Abstract
Objective: To assess the ability of an oral supplement to increase hair growth in women with thinning hair. Design: A randomized, placebo-controlled, double-blind study. Setting: One United States clinical site. Participants: Healthy women aged 21 to 75 years with Fitzpatrick I to IV photo skin types with self-perceived thinning hair. Measurements: Subjects were randomized to treatment with the study medication (N=10) or placebo (N=5) twice daily for 180 days. A 4cm2 area of scalp was selected for hair counts performed after 90±7 and 180±7 days of treatment. The primary efficacy measure was the change in terminal and vellus hairs in each target area. The secondary measure was changes in a self-assessment questionnaire. Results: The mean (SD) number of terminal vellus hairs among placebo-treated subjects at baseline was 256.0 (24.1), remaining at 245.0 (22.4) and 242.2 (26.9) after 90 and 180 days, respectively. The mean baseline number of terminal hairs in control-treated subjects was 271.0 (24.2) increasing to 571 (65.7) and 609.6 (66.6) after 90 and 180 days, respectively (for each, p<0.001 vs. placebo). The mean number of vellus hairs among placebo-and control-treated subjects did not significantly change. Significantly more control-treated subjects perceived improvements in overall hair volume, scalp coverage, and thickness of hair body after 90 days. Additional improvement after 180 days included hair shine, skin moisture retention, and skin smoothness. No adverse events were reported. Conclusion: The oral supplement assessed in this study safely and effectively promotes significant hair growth in women with temporary hair thinning.
Androgenic alopecia or male-pattern baldness is the most common form of hair loss in men and less commonly a cause of hair loss in women.1 More common causes of hair loss in women include medical conditions, such as hypothyroidism; medications including oral contraceptives; nutritional deficiencies; and physiological and emotional stresses.1,2 The causes of hair loss in elderly women may be multifactorial.3 Among 100 adult women with diffuse hair loss in one study, probable causes were determined to be psychological stress (30%), fever (33%), abortion and delivery (21%), trauma or surgical operations (13%), and hypothyroidism (10%).4 More than 50 percent of women had more than one likely cause of hair loss while a cause could not be determined in six percent.
The effects of hair loss on self-image and self-esteem have been well documented. In one study, significantly diminished quality of life among adult men and women with various forms of hair loss was significantly correlated with symptoms of clinical depression,5 and the psychological impact of hair loss among women appears to be more severe than for men.6,7 Consequently, a drug therapy that will safely and effectively increase hair growth in women is highly desirable.
An oral compound consisting of proteins and glycosaminoglycans of marine origin was found to have beneficial effects on women with sun-damaged skin. The results of two studies demonstrated improvements in the appearance of sun-damaged skin and increased skin thickness and elasticity. Brittle hair and nails returned to normal after 90 days of treatment.8,9 Subsequent studies assessed the use of a similar marine extract with the addition of other natural compounds (Viviscal® Hair Nourishment System; Lifes2good, Inc., Chicago, Illinois) for the treatment of androgenetic alopecia. Several six-month, randomized, controlled studies have demonstrated the efficacy of Viviscal (the product used in these studies was originally marketed under the brand name Hairgain® [Parexel Medstat AS, Lillestrøm, Norway]) for the treatment of androgenetic alopecia.10 Similar results were achieved in 8- and 12-month, open-label studies11,12 Since these studies enrolled men, less is known about the use of Viviscal in women with thinning hair.
The objective of this randomized, double-blind, placebo-controlled study was to test the hypothesis that the administration of this new oral supplement over a six-month period will increase hair growth in adult women with self-perceived thinning hair associated with poor diet, stress, hormonal influences, or abnormal menstrual cycles. Preliminary results of this study have been presented elsewhere.13
METHODS
Subjects. The study enrolled women 21 to 75 years of age with Fitzpatrick skin types I to IV who were in generally good health, but complained of self-perceived thinning hair. Participating subjects expressed their willingness to maintain a consistent shampooing frequency and cut and color of their hair and agreed not to substantially change their current diet, medications, or exercise routines for the duration of the study. Women of childbearing potential were required to use a medically accepted form of birth control during the study.
