Abstract
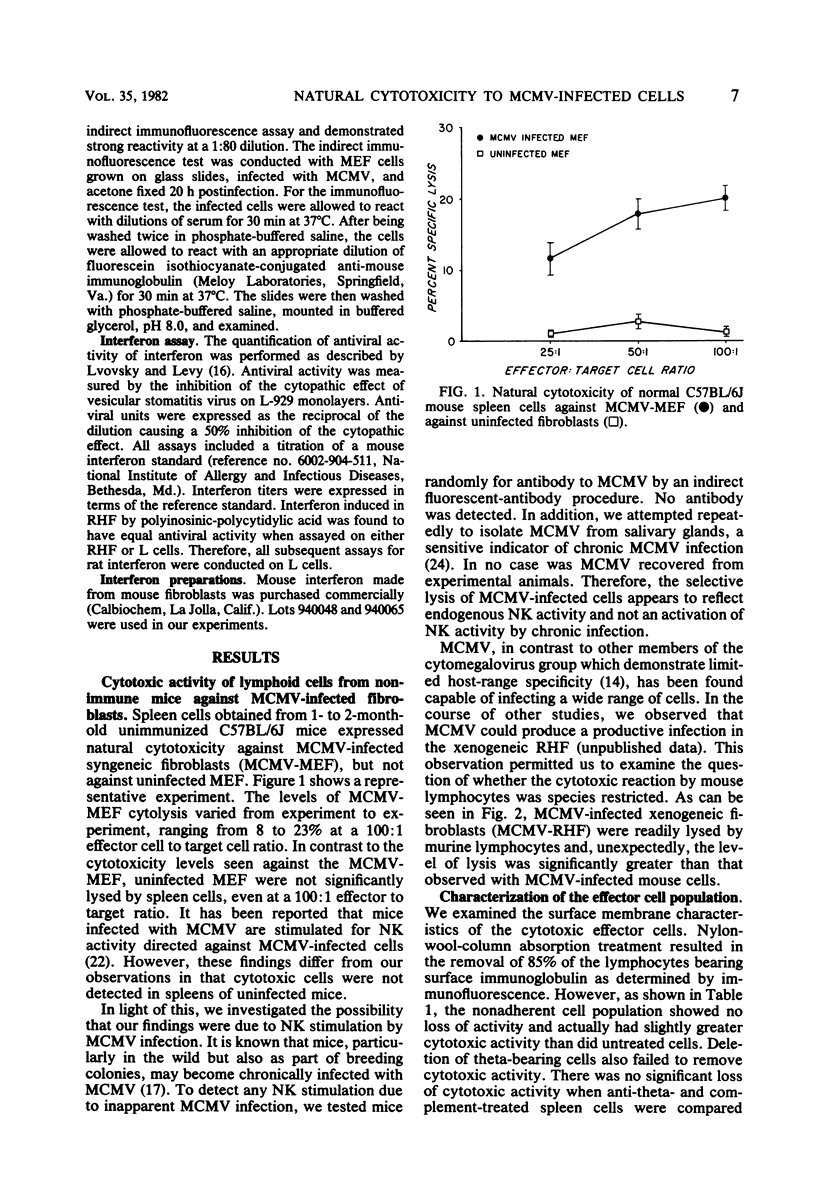
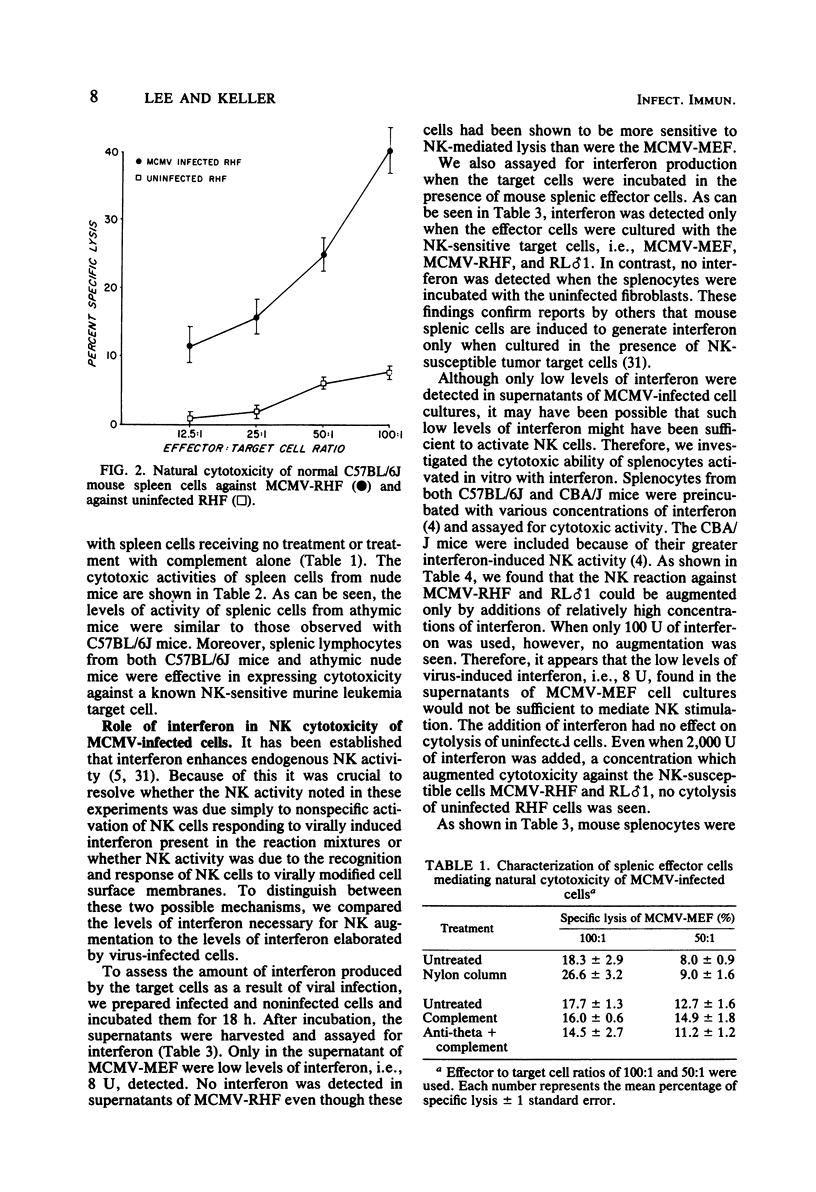
Lymphoid cells from unstimulated normal C57BL/6J mice were shown to lyse murine cytomegalovirus (MCMV)-infected syngeneic mouse embryo fibroblasts but not uninfected mouse embryo fibroblasts. This cytotoxicity by mouse effector cells was not restricted to MCMV-infected syngeneic cells since MCMV-infected xenogeneic rat heart fibroblasts were also lysed. Characterization of the effector cells mediating this cytotoxicity against MCMV-infected cells indicated that the effector cells are similar to described natural killer (NK) cells mediating lysis of tumor cells and virus-infected cells. Because of the described augmentation of NK activity by interferon, we examined the role of interferon in the NK reaction. Although low levels of virus-induced interferon were detectable in supernatants of MCMV-infected mouse embryo fibroblasts, no interferon was detectable in supernatants of MCMV-infected rat heart fibroblasts, a target significantly more sensitive to NK cytolysis than infected mouse embryo fibroblasts. We were able to augment the NK reaction against MCMV-infected cells by in vitro treatments with interferon. However, the amounts of interferon required for augmentation were significantly greater than the amounts generated by infected target cells. In vitro interferon-stimulated NK cells retained selective cytotoxic activity since they continued to remain incapable of lysing uninfected target cells. MCMV-infected rat heart fibroblasts induced more interferon and were also more susceptible to NK activity than MCMV-infected mouse embryo fibroblasts. In spite of this difference in interferon-inducing capacity, there was no augmentation of cytotoxicity of MCMV-infected mouse embryo fibroblasts when mouse splenocytes were cocultivated with both target cells. Finally, when production of interferon in the NK reaction was inhibited by the addition of actinomycin D, no reduction of NK activity was seen. Our findings suggest that native mouse NK cells can discriminate between MCMV-infected cells and uninfected cells, this ability leading to the selective lysis of the virus-infected cells. Furthermore, although we could demonstrate augmentation of NK activity by interferon, interferon activation of NK cells may not be a necessary precondition for the development of endogenous NK activity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. J. Innate cytotoxicity of CBA mouse spleen cells to Sendai virus-infected L cells. Infect Immun. 