Abstract
Rieske/cytochrome b (Rieske/cytb) complexes are proton pumping quinol oxidases that are present in most bacteria and Archaea. The phylogeny of their subunits follows closely the 16S-rRNA phylogeny, indicating that chemiosmotic coupling was already present in the last universal common ancestor of Archaea and bacteria. Haloarchaea are the only organisms found so far that acquired Rieske/cytb complexes via interdomain lateral gene transfer. They encode two Rieske/cytb complexes in their genomes; one of them is found in genetic context with nitrate reductase genes and has its closest relatives among Actinobacteria and the Thermus/Deinococcus group. It is likely to function in nitrate respiration. The second Rieske/cytb complex of Haloarchaea features a split cytochrome b sequence as do Cyanobacteria, chloroplasts, Heliobacteria, and Bacilli. It seems that Haloarchaea acquired this complex from an ancestor of the above-mentioned phyla. Its involvement in the bioenergetic reaction chains of Haloarchaea is unknown. We present arguments in favor of the hypothesis that the ancestor of Haloarchaea, which relied on a highly specialized bioenergetic metabolism, that is, methanogenesis, and was devoid of quinones and most enzymes of anaerobic or aerobic bioenergetic reaction chains, integrated laterally transferred genes into its genome to respond to a change in environmental conditions that made methanogenesis unfavorable.
Keywords: Rieske/cytb complex, Haloarchaea, bc-complex, halobacteria, evolution, bioenergetics
Introduction
Rieske/cytochrome b (Rieske/cytb) complexes are energy-converting enzymes involved in bioenergetic electron transfer chains as diverse as oxygenic and anoxygenic photosynthesis, oxygen respiration, and energy-conserving systems using further substrates such as nitrogen oxide (Suharti et al. 1984; Shapleigh and Payne 1985; Ducluzeau et al. 2009; van Lis et al. 2010), nitrous oxide (Boogerd et al. 1980; Itoh et al. 1989), sulphide (Nübel et al. 2000; Guiral et al. 2009), or hydrogen (Guiral et al. 2009). All these bioenergetic electron transfer chains are based on electrochemical disequilibria between reduced and oxidized substrates and build up a transmembrane proton motive potential as a result of the respective redox reactions. This proton motive potential drives the formation of adenosine triphosphate (ATP) through the enzyme ATP synthase via chemiosmotic coupling (Mitchell 1970, 1972). The phylogenies of a number of bioenergetic enzymes have led to the conclusion that chemiosmosis was a process of energy conversion already used by the ancestor of Archaea and bacteria (Castresana et al. 1995; Henninger et al. 1999; Schütz et al. 2000; Baymann et al. 2003; Lebrun et al. 2003; Duval et al. 2008; Ducluzeau et al. 2009; Nitschke and Russell 2009; Lane et al. 2010), frequently referred to as the last universal common ancestor (LUCA).
Rieske/cytb complexes operate between the initial electron donors and the terminal electron acceptors of bioenergetic reaction chains as bioenergetic turbochargers (a turbocharger is a device that increases the power output of an engine by recovering waste energy in the exhaust and feeding it back into the engine intake), serving to harvest the excess electrochemical energy contained in certain substrate couples (Rich 1984; Nitschke and Russell 2009). The phylogeny reconstructed for this class of enzymes was in fact among the first to indicate an operation of the chemiosmotic mechanism in LUCA as a result of its clear-cut cleavage into an archaeal and a bacterial subtree and its far-going congruency with small subunit ribosomal ribonucleic acid (rRNA)–based species trees observed for both conserved subunits, cytochrome b and the Rieske protein (Castresana et al. 1995; Henninger et al. 1999; Schütz et al. 2000; Lebrun et al. 2006).
The phylogenetic trees of cytochrome b published so far suggest an astonishingly low occurrence of lateral gene transfer with the only prominent examples provided by the enzyme of Aquificales (Schütz et al. 2003), Haloarchaea (Boucher et al. 2003), and a potential case for the green sulphur bacteria (Nitschke et al. 2010).
In this study, we confirm and interpret interdomain lateral gene transfers of the Rieske/cytb gene cluster from Bacteria into Haloarchaea taking into account recent genome sequence data.
Basic local alignment search tool (BLAST) searches in Haloarchaea using cytochrome b sequences from various bacterial and archaeal phyla (Rhodobacter, Sulfolobus, Heliobacterium, and Synechococcus) as query identified one or two gene clusters containing the two essential subunits of Rieske/cytb complexes, the Rieske iron sulfur protein and the transmembranous cytochrome b subunit, in the sequenced genomes from Haloarcula marismortui (HalMa), Halogeometricum borinquense (HalBo), Haloquadratum walsbyi (HalWa), Halomicrobium mukohataei (HalMu), Halorhabdus utahensis (HalUt), Haloferax volcanii (HalVo), Halobacterium salinarum (HalSa), Halorubrum lacusprofundi (HalLa), and Halalkalicoccus jeotgali (HalJe) (Boucher et al. 2003; Martinez-Espinosa et al. 2007; Yoshimatsu et al. 2007). All tested queries allowed to find the genes coding for Rieske proteins and cytochrome b in Haloarchaea. Already before genome sequencing, inhibitor experiments (2-heptyl-4-hydroxy quinoline-N-oxide and Antimycin A) on membranes (Cheah 1969, 1970a, 1970b; Halberg-Gradin and Colmjö 1989) indicated the presence of Rieske/cytb complexes in Haloarchaea. Sreeramulu et al. (1998) reported the only purification procedure so far for a Rieske/cytb complex from Haloarchaea that preserved both Rieske and cytochrome b subunits. Genome analysis revealed that Rieske/cytb-encoding genes are part of a larger gene cluster also encompassing the enzyme nitrate reductase (Nar). The Rieske/cytb complex was correspondingly proposed to participate in energy conversion via the denitrification chain in Haloarchaea (Martinez-Espinosa et al. 2007; Yoshimatsu et al. 2007; Bonete et al. 2008; van Lis et al. 2010). This idea was reinforced by the observation that growth with nitrate 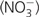 is inhibited by antimycin A at a concentration of 100 μM (Martinez-Espinosa et al. 2007). Antimycin A is a well-known inhibitor of Rieske/cytb complexes from mitochondria and proteobacteria and does not affect the bacterial Nar at these concentrations (Alefounder et al. 1981).
is inhibited by antimycin A at a concentration of 100 μM (Martinez-Espinosa et al. 2007). Antimycin A is a well-known inhibitor of Rieske/cytb complexes from mitochondria and proteobacteria and does not affect the bacterial Nar at these concentrations (Alefounder et al. 1981).
Here, we set out to analyze sequence conservation and phylogenetic positioning of the haloarchaeal Rieske/cytb complexes in detail, place our results in the context of the adaptation of the bioenergetic reaction chain to the environment, and discuss the selection pressure for integration of laterally transferred genes into the genome.