Subjects were excluded from participation if they had a history of allergy or intolerance to fish, seafood, acerola, any shampoos or hair conditioners; were nursing, pregnant, or planning to become pregnant during the study; were participating in another clinical research study; had started the use of hormones for birth control or hormone replacement therapy within the prior six months; were currently undergoing a form of treatment for thinning hair including drug or light therapy within the last three months; or used prescription drugs known to affect the hair growth cycle within the last six months. Subjects with other hair loss disorders, such as alopecia areata, scarring alopecia, and androgenetic alopecia; self-reported uncontrolled diseases, such as diabetes, hypertension, hyperthyroidism, or hypothyroidism; self-reported active hepatitis, immune deficiency, human immunodeficiency virus, or autoimmune disease; or any known active dermatological condition, which, in the opinion of the investigator, might place the subject at a greater risk or interfere with clinical evaluations, were also excluded.
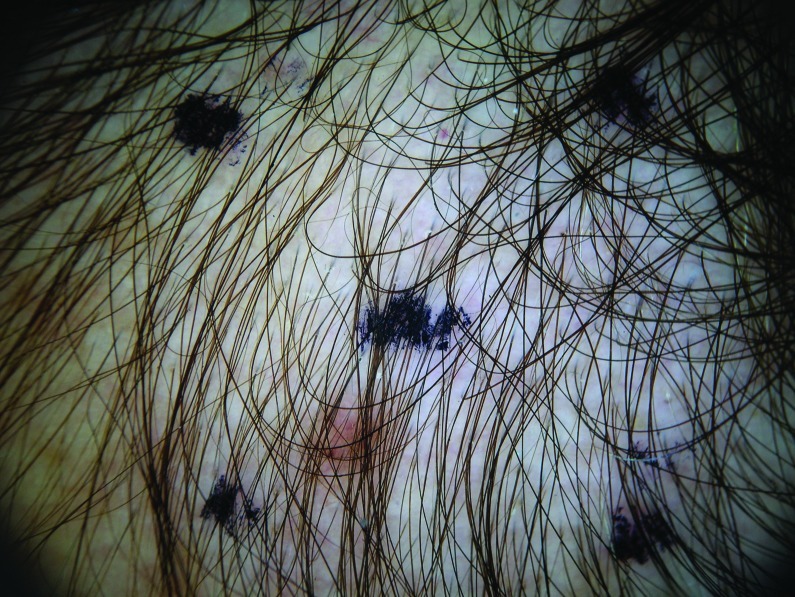
Procedures. During the baseline visit, inclusion/exclusion criteria were reviewed and each subject provided informed consent and signed a photography release form. A medical history was obtained from each subject, concomitant medications and lifestyle instructions were reviewed, and each subject underwent a physical examination and pregnancy testing. The scalp was examined to rule out the presence of any confounding scalp conditions. The investigator selected an approximately 4cm2 area of scalp along the frontalis bone at the junction of the frontal and lateral hairlines. This location was identified for further assessments using a three-point location noted on each patient according to measurements taken from medial canthus, lateral canthus, and preauricular skin pit to the hairline junction and digitally photographed (Nikkon SLR 200/300 camera with a Canfield EpiFlash). Following the baseline visit, subjects returned for evaluation after 90±7 and 180±7 days. At that time, the physical examination, vital signs, and digital photographs were repeated. In addition, subjects completed self-assessment questionnaires (Tables 1) and were queried about possible adverse events.
TABLE 1.
