the amounts of T2 protein in GK77 and GK69 were estimated to be 50% and 25% of control, respectively (Fig. 1).
Fig. 1.
Immunoblot analysis. In the cases of the controls, serial twofold dilutions from 30 to 3.75 μg were studied together with samples (30 μg) from GK68 and GK77. The first antibody was a mixture of an anti-T2 antibody and an anti-SCOT antibody. The positions of the bands for T2 and SCOT are indicated by arrows
Mutations and Their Effects on T2 Protein
Mutation screening revealed that GK69 was a compound heterozygote of c.431A>C (H144P) and c.1168T>C (S390P). Her mother had S390P heterozygously but did not have H144P. The father’s DNA was not available for analysis. GK77 had an H144P mutation homozygously, shown by mutation screening at the genomic level. Their parents and GK77b were heterozygous carriers and a homozygote of H144P, respectively. The c.431A>C (H144P) mutation creates a BmgT120I site (GGACA to GGACC). We could not find c.431A>C (H144P) in the 110 Japanese controls using the restriction enzyme assay with BmgT120I.
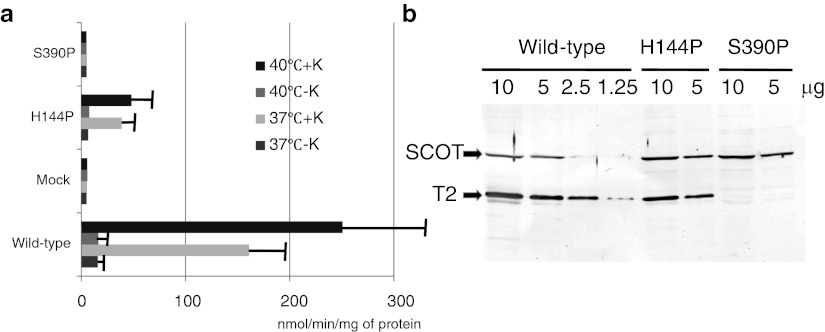
We performed transient expression analysis of wild-type and mutant cDNAs in T2-deficient SV40-transformed fibroblasts. Following expression of T2 cDNAs for 48 h at 37°C, an enzyme assay and immunoblots were performed (Fig. 2a,b). The transfection of wild-type T2 cDNA produced high potassium ion-activated acetoacetyl-CoA thiolase activity (T2 activity), whereas that of mock cDNA produced no demonstrable enzyme activity at any temperature. The H144P mutant retained a residual T2 activity of ~25% of the wild-type value (Fig. 2a). The S390P mutant did not retain any residual T2 activity. In immunoblot analysis (Fig. 2b), the H144P mutant protein was detected, whereas no S390P protein was detected. The relative amount of the H144P mutant protein, as compared to the wild-type, was estimated to be 50%. Hence, the specific activity (unit/mg of T2 protein) of the H144P mutant protein was estimated to be about 50% of the wild type. Protein-folding and post-folding stability is predicted to vary with the incubation temperature. Hence, we also performed transient expression at 40°C for 48 h. The H144P mutant in expression at 40°C had a similar level of residual activity to that at 37°C.
Fig. 2.