Materials and Methods
Protein sequences were collected by BLAST searches on completely sequenced genomes on the NCBI server. All available archaeal genomes were included in the analysis. For Bacteria, 5–10 species per phylum were selected, if available. Sequence alignments were done in several steps: A first alignment was done by Clustal (Larkin et al. 2007), and in a second step, the alignment was refined, taking into account the structural features of the protein, that were either taken directly from the 3D structure, when available, or in most cases predicted with the protein sequence analysis and modeling software package PSAAM (http://www.life.illinois.edu/crofts/ahab/psaam.html, last accessed July 26, 2012). With the help of Seaview (Galtier et al. 1996), the alignment was corrected to assure that corresponding structural elements (such as α-helices or β-sheets) were properly aligned. Each of these structural elements and the connecting sequence stretches was then submitted individually to Clustal. This procedure was crucial for the Rieske alignment as explained in detail in a previous study (Lebrun et al. 2006) and was also used to improve the alignment of the cytochrome b sequences. N- or C-terminal extensions that occur in individual species were cut from this alignment before submitting it to tree-building procedures. In the case of cytochrome b, either the entire protein sequence or the first four transmembranous helices and their connecting sequence stretches, corresponding to cytochrome b6 in Cyanobacteria and chloroplasts, were used. Both sets of sequences gave similar results, but the less conserved C-terminal part of the protein induced a higher uncertainty reflected by lower bootstrap values. The “four-helix” alignment was used to calculate the trees of cytochrome b, shown in figures 3, 4, and 5. Different algorithms such as maximum likelihood, minimum evolution, or neighbor joining were tested to calculate the trees with the program MEGA (K. Tamura, D. Peterson, N. Peterson, M. Nei, and S. Kumar). The trees obtained with these different algorithms and with the neighbor joining algorithm (Felsenstein 1997) from Clustal, corrected for multiple substitutions, were essentially identical with respect to the branching order of the different phyla. Clustal was then used to calculate the trees depicted in this article.
Fig. 3.—
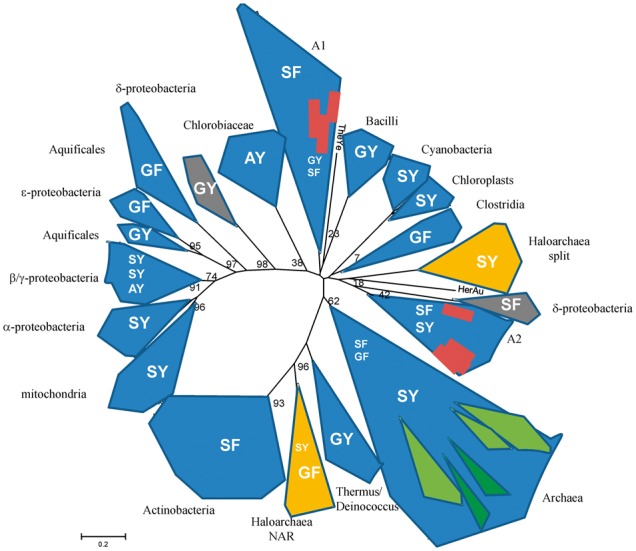
Phylogenetic tree of cytochrome b from Rieske/cytb complexes. The different forms represent the different phyla. The letters in the area of the phyla indicate the nature of the two amino acid residues that have been shown to correlate with the redox midpoint potential of the iron–sulfur center of the Rieske protein (SY: 300–350 mV; SF: 200–230 mV; AY, GY, GF: 120–160 mV). Orange, gray, green, and red (gray and white) forms indicate groups of organisms that have two phylogenetically distant Rieske/cytb complexes. Group A1 includes Candidatus Kuenenia stuttgartiensis 3, Planctomyces limnophilus, Solibacter usitatus 1 and 2, Plesiocystis pacifica, Gemmatimonas aurantiaca, Sorangium cellulosum, and Candidatus Nitrospira defluvii; group A2 includes Candidatus Kuenenia stuttgartiensis 1 and 2, Blastopirellula marina, Desulfococcus oleovorans, Solibacter usitatus 3 and 4 Parachlamydia acanthamoebae, Ktedonobacter racemifer, Waddlia chondrophila, Symbiobacterium thermophilum, and Desulfococcus oleovorans; TheYe is Thermodesulfovibrio yellowstonii and HerAu is Herpetosiphon aurantiacus.
Fig. 4.—
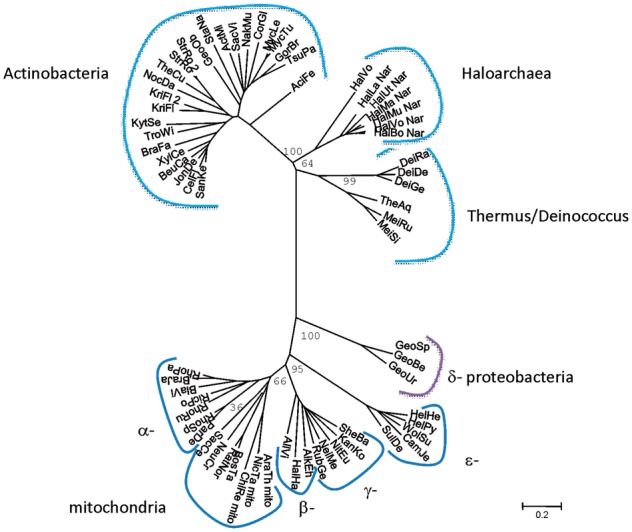
Phylogenetic tree of cytochrome b of Rieske/cytb complexes from Actinobacteria, Thermus/Deinococcus, proteobacteria, and Haloarchaea (Rieske/cytb-Nar cluster).
Fig. 5.—
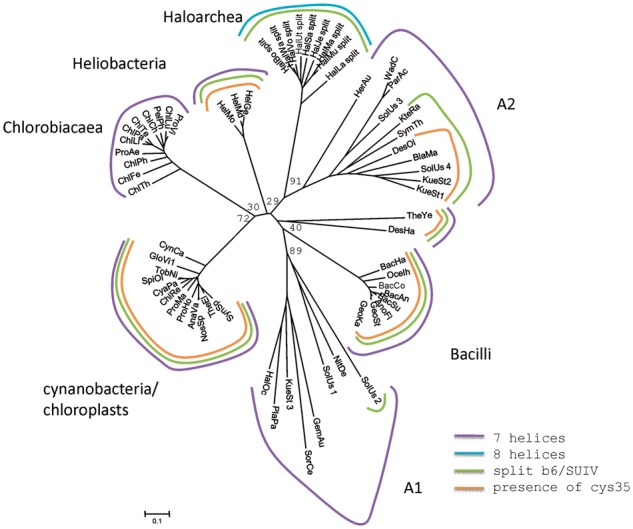
The phylogenetic tree of cytochrome b from Rieske/cytb complexes from Chlorobiaceae, Firmicutes, Cyanobacteria, and Haloarchaea in genomic context with halocyanin and the “additional” sequences. Cys stands for the cystein that covalently binds heme ci in cyanobacteria, chloroplasts, and heliobacteria.