1978 Jun;20(3):608–612. doi: 10.1128/iai.20.3.608-612.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Diamond R. D., Keller R., Lee G., Finkel D. Lysis of cytomegalovirus-infected human fibroblasts and transformed human cells by peripheral blood lymphoid cells from normal human donors. Proc Soc Exp Biol Med. 1977 Feb;154(2):259–263. doi: 10.3181/00379727-154-39650. [DOI] [PubMed] [Google Scholar]
- Djeu J. Y., Heinbaugh J. A., Holden H. T., Herberman R. B. Augmentation of mouse natural killer cell activity by interferon and interferon inducers. J Immunol. 1979 Jan;122(1):175–181. [PubMed] [Google Scholar]
- Gidlund M., Orn A., Wigzell H., Senik A., Gresser I. Enhanced NK cell activity in mice injected with interferon and interferon inducers. Nature. 1978 Jun 29;273(5665):759–761. doi: 10.1038/273759a0. [DOI] [PubMed] [Google Scholar]
- Haller O., Kiessling R., Orn A., Kärre K., Nilsson K., Wigzell H. Natural cytotoxicity to human leukemia mediated by mouse non-T cells. Int J Cancer. 1977 Jul 15;20(1):93–103. doi: 10.1002/ijc.2910200116. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Holden H. T., Lavrin D. H. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int J Cancer. 1975 Aug 15;16(2):230–239. doi: 10.1002/ijc.2910160205. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Holden H. T. Low density of Thy 1 antigen on mouse effector cells mediating natural cytotoxicity against tumor cells. J Immunol. 1978 Jul;121(1):304–309. [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Lavrin D. H. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity. Int J Cancer. 1975 Aug 15;16(2):216–229. doi: 10.1002/ijc.2910160204. [DOI] [PubMed] [Google Scholar]
- Jondal M., Pross H. Surface markers on human b and t lymphocytes. VI. Cytotoxicity against cell lines as a functional marker for lymphocyte subpopulations. Int J Cancer. 1975 Apr 15;15(4):596–605. doi: 10.1002/ijc.2910150409. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kiessling R., Klein E., Wigzell H. "Natural" killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975 Feb;5(2):112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- Kim K. S., Carp R. I. Growth of murine cytomegalovirus in various cell lines. J Virol. 1971 Jun;7(6):720–725. doi: 10.1128/jvi.7.6.720-725.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVY H. B., AXELROD D., BARON S. MESSENGER RNA FOR INTERFERON PRODUCTION. Proc Soc Exp Biol Med. 1965 Feb;118:384–385. doi: 10.3181/00379727-118-29850. [DOI] [PubMed] [Google Scholar]