Transient expression analysis of H144P and S390P mutant cDNAs. Transient expression analysis was performed at 40°C and 37°C. (a) Potassium ion-activated acetoacetyl-CoA thiolase assay. Acetoacetyl-CoA thiolase activity in the supernatant of the cell extract was measured. The mean values of acetoacetyl-CoA thiolase activity in the absence (−K) and presence (+K) of potassium ions are shown together with the SD of three independent experiments. (b) Immunoblot analysis. The protein amounts applied are indicated above the lanes. The first antibody was a mixture of an anti-T2 antibody and an anti-SCOT antibody. The positions of the bands for T2 and SCOT are indicated by arrows
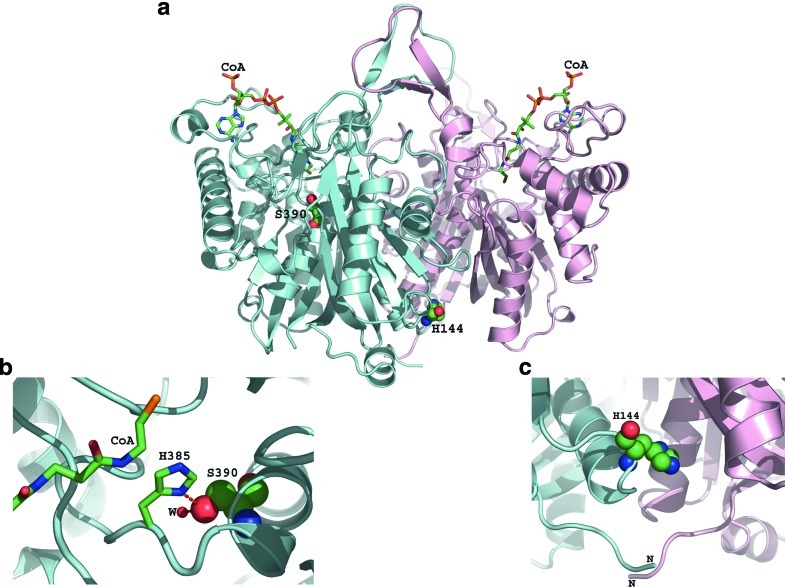
We reported the tertiary structure of the human T2 tetramer (Haapalainen et al. 2007). Figure 3a shows the positions of the H144P and S390P mutations on the dimer. As seen in the figure, S390 is close to the active site and H144 is at the dimer interface close to the surface of the protein. Figure 3b shows a zoomed-in view around S390. This mutant is located at the active site. S390 is hydrogen-bonded to catalytic histidine, H385; it could be that this serine is needed to orient histidine in a way that the histidine can stabilize the transient negative charge of the substrate optimally. S390 is also hydrogen-bonded to a water molecule that is needed in stabilizing parts of the enzyme. So, if S390 is mutated into proline, these two hydrogen bonds do not exist. Hence, this S390P is expected to bring about a serious change in T2 catalytic cavity. In our expression analysis, this S390P was also too unstable to detect mutant protein. Figure 3c shows a zoomed-in view at the dimer interface. H144 is interacting with the residues of the neighboring subunit. If this residue is mutated into Pro, there is less dimeric interaction, which in turn might destabilize the overall structure. Since this residue is far from the active site and substrate binding site, it is difficult to explain why this H144P mutant had reduced specific activity in transient expression analysis from the viewpoint of structural analysis.
Fig. 3.
The positions of H144P and S390P on the tertiary structure of human T2 dimers with substrates of coenzyme A
Urinary Organic Acid Analysis
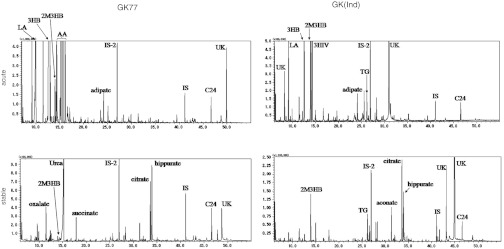
GK69 was first suspected to having T2 deficiency as a probable diagnosis; however, urinary organic acid analysis at the first ketoacidotic crisis indicated no characteristic profile for T2 deficiency such as elevated 2-methyl-3-hydroxybutyrate and tiglylglycine in 1985 (no data was available). The results of the urinary organic acid analysis of our patients are shown in comparison with those of typical T2-deficient patients, GK01 and GK(Ind) (Table 2, Fig. 4). At the age of 24 years when her condition was stable, GK69’s urinary organic acid analysis showed that there were only trace amounts of 2-methyl-3-hydroxybutyrate and tiglylglycine (Table 2). In our screening, this low level of tiglylglycine was difficult to detect. Urinary organic acid analysis during the acute crises of GK77 and GK77b showed huge amounts of 3-hydroxybutyrate and acetoacetate with elevated 2-methyl-3-hydroxybutyrate but only trace amounts of tiglylglycine. The levels of 2-methyl-3-hydroxybutyrate and tiglylglycine during a stable condition in GK77 are similar with those in GK69.