Analysis of the Haloarchaeal Rieske/cytb Genes and Their Genomic Context
The Rieske/cytb-NAR Gene Cluster
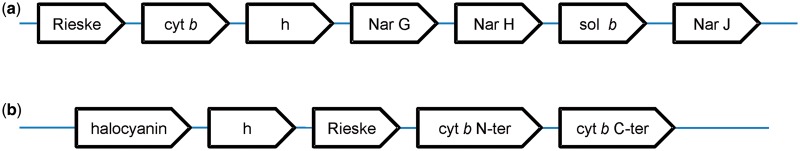
In six species of Haloarchaea (HalUt, HalMa, HalMu, HalVo (plasmid), HalBo, and HalLa), genes coding for the Rieske/cytb complex cluster with the NAR-encoding genes. In the different genomes, they are invariably arranged as follows: Rieske ► cytochrome b ► hypothetical protein ► NarG ► NarH (see fig. 1). A gene coding for cytochrome b followed by the gene coding for the Rieske protein was already described as the canonical order of gene organization for this enzyme and provided the name Rieske/cytb complex for the enzyme family (Schütz et al. 2000; Kramer et al. 2009). The Rieske [2Fe2S] cluster of the haloarchaeal Rieske/cytb-Nar clusters are predicted to be low potential (i.e., below + 200 mV) because they feature a glycine and a phenylalanine in the two positions reported to influence the redox potential of the iron–sulfur center (fig. 2b) (Denke et al. 1998; Schröter et al. 1998; Schoepp-Cothenet et al. 2009; Duval et al. 2010). In this cluster, no cytochrome c-encoding gene (such as cytochrome c1 or f, for a review see Kramer et al. (2009)) is present. As discussed by Martinez-Espinosa et al. (2007), the role of the electron shuttle between the Rieske protein and Nar probably is fulfilled by a periplasmic cytochrome b protein, also encoded in this gene cluster.
Fig. 1.—
Schematic representation of the two distinct clusters of Rieske/cytb encoding genes in Haloarchaea. (a) Genes of the first gene cluster are preceded by one or two open reading frames and succeeded by three or four proteins depending on species. h, hypothetical protein; sol b, soluble cytochrome b. (b) Genes of the second gene cluster are preceded by a hypothetical protein and succeeded by two or three other hypothetical proteins. The Rieske protein is a part of the second gene cluster in HalMa, HalMu, HalUt, HalSa, and HalJe. In HalVo, HalBo, HalWa, and HalLa, it is located somewhere else in the genome.
Fig. 2.—

Sequence comparisons in the cofactor-binding regions of Rieske/cytb complexes of Haloarchaea. Asterisks indicate amino acid residues conserved in all sequences.
The Halocyanin–Rieske/cytb Gene Cluster
The above-mentioned six species and three further haloarchaeal species harbor an additional gene cluster coding for a Rieske/cytb complex (see fig. 1b). This cluster is characterized by the presence of a gene coding for halocyanin, a soluble type I copper protein. Cytochrome b is encoded on two genes with the split between the two parts being located at the same sequence position as observed in cytochrome b6f complexes and related enzymes (Widger et al. 1984; Hauska 1986; Sone et al. 1995; Schütz et al. 2000; Nitschke et al. 2010). However, at variance with these latter cytochrome b sequences that have four and three transmembrane helices, hydrophobicity plots indicate the presence of an eighth helix at the end of the C-terminal part of the second subunit. Eight helices are found in unsplit cytochromes bs to the exception of those from Chlorobiaceae, δ-proteobacteria, and a few other species which have seven helices (members of group A1 and A2 in figs. 4 and 5, see below). The canonical gene order Rieske ► cytochrome b is present in this gene cluster in five of nine species (HalMa, HalMu, HalUt, HalSa, and HalJe); in the other four species (HalVo, HalBo, HalWa, and HalLa), a gene coding for the Rieske subunit is found elsewhere in the genome. HalMa has a second gene coding for a Rieske protein at another locus in the genome in addition to the Rieske gene in genomic context with the split cytochrome b genes. All these Rieske subunits, whether isolated in the genome or in context with the cytochrome b genes, are predicted to be of high potential (i.e., at or above +300 mV) because they have a serine and a tyrosine in the crucial positions (fig. 2b) (Denke et al. 1998; Schröter et al. 1998; Schoepp-Cothenet et al. 2009; Duval et al. 2010).
A Third Rieske/cytb Cluster in Haloferax volcanii
Haloferax volcanii is the only organism sequenced so far to have a third locus coding for a Rieske/cytb complex. The corresponding Rieske protein is high potential and precedes the gene coding for a cytochrome b, which is not split. Interestingly, this Rieske protein and the associated cytochrome b group on phylogenetic trees with the Rieske/cytb complexes that are in genomic context with Nar, but branch off at the basis of the phylogenetic clade (fig. 5).
Multiple sequence alignments of cytochrome b and the Rieske protein of the two types of gene clusters show high-sequence conservation among the sequences of each type contrasting with relatively low-sequence conservation between the two types. A multiple alignment of the sequence stretches in the vicinity of the strictly conserved heme ligating histidine residues, the most highly conserved part of the cytochrome b sequence, is shown in figure 2a and that of the well-conserved region around the Rieske cluster-binding motif in figure 2b.
Phylogenetic Affiliation of the Two Distinct Types of Rieske/cytb Complexes
Phylogenetic trees of cytochrome b and the Rieske protein are shown in figures 3, 4, 5, and 6. A schematic representation of the cytochrome b tree is shown in unrooted form in figure 3. Detailed internal phylogenies of bacterial cytochromes b can be seen in the subtrees in figures 4 and 5. Figure 4 shows a tree reconstructed from the alignment of cytochrome b sequences of Haloarchaea in genomic context with Nar and its phylogenetically closest relatives which are the sequences from Actinobacteria and Thermus/Deinococcus. Selected proteobacterial cytochrome b sequences are also included in this tree. Figure 5 shows the tree based on the alignment of the split haloarchaeal cytochrome b and the cytochrome b sequences from the green branches (Heliobacteria, Bacilli, Cyanobacteria, chloroplasts, and Chlorobiaceae, see below) and the organisms of groups A1 and A2. The Rieske tree is depicted in figure 6.