Self-assessment questionnaire
| Please review each of the parameters below and place a check mark (✓) for the most appropriate answer. | |||||||
|---|---|---|---|---|---|---|---|
| GREATLY INCREASED | MODERATELY INCREASED | SLIGHTLY INCREASED | NO CHANGE | SLIGHTLY DECREASED | MODERATELY DECREASED | GREATLY DECREASED | |
| 1. Overall Hair Volume | 1 □ | 2 □ | 3 □ | 4 □ | 5 □ | 6 □ | 7 □ |
| 2. Scalp Coverage | 1 □ | 2 □ | 3 □ | 4 □ | 5 □ | 6 □ | 7 □ |
| 3. Thickness of Hair Body | 1 □ | 2 □ | 3 □ | 4 □ | 5 □ | 6 □ | 7 □ |
| 4. Softness of Hair Body | 1 □ | 2 □ | 3 □ | 4 □ | 5 □ | 6 □ | 7 □ |
| 5. Hair Shine | 1 □ | 2 □ | 3 □ | 4 □ | 5 □ | 6 □ | 7 □ |
| 6. Number of Hairs Lost on Average Day | 7 □ | 6 □ | 5 □ | 4 □ | 3 □ | 2 □ | 1 □ |
| 7. Nail Strength | 1 □ | 2 □ | 3 □ | 4 □ | 5 □ | 6 □ | 7 □ |
| 8. Nail Growth Rate | 1 □ | 2 □ | 3 □ | 4 □ | 5 □ | 6 □ | 7 □ |
| 9. Skin Moisture Retention | 1 □ | 2 □ | 3 □ | 4 □ | 5 □ | 6 □ | 7 □ |
| 10. Facial Fine Lines and Wrinkles | 1 □ | 2 □ | 3 □ | 4 □ | 5 □ | 6 □ | 7 □ |
| 11. Skin Softness | 1 □ | 2 □ | 3 □ | 4 □ | 5 □ | 6 □ | 7 □ |
| 12. Skin Resiliency | 7 □ | 6 □ | 5 □ | 4 □ | 3 □ | 2 □ | 1 □ |
| 13. Growth of Eyebrow Hair | 1 □ | 2 □ | 3 □ | 4 □ | 5 □ | 6 □ | 7 □ |
| 14. Growth of Eyelashes | 1 □ | 2 □ | 3 □ | 4 □ | 5 □ | 6 □ | 7 □ |
| 15. Skin Smoothness | 1 □ | 2 □ | 3 □ | 4 □ | 5 □ | 6 □ | 7 □ |
| 16. Overall Skin Health | 7 □ | 6 □ | 5 □ | 4 □ | 3 □ | 2 □ | 1 □ |
Test material. Subjects were randomized in double-blind fashion to receive the new oral supplement (Viviscal® Maximum Strength) or placebo. Viviscal contains AminoMar C™ marine complex, a proprietary blend of shark and mollusk powder, an organic form of silica derived from Equisetum sp. (horsetail), vitamin C derived from Malpighia emarginata (acerola cherry), microcrystalline cellulose (E460), natural orange flavor, magnesium stearate, hypromellose, and glycerol. Placebo treatment consisted of inert tablets with similar appearance. Subjects were instructed to take one tablet of their assigned treatment each morning and one tablet each evening with water following a meal.
Efficacy measures. The primary endpoint was the change in the number of terminal and vellus hairs in the target area of the scalp. The secondary endpoint was the change in patient self-assessment questionnaires following treatment (Tables 1).
Safety measures. Safety measures included spontaneous reports of adverse events and any adverse events disclosed during clinic evaluations and any changes noted during physical examinations.
Statistical analysis. The primary endpoint parameters measured during each evaluation were compared to baseline data using a paired t-test. Comparisons between active and placebo treatments were made using analysis of variance (ANOVA). Secondary endpoint parameters were compared using top box analysis. Differences were considered significant at the level of p≤0.05.
Ethics. This study protocol and informed consent agreement were reviewed and approved by an institutional review board. Written consent was obtained from all participants prior to their participation in any study-related activities. This study was conducted in accordance with applicable guidelines for the protection of human subjects for research as outlined in the United States Food and Drug Administration (FDA) 21 CFR Part 50, with the accepted standards for Good Clinical Practices and with the standard practices of the Ablon Skin Institute Research Center.
RESULTS
Efficacy. Subjects were randomized to receive the active medication (N=10) or placebo (N=5). The mean (SD) age of subjects in the active and placebo treatment groups were 49.9 (8.5) years and 47.6 (17.0) years, respectively, and were not significantly different from one another. All subjects described themselves as Caucasian, except one who was Hispanic.
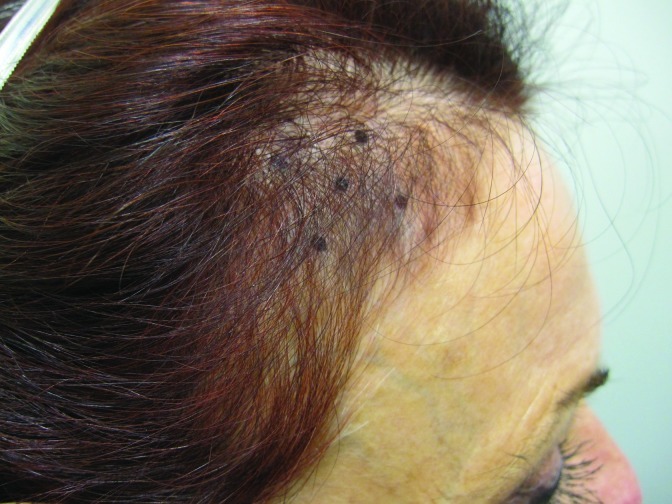
At baseline, the mean number of terminal hairs among placebo-treated subjects was 256.0 (24.1) and remained at 245.0 (22.4) and 242.2 (26.9) after 90 and 180 days, respectively (Tables 2). In contrast, the mean number of terminal hairs in the study medication-treated subjects was 271.0 (24.2) at baseline, increasing to 571 (65.7) and 609.6 (66.6) after 90 and 180 days, respectively (for each, p<0.001 vs. placebo) (Figure 1). Digital images reveal the visible improvements in two subjects treated with the new oral supplement (Figures 2–7).