Table 2.
Quantitative analysis of urinary organic acid analysis during acute crises and stable conditions
| Patients | Acute crises | Stable conditions | ||
|---|---|---|---|---|
| 2M3HB | Tiglylglycine | 2M3HB | Tiglylglycine | |
| GK69 | NA | NA | 14.0 | 13.3 |
| GK77b | 405.7 | 45.8 | NA | NA |
| GK77 | 160.2 | 6.7 | 27.3 | 14.8 |
| GK01 | NA | NA | 399.1 | 732.1 |
| GK(Ind) | 484.6 | 503.9 | 195.1 | 797.6 |
| Controls (n = 42) | 10.7 ± 7.6 | 24.6 ± 14.6 | ||
Values are expressed as mmol/mol creatinine
NA means that samples were not available for the analysis. GK01 is a compound heterozygote of c.149delC and A333P, which retained no residual activity (Fukao et al. 1998). GK(Ind) indicates a patient with typical T2-deficient profiles of urinary organic acids in our screening
Fig. 4.
Urinary organic acid profiles of GK77 during the acute episode and an asymptomatic period in comparison with those of a typical T2-deficient patient (GK(Ind)). LA Lactate, 3HB 3-OH-butyrate, 3HIV 3-OH-isovalerate, AA Acetoacetate, 2M3HB 2-Methyl-3-OH-butyrate, TG Tiglylglycine, IS-2 and IS Internal standards, UK Unknown. Since acetoacetate is unstable and samples from GK(Ind) were shipped on filter papers after thoroughly drying, the levels of acetoacetate are likely underestimated
In cases of typical T2-deficient patients, it is easy to suspect T2 deficiency based on large amounts of 2-methyl-3-hydroxybutyrate and tiglylglycine as shown in Fig. 4. However, even in cases of trace amounts of tiglylglycine (possibly under the detection limit), T2 deficiency cannot be excluded. An H144P mutation, which retained high residual activity, may contribute to atypical profiles in the presented cases. These findings strengthen our previous observations that some T2-deficient patients with mutations, which retain some residual activity do not show typical urinary organic acid profiles (Fukao et al. 2001, 2003).
Blood and Serum Acylcarnitine Analyses
Acylcarnitine analysis was done using samples during the acute crises of GK77 and GK77b. Table 3 shows the results in comparison with those of typical T2-deficient patients (R208X homozygotes) (Fukao et al. 2010b). C5:1 and C5OH elevation in blood spots, characteristic for T2 deficiency, was clearly detected in the samples from the typical T2-deficient patients but was absent in samples from GK77 and GK77b. We previously reported that the abnormality of the acylcarnitine profiles in T2-deficient patients with mutations which retain some residual activity is subtle during nonepisodic conditions (Fukao et al. 2003), but the present study clearly showed that it could be also subtle even during severe ketoacidotic episodes. This means that acylcarnitine analysis using blood spots cannot detect some T2-deficient patients like GK77 and GK77b. Serum acylcarnitine analysis might detect elevation of these compounds to some extent, but we need to analyze more cases to clarify the usefulness of serum acylcarnitine analysis in such T2-deficient patients with mutations which retain some residual activity.
Table 3.
C5-OH and C5:1 carnitines in blood filters and serum samples from GK77 and GK77b during acute crises
| Patients | Dried blood spots | Serum | ||
|---|---|---|---|---|
| C5:1 | C5-OH | C5:1 | C5-OH | |
| GK77b | 0.027 | 0.11 | ND | 0.12 |
| GK77 | 0.012 | 0.11 | 0.044 | 0.10 |
| R208X homozygotes | ||||
| GK75 (acute) | 0.89 | 2.89 | NA | NA |
| GK79 (stable) | 1.20 | 2.35 | NA | NA |
| Controls (n = 30) | ||||
| Average ± SD | 0.015 ± 0.016 | 0.26 ± 0.15 | 0.015 ± 0.013 | 0.059 ± 0.024 |
ND not detected, NA not applicable
The values are expressed as μmol/L
GK75 and GK79 are positive controls for T2 deficient patients who are R208X homozygotes (Fukao et al. 2010b)
T2 deficiency cannot be excluded even if acylcarnitine profiles during acute episodes are within normal ranges. Careful evaluation of urinary organic acids, especially for the presence of 2-methyl-3-hydroxybutyrate, is necessary not to overlook T2 deficiency.