Fig. 6.—
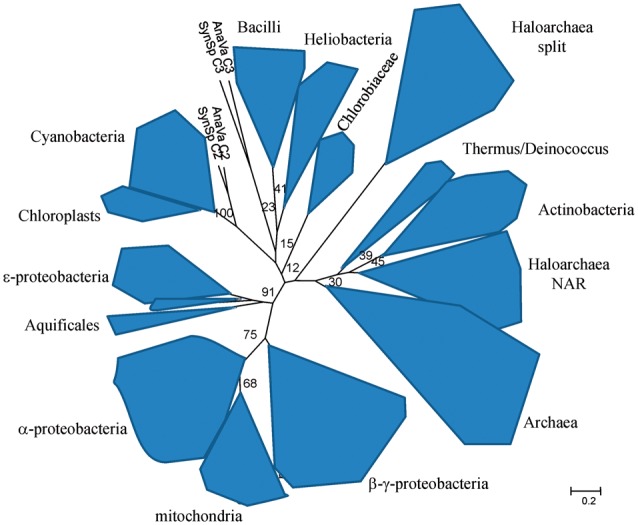
The phylogenetic tree of the Rieske subunit form Rieske/cytb complexes (as published in the study by Lebrun et al. [2006]), enriched by the sequences of the two haloarchaeal Rieske proteins.
The Cytochrome b Tree
Rather than clustering with the archaeal subtree of the entire phylogeny, the two groups of haloarchaeal cytochromes b are located within bacterial phyla. Cytochrome b genes encoded in Rieske/cytb-Nar clusters emerge from within a common clade comprising Actinobacteria and the Thermus/Deinococcus group (figs. 3 and 4). The second class of sequences, characterized by a split cytochrome b gene, is affiliated with a region in the tree containing the b6f complex and related enzymes, that is, the “green clade” of Rieske/cytb complexes (Nitschke et al. 2010). The phyla belonging to the “green clade,” as defined in Nitschke et al. (2010) are Heliobacteria, Bacilli, Cyanobacteria, chloroplasts, and Chlorobiaceae. Although the detailed position of cytochrome b of the haloarchaeal Rieske/cytb-Nar cluster within the Actinobacteria/Deinococcus/Thermus group is robust and characterized by reasonably high bootstrap values (>90), the exact branching point of the haloarchaeal split cytochrome b sequences (figs. 3 and 5) varies substantially as a function of the ensemble of sequences of the “green clade” included in the analysis. The low robustness of the internal topology of the “green clade” and low bootstrap values (about 50) in this region of the tree have already been noticed (Nitschke et al. 2010). Our recent genome analysis revealed a number of “additional species” that branch off in this part of the phylogenetic tree according to phylogenetic analysis of their cytochromes b (groups A1 and A2 in fig. 3). They belong to a variety of phylogenetic groups, namely Nitrospiraceae, δ-proteobacteria, Acidobacteria, Clamydia, Planctomycetes, Deferribacteres, Chloroflexaceae, Firmicutes, and Gemmatimonadetes. Some of these species have more than one Rieske/cytb complex and they show part or all of the characteristics that we consider as phylogenetic marker traits of the “green clade,” such as a split cytochrome b, seven transmembrane helices in cytochrome b, and the presence of the putative cysteine ligand to heme ci (see below). Their position on the phylogenetic tree of cytochrome b is not in agreement with their position on the 16S-rRNA tree, indicating that these species acquired their Rieske/cytb complex via lateral gene transfer. Among these species is a group of δ-proteobacteria (Geobacter species). These bacteria encode two Rieske/cytb complexes in their genome, one that is located in a position of the phylogenetic tree that is in agreement with its position on the 16S-rRNA tree and a second Rieske/cytb complex with a split cytochrome b that can be found on the tree among the members of the “green clade.” If we include the Rieske/cytb complexes of these species (groups A1 and A2 in fig. 3) in the analysis, they are positioned among the members of the “green clade.” In the resulting tree, the bootstrap values in this part of the tree drop and the phyla belonging to the “green clade” no longer group on a single branch of the phylogenetic tree. Therefore, they cannot be named a clade anymore and will be referred to as the “green branches” in the following. Within the subtree of group A1 or group A2, the bootstrap values are relatively high (>50), but we cannot exclude that these reasonable bootstrap values and the distribution of the corresponding species into two groups are influenced by long-branch attraction. Despite these possible problems in phylogenetic tree reconstruction, we consider the phylogenetic proximity of the haloarchaeal split cytochrome b sequences to the “green branches” as real, because these sequences were under all circumstances (i.e., set of sequences reconstruction method) found in the region of the tree where the “green branches” are located.
The split cytochrome b gene was previously suggested as a phylogenetic marker characterizing all representatives of the “green branches” except its most basally branching cluster, that is, the green sulphur bacterial sequences (Nitschke et al. 2010). Indeed, split cytochromes b are found only among the “green branches” and in some of the species of groups A1 and A2, namely in two sequences of Candidatus Kuenenia stuttgartiensis, in Blastopirellula marina, Desulfococcus oleovorans, Pirellula staleyi, and Denitrovibrio acetiphilus, in one of the four cytochrome b sequences from Solibacter usitatus, in Ktedonobacter racemifer, Symbiobacterium thermophilum, and in one group of Geobacter sequences. The fact that the haloarchaeal group of split cytochromes b again phylogenetically emanates from the region of the tree that harbors the “green branches” reinforces the molecular marker property of this trait. Archaea and the remaining bacterial phyla among which are found the lowest branching groups of the bacterial domain have an unsplit cytochrome b corroborating the conclusion that the ancestral cytochrome b indeed existed as a single gene entity (Kramer et al. 2009). The fact that the split cytochrome b occurs only among members of the “green branches” and that all sequences are split between the forth and the fifth transmembrane helix may suggest that the split occurred only once during the history of this enzyme. However, in all cytochrome b trees, some species of groups A1 and A2 with an unsplit cytochrome b gene are positioned among the species with a split cytochrome. This precludes a clearer statement on the singularity of the splitting event.
The number of transmembrane helices was proposed to be another marker trait of the “green branches” (Schütz et al. 2000; Nitschke et al. 2010). All members of the green branches were reported to have seven helices, the remaining Rieske/cytb enzymes harboring cytochrome b subunits with eight helices. In haloarchaeal split cytochrome b, however, the hydrophobic stretches in the two subunits sum up to eight, and during sequence analysis for this study, we realized that the cytochrome b sequences of the two Rieske/cytb complexes present in the so far sequenced Geobacter species (Geobacter sp., G. bemidjiensis, G. metallireducens, G. sulfurreducens, and G. uranireducens) have only seven hydrophobic (putative transmembranous) stretches. Therefore, we now suppose that loss of a C-terminal α-helix occurred several times during the evolutionary history of this subunit.