- Lvovsky E., Levy H. B. Interferon assay of high sensitivity. Proc Soc Exp Biol Med. 1976 Dec;153(3):511–513. doi: 10.3181/00379727-153-39580. [DOI] [PubMed] [Google Scholar]
- MANNINI A., MEDEARIS D. N., Jr Mouse salivary gland virus infections. Am J Hyg. 1961 May;73:329–343. doi: 10.1093/oxfordjournals.aje.a120192. [DOI] [PubMed] [Google Scholar]
- McCarl R. L., Margossian S. S. Restoration of beating and enzymatic response of cultured rat heart cells to cortisol acetate. Arch Biochem Biophys. 1969 Mar;130(1):321–325. doi: 10.1016/0003-9861(69)90039-3. [DOI] [PubMed] [Google Scholar]
- Nunn M. E., Djeu J. Y., Glaser M., Lavrin D. H., Herberman R. B. Natural cytotoxic reactivity of rat lymphocytes against syngeneic Gross virus-induced lymphoma. J Natl Cancer Inst. 1976 Feb;56(2):393–399. doi: 10.1093/jnci/56.2.393. [DOI] [PubMed] [Google Scholar]
- Oie H. K., Easton J. M., Ablashi D. V., Baron S. Murine cytomegalovirus: induction of and sensitivity to interferon in vitro. Infect Immun. 1975 Nov;12(5):1012–1017. doi: 10.1128/iai.12.5.1012-1017.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinnan G. V., Manischewitz J. E. The role of natural killer cells and antibody-dependent cell-mediated cytotoxicity during murine cytomegalovirus infection. J Exp Med. 1979 Dec 1;150(6):1549–1554. doi: 10.1084/jem.150.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg E. B., McCoy J. L., Green S. S., Donnelly F. C., Siwarski D. F., Levine P. H., Herberman R. B. Destruction of human lymphoid tissue-culture cell lines by human peripheral lymphocytes in 51Cr-release cellular cytotoxicity assays. J Natl Cancer Inst. 1974 Feb;52(2):345–352. doi: 10.1093/jnci/52.2.345. [DOI] [PubMed] [Google Scholar]
- Ruebner B. H., Hirano T., Slusser R., Osborn J., Medearis D. N., Jr Cytomegalovirus infection. Viral ultrastructure with particular reference to the relationship of lysosomes to cytoplasmic inclusions. Am J Pathol. 1966 Jun;48(6):971–989. [PMC free article] [PubMed] [Google Scholar]
- Saksela E., Imir T., Mäkelä O. Spontaneous, augmentable cell-mediated cytotoxicity with limited target cell specificity in human blood. Eur J Immunol. 1977 Mar;7(3):126–130. doi: 10.1002/eji.1830070303. [DOI] [PubMed] [Google Scholar]