TABLE 2.
Changes in the number terminal and vellus hairs, mean (SD)
| STUDY MEDICATION (N=10) | PLACEBO (N=5) | |||||
|---|---|---|---|---|---|---|
| Day 0 | Day 90 | Day 180 | Day 0 | Day 90 | Day 180 | |
| Terminal hairs | 271.0 (24.2) | 571.0 (65.7)a | 609.5 (66.6)a | 256.0 (24.1) | 245.0 (22.4) | 242.2 (26.9) |
| Vellus hairs | 46.5 (17.7) | 48.0 (16.2) | 46.5 (14.4) | 57.0 (32.1) | 68.0 (21.4) | 65.8 (16.6) |
p<0.0001, each vs. placebo; repeated measures ANOVA
Figure 1.
Change in the number of vellus hairs. The use of a new oral supplement was associated with a significant increase in the number of terminal hairs after 90 and 180 days of treatment.
*Denotes p<0.001 vs. placebo
Figures 2–4.
The use of a new oral supplement was associated with a visible increase in hair growth after 90 and 180 days. Figures 2A and 2B = Day 0; Figures 3A and 3B = Day 90; Figures 4A and 4B = Day 180
Figures 5–7.
The use of a new oral supplement was associated with a visible increase in hair growth after 90 and 180 days. Figures 5A and 5B = Day 0; Figures 6A and 6B = Day 90; Figures 7A and 7B = Day 180
The mean number of vellus hairs among placebo-treated subjects was 57.0 (32.1) at baseline and 68.0 (21.4) and 65.8 (16.6) after 90 and 180 days, respectively (Table 2). The mean number of vellus hairs among oral supplement-treated subjects was 46.5 (17.7) at baseline and 48.0 (16.2) and 46.5 (14.4) after 90 and 180 days, respectively.
With respect to subject self-assessments, significantly more study medication-treated subjects perceived improvements in overall hair volume, scalp coverage, and thickness of hair body after 90 days (Table 3). Additional improvements after 180 days included hair shine, skin moisture retention, and skin smoothness.
TABLE 3.
Changes in self-assessment questionnaire, mean (SD)
| STUDY MEDICATION (N=10) | PLACEBO (N=5) | |||
|---|---|---|---|---|
| 90 Days | 180 Days | 90 Days | 180 Days | |
| Overall hair volume | 2.8 (0.9)a | 1.8 (0.8)d | 4.2 (0.4) | 3.8 (0.4) |
| Scalp coverage | 2.6 (0.8)b | 1.5 (0.9)d | 4.2 (0.4) | 4.0 (0.0) |
| Thickness of hair body | 2.9 (0.7)c | 2.0 (0.9)d | 4.2 (0.4) | 4.0 (0.0) |
| Hair shine | 2.9 (1.1) | 2.1 (1.3)e | 3.6 (0.9) | 3.6 (0.9) |
| Skin moisture retention | 3.6 (0.8) | 3.0 (0.8)e | 4.0 (0.0) | 4.0 (0.0) |
| Skin smoothness | 3.5 (0.7) | 3.0 (0.8)e | 4.0 (0.0) | 4.0 (0.0) |
p = 0.007
p = 0.002
p = 0.003
p < 0.0001
p < 0.05, each vs. placebo; ANOVA
Safety. No adverse events were reported and no changes were observed during physical examinations.