The presence of the conserved cysteine residue that covalently binds heme ci in Cyanobacteria (Kurisu et al. 2003) and chloroplasts (Stroebel et al. 2003) is also observed in all members of Heliobacteria and Bacilli. In addition, it occurs in one of the two Rieske/cytb complexes encoded in the genome of Geobacter species and in some of the species of groups A1 and A2, namely in two sequences from Candidatus Kuenenia stuttgartiensis, in Blastopirellula marina, Ktedonobacter racemifer, Pirellula staleyi, Desulfococcus oleovorans and in one of the four cytochrome b sequences from Solibacter usitatus. In all these cases, the presence of the cysteine residue is concomitant to the presence of the split cytochrome b sequence with seven helices in total. Haloarchaea, Symbiobacterium thermophilum and Denitrovibrio acetiphilus are the only organisms known so far that feature a split of the cytochrome b sequence but not the conserved cysteine residue as a putative ligand to heme ci. So far, only for Heliobacteria, it has been confirmed experimentally that the presence of the cysteine indeed correlated with the presence of heme ci (Ducluzeau et al. 2008). Further work is required to extend this correlation to other phyla.
The Rieske Tree
Phylogenetic studies of the Rieske subunit are hampered by features inherent to this protein (Lebrun et al. 2006) as there are its short sequence and the presence of insertions of different length that most probably occurred several times independently in different lineages and in different well-defined positions in the protein sequence. Therefore, structural information is necessary to guide the sequence alignment. We constructed a phylogenetic tree of the Rieske subunit in 2006 (Lebrun et al. 2006), based on the available crystal structures or the structure predictions for proteins of phyla where no structures could be obtained so far. Further work on the phylogeny of this protein subunit is awaiting new crystal structures of representatives of so far unrepresented phyla.
Therefore, for the time being, we base our discussion of the phylogeny of the Rieske subunit on the tree published in 2006 to which we added the sequences of the two types of Rieske subunits of Haloarchaea (see fig. 6).
In the Rieske tree, as in the tree of cytochrome b, sequences from most of the organisms that belong to a phylogenetic group cluster together, and some of the above-discussed cases of lateral gene transfer can be clearly detected: Archaea and bacteria are well separated on the tree to the exception of the Haloarchaea. The Proteobacteria form a well-defined clade and the Aquificales are affiliated to the ε-proteobacteria, as observed for cytochrome b. The Rieske protein that is in genomic context with Nar in Haloarchaea groups together with the Actinobacteria on the Rieske tree as on the cytochrome b tree. The Rieske proteins affiliated to the split cytochrome b gene are located among the bacterial sequences on the tree in a place differing from that of the Rieske/cytb-Nar cluster, indicating that both complexes have been acquired by independent events of lateral gene transfer.
Despite the shortcomings of the Rieske tree, we take the similarities between the Rieske and the cytochrome b trees and the fact that the two genes are nearly always organized in the order Rieske ► cytochrome b as an indication that the Rieske subunit and the cytochrome b subunit co-evolved.
Discussion
Phylogenetic analysis indicated that Haloarchaea acquired their Rieske/cytb complexes twice independently from different bacterial sources. The presence of this enzyme allows optimizing the energy yield of diverse respiratory metabolisms, due to the efficient proton pumping capacity of the complex. Rieske/cytb complexes and their functional role in bioenergetic chains are reasonably well characterized in Heliobacteria (Kramer et al. 1997; Ducluzeau et al. 2008), Bacilli (Yu and Le Brun 1988; Sone and Fujiwara 1991; Liebl et al. 1992; Tanaka et al. 1996), Actinobacteria (Sone et al. 2001, 2003), and Aquificales (Schütz et al. 2003). They are extremely well studied in proteobacteria and mitochondria (p.e. Xia et al. 1984; Zhang et al. 1998; Cape et al. 2006; Swierczek et al. 2010), chloroplasts, and cyanobacteria (p.e. Joliot and Joliot 1986; Kurisu et al. 2003; Stroebel et al. 2003; Alric et al. 2005; Baymann et al. 2007). However, our knowledge on archaeal representatives of this enzyme family remains poor. Biochemical and biophysical characterizations of archaeal representatives of this enzyme have been reported for the Crenarchaeota Sulfolobus acidocaldarius and sp. (Lübben et al. 1994; Brugna et al. 1999; Bönisch et al. 2003; Hiller et al. 2003; Iwasaki et al. 2004) and for Acidianus ambivalens (Bandeiras et al. 2009). For the Sulfolobus species, a functional supercomplex of the Rieske/cytb complex with an oxidase has been obtained (Iwasaki et al. 1995; Komorowski et al. 2002), and a study on Pyrobaculum oguniense (Nunoura et al. 2003) showed that the Rieske/cytb complex is expressed together with a SoxB-type oxidase under anaerobic growth conditions.
In the case of Haloarchaea, a detailed scenario for the involvement of the Rieske/cytb enzymes encoded by the Rieske/cytb-Nar gene clusters in the denitrifying chain has been proposed (Martinez-Espinosa et al. 2007). In this model, the Rieske/cytb complex delivers electrons via a periplasmic high-potential b-type cytochrome to the iron–sulphur subunit NarH and eventually to the catalytic subunit NarG of the Nar-type nitrate reductase. In agreement with this electron transfer chain in Haloarchaea, Nar appears to be located in the periplasm in contrast to the characterized denitrification chains of the domain Bacteria, where nitrate reduction via Nar occurs in the cytoplasm and where electron donation to Nar bypasses the Rieske/cytb complex (Yoshimatsu et al. 2002; Martinez-Espinosa et al. 2007; van Lis et al. 2010). However, this mode of operation of the haloarchaeal Rieske/cytb-Nar enzymes so far relies mainly on bioinformatic data (Martinez-Espinose et al. 2007; Bonete et al. 2008) and needs to be tested experimentally.
For the second Rieske/cytb complex in the genome of Haloarchaea, featuring a split cytochrome b and a high-potential Rieske protein, no physiological role has been proposed so far. Halocyanin encoded in the same gene cluster may be an electron acceptor of the Rieske/cytb complex and has been proposed to be a donor to a cytochrome oxidase (Scharf et al. 1997). An implication of this complex in aerobic respiration seems the most likely candidate. We would like to point out in this context that the presence of a high-potential Rieske protein in an organisms for which low-potential menaquinone is the only quinone detected (Collins and Jones 1981; de Rosa and Gambacorta 1988; Wainø et al. 2000; Gruber et al. 2004) is unusual. In general, the redox potential of the Rieske cluster correlates with the redox potential of its electron donor, the quinone (Schoepp-Cothenet et al. 2009). Experimental results on bc1 complexes that have high-potential Rieske proteins and normally react with high-potential ubiquinones showed that low-potential quinones as substrate resulted in enhanced superoxide production (Cape et al. 2005). However, this combination appears to occur in nature in Haloarchaea that grow for the most part aerobically.