Clinical Issues
Since they were confirmed as identical twins by DNA analysis (data not shown), their genetic backgrounds were identical and most environmental factors were also very similar between them. One died during the ketoacidotic crisis and the other survived.
In Japan, intravenous infusion therapy for vomiting, appetite loss, and dehydration is commonly performed with commercially available initial infusion solution, such as Solita T1 (2.6% glucose) followed by maintenance solution, such as Solita T2 and T3 (4.3% glucose). These solutions are effective for physiological ketosis. However, in the case of T2 deficiency, a higher concentration of glucose may be necessary. Accordingly, we had the impression that GK77 became much better after the glucose concentration was changed from 5% to 10%. In the case of prolonged ketoacidosis, consideration should be given to increasing the infusion rate of glucose to ensure high normal blood glucose level to suppress ketone body synthesis and isoleucine catabolism via insulin secretion.
Acknowledgments
We thank professor Jörn Oliver Sass (Freiburg Univ) for quantification of urinary 2-methyl-3-hydroxybutyrate and tiglylglycine, Drs Hironori Kobayashi and Yuichi Mushimoto (Shimane University) for urinary organic acid analysis and tandem mass analysis, Dr Tamayo Ishikawa (Kagoshima University) for patients’ care, and Ms Keiko Murase and Ms Naomi Sakaguchi (Gifu University) for technical assistance. We also thank Paul Langman, PhD for his assistance with scientific English usage.
This study was in part supported by Health and Labor Science Research Grants for Research on Intractable Diseases and for Research on Children and Families from The Ministry of Health, Labor and Welfare of Japan and by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan.
Abbreviations
- SCOT
Succinyl-CoA:3-ketoacid CoA transferase
- T2
Mitochondrial acetoacetyl-CoA thiolase
Concise One-Sentence Take-Home Message
Patients with beta-ketothiolase deficiency having a mutation which retains some residual activity showed subtle abnormality in urinary organic acid analysis and blood acylcarnitine analysis even during acute ketoacidotic episodes.
Details of the Contributions of Individual Authors
Toshiyuki Fukao and Naomi Kondo performed the enzyme assays, immunoblot/mutation analysis, and expression analysis of cDNAs. Toshiyuki Fukao mainly wrote this manuscript. Shinsuke Maruyama, Toshihiro Ohura, Mitsuo Toyoshima, Naomi Kuwada, and Mari Imamura are the physicians responsible for the patients. Yuki Hasegawa and Seiji Yamaguchi performed gas chromatography-mass spectrometry and tandem mass spectrometry analyses and first suspected the disorder. Isao Yuasa confirmed GK77 and 77b as identical twins by DNA analyses. Antti M Haapalainen and Rik K Wierenga analyzed the tertiary structural effects of the mutations.
References to Electronic Databases
Alpha-methylacetoacetic acidura, mitochondrial acetoacetyl-CoA thiolase deficiency (OMIM 203750, 607809)
Mitochondrial acetoacetyl-CoA thiolase, acetyl-CoA acetyltransferase 1 (EC 2.3.1.9)
ACAT1 gene (gene ID 38, NM_000019.3)
Details of Funding
This study was in part supported by Health and Labor Science Research Grants for Research on Intractable Diseases and Research on Children and Families from the Ministry of Health, Labor and Welfare of Japan and by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan
Details of Ethics Approval
This study has been approved by the Ethical Committee of the Graduate School of Medicine, Gifu University.
Footnotes
Competing interests: None declared.