DISCUSSION
When women with thinning hair were treated with the study medication, the mean number of terminal hairs in the target scalp area increased from 271.0 at baseline to 571 after three months of treatment and increased further to 609.6 after six months. Both were significantly greater than the mean number of terminal hairs among placebo-treated subjects at baseline (256.0), which remained unchanged throughout the study. These results support the hypothesis that Viviscal increases hair growth in women with thinning hair. These results are in agreement with studies demonstrating the drug’s beneficial effect in the treatment of sun-damaged skin in women8,9 and androgenetic alopecia in men.10–12
In addition to objective measures of increased hair growth, significantly more women treated with the study medication perceived improvements in overall hair volume and thickness and scalp coverage after three months of treatment. Other improvements occurred after three additional months of treatment including hair shine and skin smoothness and moisture retention, suggesting additional hair and skin improvements may occur with continued product use. These results may represent the first description of increased hair growth in women associated with the use of a nutritional supplement. The results of work by others has demonstrated an oral supplement containing natural ingredients including marine-derived protein (shark cartilage) and fish oil (omega-3 polyunsaturated fatty acids) significantly reduced hair loss in women; however, it did not promote hair growth.14
Other natural products, such as biotin and zinc, have also been advocated for the treatment of hair loss. Biotin is a water-soluble vitamin and an essential coenzyme for several important enzymes15 while zinc is an essential micronutrient that is responsible for the normal functioning of hundreds of enzymes.16,17 The use of these agents for hair loss is based on the observation that alopecia is one of many consequences associated with biotin15 and zinc deficiencies.18 In one case report, a child with alopecia due to zinc deficiency was administered a zinc supplement and her hair loss stopped in three weeks18; however, the use of a zinc supplement in a group of 15 patients with alopecia areata and low serum zinc levels did not result in significant hair growth.19 A literature search did not reveal any studies describing the use of biotin supplementation for the treatment of hair loss.
Iron deficiency is also believed to be a cause of hair loss in women, but literature reports are inconsistent. One report suggests women with iron deficiency status are at a risk of telogen hair loss,20 and among more than 5,000 women, a larger proportion with excessive hair loss (59%) had low iron stores compared to women with moderate or no hair loss.21 However, in another study, the incidence of iron deficiency was not increased among women with female pattern hair loss or chronic telogen effluvium.22 A literature search did not reveal any studies describing the use of iron supplementation for the treatment of hair loss.
Based on these promising results, ongoing research is studying how the AminoMar C extract affects hair fiber formation within the hair follicle organ using an ex vivo culture model. Additional clinical studies designed to further assess the use of Viviscal to increase hair thickness and hair counts using larger patient populations are currently under way. Together, these studies will help provide a more detailed understanding of the mechanism of this new drug to promote hair growth and add to the existing body of clinical evidence.
CONCLUSION
The daily administration of a proprietary nutritional supplement significantly increased hair growth after 90 and 180 days. Self-perceived improvements after 90 days were increased after 180 days of additional treatment, suggesting continued improvements may occur with ongoing treatment. No adverse events were reported. These results may represent the first description of increased hair growth in women associated with the use of a nutritional supplement.
ACKNOWLEDGMENT
Funding for this study was provided by Lifes2good, Inc., Chicago, Illinois. The author acknowledges the assistance of Dr. Carl Hornfeldt during the preparation of this manuscript.
Footnotes
DISCLOSURE:Dr. Ablon received a research grant from Lifes2good, Inc., Chicago, Illinois. Funding for this study was provided by Lifes2good, Inc.