Haloarchaea are hitherto the only recognized representatives of Archaea that imported their Rieske/cytb complexes from the domain of Bacteria. Lateral gene transfer out of the bacterial domain was deduced earlier from the genome sequence of the first sequenced Haloarchaeal genome, Halobacterium NARC-1, for six genes of menaquinone synthesis, cytochrome c oxidase, and NADH dehydrogenase (Kennedy et al. 2001). A later investigation of a few additional halobacterial species confirmed these results (Boucher et al. 2003). Further phylogenetic studies revealed that in addition to the Rieske/cytb complexes, the enzyme nitric oxide reductase also appear to have been imported into Haloarchaea via lateral gene transfer from a donor related to Actinobacteria (Ducluzeau et al. 2009; van Lis et al. 2010). It has been argued that Haloarchaea are specifically prone to lateral gene transfer from other halophilic species, both archaeal and bacterial, due to sharing a very confined, restricted extreme habitat (Mongodin et al. 2005). Certainly, this is a necessary condition for lateral gene transfer to occur but the question of the evolutionary advantage, that is, the selection pressure for the laterally transferred genes to remain and integrate the genome of the receptor species, remains unsolved. A close inspection of recent 16S-rRNA phylogenetic trees of the archaeal domain (Yarza et al. 2008; Brochier-Armanet et al. 2011; Kelly et al. 2011) prompted us to propose a hypothetical answer to this question. In these species phylogenies, Haloarchaea cluster together with Methanosarcinales and Methanomicrobiales, that is, with archaeal species having a very specialized energy metabolism, that is, methanogenesis. Due to the strongly reducing electrochemical properties of the involved substrates, methanogenic electron transfer chains operate at substantially lower redox potentials than typical aerobic and anaerobic respiratory chains. Methanosarcinales have methanophenazine, a hydrophobic molecule that fulfills the electron and proton shuttle function of quinones while featuring a much lower redox potential (Thauer et al. 2008). Methanomicrobiales do not have this molecule and like all methanogens are devoid of enzymes involved in the higher potential aerobic or anaerobic respiratory chains to the exception of the ATPase. Therefore, the common ancestor of extant Haloarchaea was restricted in its bioenergetic possibilities to methanogenesis using CO2 as an electron acceptor and hydrogen, actetate, pyruvate, formate, alcohol, or methyl compounds as electron donors. The ΔG available from these reactions is low (136 kJ at best with H2 as an electron donor, due to the low ΔEm of the redox couples CH4/CO2 and H+/H2 of 150 mV (Thauer et al. 2008)). If other electron acceptors such as sulphate, nitrate FeII, or MnIV are available, methanogens will have to compete for H2 against organisms relying on anaerobic respiration of these oxidized molecules, that is, on a much more energy-rich metabolism.
Environmental changes due to either global geochemical transitions of migration to new habitats, which resulted in a shortage in hydrogen, and/or the appearance of different electron acceptors and bacteria that metabolize them may have rendered the methanogenic lifestyle insufficiently competitive. Devoid of nearly all bioenergetic enzymes for metabolisms besides methanogenesis, the ancestor of Haloarchaea may have found a way to enhance its fitness by taking in the bioenergetic equipment of Bacteria that shared the habitat. Integrating these genes into its genome allowed the Haloarchaea to radiate to the metabolical diversity found today.
The phylogeny of the Rieske/cytb complex is a good example for dominant vertical gene transfer to the exception of a few specific cases such as Haloarchaea, where laterally acquired genes provided an evolutionary advantage to the parent species and therefore got stabilized in the genome. Today, only a few other enzymes of the bioenergetic reaction chain of the Haloarchaea are studied by bioinformatic means. The investigation of further enzymes and a biochemical and biophysical characterization of the energy metabolism of Haloarchaea will be necessary to back up our hypothesis and to draw further conclusions. In the meantime, we would like to summarize available information. First, lateral gene transfer between organisms even phylogenetically as distant as Archaea and Bacteria does occur and may involve numerous genes. Second, there are bioenergetic enzymes such as the Rieske/cytb complexes that show only a few cases of lateral gene transfer indicating that in most cases, laterally transferred genes are lost rapidly. Third, for the example analyzed here, Haloarchaea, the bioenergetic equipment of its ancestor was highly specialized and adapted to a metabolism with poor energy yield. At one point, in history, laterally transferred genes could have enlarged the metabolic capacities of individual cells and therefore may have conferred an evolutionary advantage to single organisms in their specific environment. Selection pressure could have operated on the difference between individuals, and as a consequence, organism with acquired metabolic capacities took over in the population and the imported gene became finally part of the genome of the species. Therefore, events of lateral gene transfer do not necessarily fully blur the evolutionary history of enzymes and their parent organisms but may bear witness to the selection pressure that resulted in their integration in the genome. If we can pinpoint these events on a phylogenetic tree of an enzyme and if we know the function of the enzyme and the bioenergetic reaction chains it is operating in, these events may hold information concerning the geochemical and biological history that shaped the organism.
Acknowledgments
This work was supported by the French Agence Nationale de la Recherche grant BLAN 1506 01 to F.B. and W.N.