References
- Daum RS, Lamm PH, Mamer OA, Scriver CR. A “new” disorder of isoleucine catabolism. Lancet. 1971;2:1289–1290. doi: 10.1016/S0140-6736(71)90605-2. [DOI] [PubMed] [Google Scholar]
- Fukao T, Yamaguchi S, Kano M, et al. Molecular cloning and sequence of the complementary DNA encoding human mitochondrial acetoacetyl-coenzyme A thiolase and study of the variant enzymes in cultured fibroblasts from patients with 3-ketothiolase deficiency. J Clin Invest. 1990;86:2086–2092. doi: 10.1172/JCI114946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao T, Yamaguchi S, Orii T, Hashimoto T. Molecular basis of beta-ketothiolase deficiency: mutations and polymorphisms in the human mitochondrial acetoacetyl-coenzyme A thiolase gene. Hum Mutat. 1995;5:113–120. doi: 10.1002/humu.1380050203. [DOI] [PubMed] [Google Scholar]
- Fukao T, Song XQ, Mitchell GA, et al. Enzymes of ketone body utilization in human tissues: protein and messenger RNA levels of succinyl-coenzyme A (CoA):3-ketoacid CoA transferase and mitochondrial and cytosolic acetoacetyl-CoA thiolases. Pediatr Res. 1997;42:498–502. doi: 10.1203/00006450-199710000-00013. [DOI] [PubMed] [Google Scholar]
- Fukao T, Nakamura H, Song XQ, et al. Characterization of N93S, I312T, and A333P missense mutations in two Japanese families with mitochondrial acetoacetyl-CoA thiolase deficiency. Hum Mutat. 1998;12:245–254. doi: 10.1002/(SICI)1098-1004(1998)12:4<245::AID-HUMU5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Fukao T, Scriver CR, Kondo N. The clinical phenotype and outcome of mitochondrial acetoacetyl-CoA thiolase deficiency (beta-ketothiolase or T2 deficiency) in 26 enzymatically proved and mutation-defined patients. Mol Genet Metab. 2001;72:109–114. doi: 10.1006/mgme.2000.3113. [DOI] [PubMed] [Google Scholar]
- Fukao T, Nakamura H, Nakamura K, et al. Characterization of six mutations in five Spanish patients with mitochondrial acetoacetyl-CoA thiolase deficiency: effects of amino acid substitutions on tertiary structure. Mol Genet Metab. 2002;75:235–243. doi: 10.1006/mgme.2001.3288. [DOI] [PubMed] [Google Scholar]
- Fukao T, Zhang GX, Sakura N, et al. The mitochondrial acetoacetyl-CoA thiolase (T2) deficiency in Japanese patients: urinary organic acid and blood acylcarnitine profiles under stable conditions have subtle abnormalities in T2-deficient patients with some residual T2 activity. J Inherit Metab Dis. 2003;26:423–431. doi: 10.1023/A:1025117226051. [DOI] [PubMed] [Google Scholar]
- Fukao T, Shintaku H, Kusubae R, et al. Patients homozygous for the T435N mutation of succinyl-CoA:3-ketoacid CoA Transferase (SCOT) do not show permanent ketosis. Pediatr Res. 2004;56:858–863. doi: 10.1203/01.PDR.0000145297.90577.67. [DOI] [PubMed] [Google Scholar]
- Fukao T, Zhang G, Rolland MO, et al. Identification of an Alu-mediated tandem duplication of exons 8 and 9 in a patient with mitochondrial acetoacetyl-CoA thiolase (T2) deficiency. Mol Genet Metab. 2007;92:375–378. doi: 10.1016/j.ymgme.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Fukao T, Boneh A, Aoki Y, Kondo N. A novel single-base substitution (c.1124A > G) that activates a 5-base upstream cryptic splice donor site within exon 11 in the human mitochondrial acetoacetyl-CoA thiolase gene. Mol Genet Metab. 2008;94:417–421. doi: 10.1016/j.ymgme.2008.04.014. [DOI] [PubMed] [Google Scholar]