REFERENCES
- 1.Harrison S, Bergfeld W. Diffuse hair loss: its triggers and management. Cleve Clin J Med. 2009;76:361–367. doi: 10.3949/ccjm.76a.08080. [DOI] [PubMed] [Google Scholar]
- 2.Thiedke CC. Alopecia in women. Am Fam Physician. 2003;67:1007–1014. [PubMed] [Google Scholar]
- 3.Chen W, Yang CC, Todorova A, et al. Hair loss in elderly women. Eur J Dermatol. 2010;20:145–151. doi: 10.1684/ejd.2010.0828. [DOI] [PubMed] [Google Scholar]
- 4.Jain VK, Kataria U, Dayal S. Study of diffuse alopecia in females. Indian J Dermatol Venereol Leprol. 2000;66:65–68. [PubMed] [Google Scholar]
- 5.Williamson D, Gonzalez M, Finlay AY. The effect of hair loss on quality of life. J Eur Acad Dermatol Venereol. 2001;15:137–139. doi: 10.1046/j.1468-3083.2001.00229.x. [DOI] [PubMed] [Google Scholar]
- 6.van der Donk J, Passchier J, Knegt-Junk C, et al. Psychological characteristics of women with androgenetic alopecia: a controlled study. Br J Dermatol. 1991;125:248–252. doi: 10.1111/j.1365-2133.1991.tb14749.x. [DOI] [PubMed] [Google Scholar]
- 7.Reid EE, Haley AC, Borovicka JH, et al. Clinical severity does not reliably predict quality of life in women with alopecia areata, telogen effluvium, or androgenic alopecia. J Am Acad Dermatol. 2012;66:e97–e102. doi: 10.1016/j.jaad.2010.11.042. [DOI] [PubMed] [Google Scholar]
- 8.Lassus A, Jeskanen L, Happonen HP, Santalahti J. Imedeen for the treatment of degenerated skin in females. J Int Med Res. 1991;19:147–152. doi: 10.1177/030006059101900208. [DOI] [PubMed] [Google Scholar]
- 9.Eskelinin A, Santalahti J. Special natural cartilage polysaccharides for the treatment of sun-damaged skin in females. J Int Med Res. 1992;20:99–105. doi: 10.1177/030006059202000201. [DOI] [PubMed] [Google Scholar]
- 10.Thom E. Efficacy and tolerability of Hairgain in individuals with hair loss: a placebo-controlled, double-blind study. J Int Med Res. 2001;29:2–6. doi: 10.1177/147323000102900101. [DOI] [PubMed] [Google Scholar]
- 11.Pereira JM. Uso do extrato de protefnas e polissacarfdeos de origem marinha no tratamento da alopecia androgenetica. Rev Bras Med. 1997;53:144–155. [Google Scholar]
- 12.Majas M, Puuste O, Prästhacka B, Brorsdotter-Johansson P. 1996. Treatment of alopecia areata, alopecia totalis and alopecia universalis with oral ViviScal for 12 months. Swedish Association for Alopecia.
- 13.Ablon G. Efficacy of an oral supplement in women with self-perceived thinning hair: preliminary results from a double-blind, placebo-controlled clinical study. Presented at: South Beach Clinical Dermatology Symposium; February 16-20, 2012; Miami Beach, Florida.
- 14.Jacquet A, Coolen V, Vandermander J. Effect of dietary supplementation with INVERSION Femme on slimming, hair loss, and skin and nail parameters in women. Adv Ther. 2007;24:1154–1171. doi: 10.1007/BF02877721. [DOI] [PubMed] [Google Scholar]
- 15.Zempleni J, Hassan YI, Wijeratne SS. Biotin and biotinidase deficiency. Expert Rev Endocrinol Metab. 2008;3:715–724. doi: 10.1586/17446651.3.6.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saper RB, Rash R. Zinc: an essential micronutrient. Am Fam Physician. 2009;79:768–772. [PMC free article] [PubMed] [Google Scholar]
- 17.Yanagisawa H. Zinc deficiency and clinical practice—validity of zinc preparations. Yakugaku Zasshi. 2008;128:333–339. doi: 10.1248/yakushi.128.333. [DOI] [PubMed] [Google Scholar]
- 18.Alhaj E, Alhaj N, Alhaj NE. Diffuse alopecia in a child due to dietary zinc deficiency. Skinmed. 2007;6:199–200. doi: 10.1111/j.1540-9740.2007.05881.x. [DOI] [PubMed] [Google Scholar]
- 19.Park H, Kim CW, Kim SS, Park CW. The therapeutic effect and the changed serum zinc level after zinc supplementation in alopecia areata patients who had a low serum zinc level. Ann Dermatol. 2009;21:142–146. doi: 10.5021/ad.2009.21.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moeinvaziri M, Mansoori P, Holakooee K, Safaee Naraghi Z, Abbasi A. Iron status in diffuse telogen hair loss among women. Acta Dermatovenerol Croat. 2009;17:279–284. [PubMed] [Google Scholar]
- 21.Deloche C, Bastien P, Chadoutaud S, et al. Low iron stores: a risk factor for excessive hair loss in non-menopausal women. Eur J Dermatol. 2007;17:507–512. doi: 10.1684/ejd.2007.0265. [DOI] [PubMed] [Google Scholar]
- 22.Olsen EA, Reed KB, Cacchio PB, Caudill L. Iron deficiency in female pattern hair loss, chronic telogen effluvium, and control groups. J Am Acad Dermatol. 2010;63:991–999. doi: 10.1016/j.jaad.2009.12.006. [DOI] [PubMed] [Google Scholar]