Literature Cited
- Alefounder PR, McCarthy JEG, Ferguson SJ. The basis of the control of nitrate reduction by oxygen in Paracoccus denitrificans. FEMS Microbiol Lett. 1981;12:321–326. [Google Scholar]
- Alric J, Pierre Y, Picot D, Lavergne J, Rappaport F. Spectral and redox characterization of the heme ci of the cytochrome b6f complex. Proc Natl Acad Sci U S A. 2005;102:15860–15865. doi: 10.1073/pnas.0508102102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandeiras TM, et al. The cytochrome ba complex from the thermoacidophilic crenarchaeote Acidianus ambivalens is an analog of bc(1) complexes. Biochim Biophys Acta. 2009;1787:37–45. doi: 10.1016/j.bbabio.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Baymann F, et al. The redox protein construction kit: pre-LUCA evolution of energy conserving enzymes. Phil Trans R Soc Lond B. 2003;358:267–274. doi: 10.1098/rstb.2002.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baymann F, Giusti F, Picot D, Nitschke W. The ci /bH-moiety in the cytochrome b6f complex studied by EPR. A pair of strongly interacting hemes. Proc Natl Acad Sci U S A. 2007;104:519–524. doi: 10.1073/pnas.0606369104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bönisch H, Schmidt SL, Schäfer G, Ladenstein R. The structure of the soluble domain of an archaeal Rieske iron-sulfur protein at 1.1 A resolution. J Mol Biol. 2003;319:791–805. doi: 10.1016/S0022-2836(02)00323-6. [DOI] [PubMed] [Google Scholar]
- Boogerd FC, van Verseveld HW, Stouthamer AH. Electron transport to nitrous oxide in Paracoccus enitrificans. FEBS Lett. 1980;113:279–284. doi: 10.1016/0014-5793(80)80609-0. [DOI] [PubMed] [Google Scholar]
- Bonete JM, Martinez-Espinosa RM, Pire C, Zafrilla B, Richardson DJ. Nitrogen metabolism in haloarchaea. Saline Syst. 2008;4:9. doi: 10.1186/1746-1448-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher Y, et al. Lateral gene transfer and the origin of prokaryotic groups. Annu Rev Genet. 2003;37:283–328. doi: 10.1146/annurev.genet.37.050503.084247. [DOI] [PubMed] [Google Scholar]
- Brochier-Armanet C, Forterre P, Gribaldo S. Phylogeny and evolution of the Archaea: one hundred genomes later. Curr Opin Microbiol. 2011;14:274–281. doi: 10.1016/j.mib.2011.04.015. [DOI] [PubMed] [Google Scholar]
- Brugna M, et al. Redox components of cytochrome bc-type enzymes in acidophilic prokaryotes. II. The Rieske protein of phylogenetically distant acidophilic organisms. J Biol Chem. 1999;274:16766–16772. doi: 10.1074/jbc.274.24.16766. [DOI] [PubMed] [Google Scholar]
- Cape JL, et al. The respiratory substrate rhodoquinol induces Q-cycle bypass reactions in the yeast cytochrome bc1 complex: mechanistic and physiological implications. J Biol Chem. 2005;280:34654–34660. doi: 10.1074/jbc.M507616200. [DOI] [PubMed] [Google Scholar]
- Cape JL, Bowman MK, Kramer DM. Understanding the cytochrome bc complexes by what they do not do. The Q-cycle at 30. Trends Biochem Sci. 2006;11:46–55. doi: 10.1016/j.tplants.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Castresana J, Lübben M, Saraste M. New archaebacterial genes coding for redox proteins: implications for the evolution of aerobic metabolism. J Mol Biol. 1995;250:202–210. doi: 10.1006/jmbi.1995.0371. [DOI] [PubMed] [Google Scholar]
- Cheah KS. Properties of electron transport particles from Halobacterium culirubrum. The respiratory chain system. Biochim Biophys Acta. 1969;180:320–333. doi: 10.1016/0005-2728(69)90117-0. [DOI] [PubMed] [Google Scholar]
- Cheah KS. The membrane-bound ascorbate oxidase system of Halobacterium halobium. Biochim Biophys Acta. 1970a;205:148–160. doi: 10.1016/0005-2728(70)90245-8. [DOI] [PubMed] [Google Scholar]
- Cheah KS. Properties of the membrane-bound respiratory chain system of Halobacterium salinarum. Biochim Biophys Acta. 1970b;216:43–53. doi: 10.1016/0005-2728(70)90157-x. [DOI] [PubMed] [Google Scholar]
- Collins MD, Jones D. Distribution of isoprenoid quinone structural types in bacteria and their taxonomic implications. Microbiol Rev. 1981;45:316–354. doi: 10.1128/mr.45.2.316-354.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denke E, et al. Alteration of the midpoint potential and catalytic activity of the rieske iron-sulfur protein by changes of amino acids forming hydrogen bonds to the iron-sulfur cluster. J Biol Chem. 1998;273:9085–9093. doi: 10.1074/jbc.273.15.9085. [DOI] [PubMed] [Google Scholar]
- de Rosa M, Gambacorta A. The lipids of archaebacteria. Prog Lipid Res. 1988;27:153–175. doi: 10.1016/0163-7827(88)90011-2. [DOI] [PubMed] [Google Scholar]
- Ducluzeau AL, Chenu E, Capowiez L, Baymann F. The Rieske/cytochrome b complex of Heliobacteria. Biochim Biophys Acta. 2008;1777:1140–1146. doi: 10.1016/j.bbabio.2008.04.026. [DOI] [PubMed] [Google Scholar]
- Ducluzeau A-L, et al. Was nitric oxide the first deep electron sink? Trends in Biochem Sci. 2009;34:9–15. doi: 10.1016/j.tibs.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Duval S, Ducluzeau A-L, Nitschke W, Schoepp-Cothenet B. Enzyme phylogenies as markers for the oxidation state of the environment: the case of the respiratory arsenate reductase and related enzymes. BMC Evol Biol. 2008;8:206. doi: 10.1186/1471-2148-8-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval S, Santini JM, Nitschke W, Hille R, Schoepp-Cothenet B. The small subunit AroB of arsenite oxidase: lessons on the [2Fe2S] Rieske protein superfamily. J Biol Chem. 2010;285:20442–20451. doi: 10.1074/jbc.M110.113811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. An alternating least squares approach to inferring phylogenies from pairwise distances. Syst Biol. 1997;46:101–11. doi: 10.1093/sysbio/46.1.101. [DOI] [PubMed] [Google Scholar]
- Galtier N, Gouy M, Gautier C. SEAVIEW and PHYLO_WIN: two graphic tools for sequence alignment and molecular phylogeny. Comput Appl Biosci. 1996;12:543–548. doi: 10.1093/bioinformatics/12.6.543. [DOI] [PubMed] [Google Scholar]
- Guiral M, et al. New insights into the respiratory chains of the chemolithoautotrophic and hyperthermophilic bacterium Aquifex aeolicus. J Prot Res. 2009;8:1717–1730. doi: 10.1021/pr8007946. [DOI] [PubMed] [Google Scholar]
- Gruber C, et al. Halobacterium noricense sp. nov., an archaeal isolate from a bore core of an alpine Permian salt deposit, classification of Halobacterium sp. NRC-1 as a strain of H. salinarum and emended description of H. salinarum. Extremophiles. 2004;8:431–439. doi: 10.1007/s00792-004-0403-6. [DOI] [PubMed] [Google Scholar]