- Santoli D., Trinchieri G., Lief F. S. Cell-mediated cytotoxicity against virus-infected target cells in humans. I. Characterization of the effector lymphocyte. J Immunol. 1978 Aug;121(2):526–531. [PubMed] [Google Scholar]
- Sendo F., Aoki T., Boyse E. A., Buafo C. K. Natural occurrence of lymphocytes showing cytotoxic activity to BALB/c radiation-induced leukemia RL male 1 cells. J Natl Cancer Inst. 1975 Sep;55(3):603–609. doi: 10.1093/jnci/55.3.603. [DOI] [PubMed] [Google Scholar]
- Shellam G. R. Gross-virus-induced lymphoma in the rat. V. Natural cytotoxic cells are non-T cells. Int J Cancer. 1977 Feb 15;19(2):225–235. doi: 10.1002/ijc.2910190212. [DOI] [PubMed] [Google Scholar]
- Tai A., Burton R. C., Warner N. L. Differential natural killer cell reactivity against T cell lymphomas by cells from normal or stimulated mice. J Immunol. 1980 Apr;124(4):1705–1711. [PubMed] [Google Scholar]
- Takasugi M., Mickey M. R., Terasaki P. I. Reactivity of lymphocytes from normal persons on cultured tumor cells. Cancer Res. 1973 Nov;33(11):2898–2902. [PubMed] [Google Scholar]
- Trinchieri G., Santoli D. Anti-viral activity induced by culturing lymphocytes with tumor-derived or virus-transformed cells. Enhancement of human natural killer cell activity by interferon and antagonistic inhibition of susceptibility of target cells to lysis. J Exp Med. 1978 May 1;147(5):1314–1333. doi: 10.1084/jem.147.5.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh R. M., Jr, Hallenbeck L. A. Effect of virus infections on target cell susceptibility to natural killer cell-mediated lysis. J Immunol. 1980 May;124(5):2491–2497. [PubMed] [Google Scholar]
- Welsh R. M., Jr, Zinkernagel R. M. Heterospecific cytotoxic cell activity induced during the first three days of acute lymphocytic choriomeningitis virus infection in mice. Nature. 1977 Aug 18;268(5621):646–648. doi: 10.1038/268646a0. [DOI] [PubMed] [Google Scholar]
- West W. H., Cannon G. B., Kay H. D., Bonnard G. D., Herberman R. B. Natural cytotoxic reactivity of human lymphocytes against a myeloid cell line: characterization of effector cells. J Immunol. 1977 Jan;118(1):355–361. [PubMed] [Google Scholar]
- Zarling J. M., Nowinski R. C., Bach F. H. Lysis of leukemia cells by spleen cells of normal mice. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2780–2784. doi: 10.1073/pnas.72.7.2780. [DOI] [PMC free article] [PubMed] [Google Scholar]