- Fukao T, Horikawa R, Naiki Y, et al. A novel mutation (c.951 C > T) in an exonic splicing enhancer results in exon 10 skipping in the human mitochondrial acetoacetyl-CoA thiolase gene. Mol Genet Metab. 2010;100:339–344. doi: 10.1016/j.ymgme.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Fukao T, Nguyen HT, Nguyen NT, et al. A common mutation, R208X, identified in Vietnamese patients with mitochondrial acetoacetyl-CoA thiolase (T2) deficiency. Mol Genet Metab. 2010;100:37–41. doi: 10.1016/j.ymgme.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Haapalainen AM, Merilainen G, Pirila PL, Kondo N, Fukao T, Wierenga RK. Crystallographic and kinetic studies of human mitochondrial acetoacetyl-CoA thiolase: the importance of potassium and chloride ions for its structure and function. Biochemistry. 2007;46:4305–4321. doi: 10.1021/bi6026192. [DOI] [PubMed] [Google Scholar]
- Kano M, Fukao T, Yamaguchi S, Orii T, Osumi T, Hashimoto T. Structure and expression of the human mitochondrial acetoacetyl-CoA thiolase-encoding gene. Gene. 1991;109:285–290. doi: 10.1016/0378-1119(91)90623-J. [DOI] [PubMed] [Google Scholar]
- Kimura M, Yamamoto T, Yamaguchi S. Automated metabolic profiling and interpretation of GC/MS data for organic acidemia screening: a personal computer-based system. Tohoku J Exp Med. 1999;188:317–334. doi: 10.1620/tjem.188.317. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Hasegawa Y, Endo M, Purevsuren J, Yamaguchi S. A retrospective ESI-MS/MS analysis of newborn blood spots from 18 symptomatic patients with organic acid and fatty acid oxidation disorders diagnosed either in infancy or in childhood. J Inherit Metab Dis. 2007;30:606. doi: 10.1007/s10545-007-0642-7. [DOI] [PubMed] [Google Scholar]
- Lehnert W. Long-term results of selective screening for inborn errors of metabolism. Eur J Pediatr. 1994;153:S9–S13. doi: 10.1007/BF02138770. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Fukao T, Perez-Cerda C, et al. A novel single-base substitution (380 C > T) that activates a 5-base downstream cryptic splice-acceptor site within exon 5 in almost all transcripts in the human mitochondrial acetoacetyl-CoA thiolase gene. Mol Genet Metab. 2001;72:115–121. doi: 10.1006/mgme.2000.3125. [DOI] [PubMed] [Google Scholar]
- Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-D. [DOI] [PubMed] [Google Scholar]
- Robinson BH, Sherwood WG, Taylor J, Balfe JW, Mamer OA. Acetoacetyl CoA thiolase deficiency: a cause of severe ketoacidosis in infancy simulating salicylism. J Pediatr. 1979;95:228–233. doi: 10.1016/S0022-3476(79)80658-7. [DOI] [PubMed] [Google Scholar]
- Sakurai S, Fukao T, Haapalainen AM, et al. Kinetic and expression analyses of seven novel mutations in mitochondrial acetoacetyl-CoA thiolase (T2): identification of a Km mutant and an analysis of the mutational sites in the structure. Mol Genet Metab. 2007;90:370–378. doi: 10.1016/j.ymgme.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Wakazono A, Fukao T, Yamaguchi S, et al. Molecular, biochemical, and clinical characterization of mitochondrial acetoacetyl-coenzyme A thiolase deficiency in two further patients. Hum Mutat. 1995;5:34–42. doi: 10.1002/humu.1380050105. [DOI] [PubMed] [Google Scholar]
- Zhang GX, Fukao T, Rolland MO, et al. Mitochondrial acetoacetyl-CoA thiolase (T2) deficiency: T2-deficient patients with “mild” mutation(s) were previously misinterpreted as normal by the coupled assay with tiglyl-CoA. Pediatr Res. 2004;56:60–64. doi: 10.1203/01.PDR.0000129657.48122.52. [DOI] [PubMed] [Google Scholar]
- Zhang G, Fukao T, Sakurai S, Yamada K, Michael Gibson K, Kondo N. Identification of Alu-mediated, large deletion-spanning exons 2–4 in a patient with mitochondrial acetoacetyl-CoA thiolase deficiency. Mol Genet Metab. 2006;89:222–226. doi: 10.1016/j.ymgme.2006.06.010. [DOI] [PubMed] [Google Scholar]