- Hallberg-Gradin C, Colmjö A. Four different b-type cytochromes in the halophilic Archaebacterium Halobacterium halobium. Arch Biochem Biophys. 1989;272:130–136. doi: 10.1016/0003-9861(89)90203-8. [DOI] [PubMed] [Google Scholar]
- Hauska G. Composition and structure of cytochrome bc1 and b6f complexes. Enz Plant Physiol. 1986;19:496–507. [Google Scholar]
- Henninger T, et al. A novel Rieske iron-sulfur protein from the hyperthermophilic crenarchaeon Pyrobaculum aerophilum: sequencing of the gene, expression in E. coli and characterization of the protein. J Bioenerg Biomembr. 1999;31:119–128. doi: 10.1023/a:1005447710894. [DOI] [PubMed] [Google Scholar]
- Hiller A, Henninger T, Schäfer G, Schmidt CL. New genes encoding subunits of a cytochrome bc1-analogous complex in the respiratory chain of the hyperthermoacidophilic crenarchaeon Sulfolobus acidocaldarius. J Bioenerg Biomembr. 2003;35:121–131. doi: 10.1023/a:1023742002493. [DOI] [PubMed] [Google Scholar]
- Itoh M, Matsuura K, Satoh T. Involvement of cytochrome bc1 complex in the electron transfer pathway for N2O reduction in a photodenitrifier, Rhodobacter sphaeroides f. s. denitrificans. FEBS Lett. 1989;251:104–108. [Google Scholar]
- Iwasaki T, Matsuura K, Oshima T. Resolution of the aerobic respiratory system of the thermoacidophilic Archaeon, Sulfolobus sp. Strain7. J Biol Chem. 1995;270:30881–30892. doi: 10.1074/jbc.270.52.30881. [DOI] [PubMed] [Google Scholar]
- Iwasaki T, Kounosu A, Uzawa T, Samoilova RI, Dikanov SA. Orientation-selected 15N-HYSCORE detection of weakly coupled nitrogens around the archaeal Rieske [2Fe-2S] center. J Am Chem Soc. 2004;126:13902–13903. doi: 10.1021/ja045898x. [DOI] [PubMed] [Google Scholar]
- Joliot P, Joliot A. Proton pumping and electron transfer in the cytochrome b/f complex of algae. Biochim Biophys Acta. 1986;849:211–222. [Google Scholar]
- Kelly S, Wickstead B, Gull K. Archaeal phylogenies provide evidence in support of a methanogenic origin of the Archaea and a thaumarchaeal origin of the eukaryotes. Proc Biol Sci. 2011;278:1009–1018. doi: 10.1098/rspb.2010.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy SP, Ng WV, Salzberg SL, Hood L, DasSarma S. Understanding the adaptation of Halobacterium species NRC-1 to its extreme environment through computational analysis of its genome sequence. Gen Res. 2001;11:1641–1650. doi: 10.1101/gr.190201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komorowski L, Verheyen W, Schäfer G. The Archaeal respiratory supercomplex SoxM from S. acidocaldarius combines features of quinol and cytochrome c oxidases. J Biol Chem. 2002;383:1791–1799. doi: 10.1515/BC.2002.200. [DOI] [PubMed] [Google Scholar]
- Kramer DM, Schoepp B, Liebl U, Nitschke W. Cyclic electron transfer in Heliobacillus mobilis involving a menaquinol-oxidizing cytochrome bc complex and an RCI-type reaction center. Biochemistry. 1997;36:4203–4211. doi: 10.1021/bi962241i. [DOI] [PubMed] [Google Scholar]
- Kramer DM, Nitschke W, Cooles JW. The cytochrome bc1 and related bc complexes: Rieske/cytochrome b complexes as the functional core of a central electron/proton transfer complex. In: Hunter N, Daldal F, Thurnauer MC, Beatty JT, editors. The purple photosynthetic bacteria. Dordrecht (The Netherlands): Springer Science + Business Media BV; 2009. pp. 451–473. [Google Scholar]
- Kurisu G, Zhang H, Smith JL, Cramer WA. Structure of the cytochrome b6f complex of oxygenic photosynthesis: tuning the cavity. Science. 2003;302:1009–1014. doi: 10.1126/science.1090165. [DOI] [PubMed] [Google Scholar]
- Lane N, Allen JF, Martin W. How did LUCA make a living? Chemiosmosis in the origin of life. BioEssays. 2010;32:271–280. doi: 10.1002/bies.200900131. [DOI] [PubMed] [Google Scholar]
- Larkin MA, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Lebrun E, et al. Arsenite oxidase, an ancient bioenergetic enzyme. Mol Biol Evol. 2003;20:686–693. doi: 10.1093/molbev/msg071. [DOI] [PubMed] [Google Scholar]
- Lebrun E, et al. The Rieske protein: a case study on the pitfalls of multiple sequence alignments and phylogenetic reconstruction. Mol Biol Evol. 2006;23:1180–1191. doi: 10.1093/molbev/msk010. [DOI] [PubMed] [Google Scholar]
- Liebl U, Pezennec S, Riedel A, Kellner E, Nitschke W. The Rieske FeS center from the gram-positive bacterium PS3 and its interaction with the menaquinone pool studied by EPR. J Biol Chem. 1992;267:14068–14072. [PubMed] [Google Scholar]
- Lübben M, et al. A second terminal oxidase in Sulfolobus acidocaldarius. Eur J Biochem. 1994;224:151–159. doi: 10.1111/j.1432-1033.1994.tb20006.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Espinosa RM, et al. Look on the positive side! The orientation, identification and bioenergetics of “Archaeal” membrane-bound nitrate reductases. FEMS Microbiol Lett. 2007;276:129–139. doi: 10.1111/j.1574-6968.2007.00887.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Aspects of the chemiosmotic hypothesis. Biochem J. 1970;116:5P–6P. doi: 10.1042/bj1160005p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in energy transduction: a logical development of biochemical knowledge. J Bioenerg. 1972;3:5–24. doi: 10.1007/BF01515993. [DOI] [PubMed] [Google Scholar]
- Mongodin EF, et al. The genome of Salinibacter ruber: convergence and gene exchange among hyperhalophilic bacteria and archaea. Proc Natl Acad Sci U S A. 2005;102:18147–18152. doi: 10.1073/pnas.0509073102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke W, Russell MJ. Hydrothermal focusing of chemical and chemiosmotic energy, supported by delivery of catalytic Fe, Ni, Mo/W, Co, S and Se, forced life to emerge. J Mol Evol. 2009;69:481–496. doi: 10.1007/s00239-009-9289-3. [DOI] [PubMed] [Google Scholar]
- Nitschke W, van Lis R, Schoepp-Cothenet B, Baymann F. The “green” phylogenetic clade of Rieske/cytb complexes. Photosynth Res. 2010;104:347–355. doi: 10.1007/s11120-010-9532-1. [DOI] [PubMed] [Google Scholar]
- Nübel T, Klughammer C, Hube R, Hauska G, Schütz M. Sulfide:quinone oxidoreductase in membranes of the hyperthermophilic bacterium Aquifex aeolicus (VF5) Arch Microbiol. 2000;173:233–244. doi: 10.1007/s002030000135. [DOI] [PubMed] [Google Scholar]
- Nunoura T, Sako Y, Wakagi T, Uchida A. Regulation of the aerobic respiratory chain in the facultatively aerobic and hyperthermophilic archaeaon Pyrobaculum oguniense. Microbiology. 2003;149:673–688. doi: 10.1099/mic.0.26000-0. [DOI] [PubMed] [Google Scholar]
- Rich PR. Electron and proton transfers through quinones and cytochrome bc complexes. Biochim Biophys Acta. 1984;768:53–79. doi: 10.1016/0304-4173(84)90007-7. [DOI] [PubMed] [Google Scholar]
- Scharf B, Wittenberg R, Engelhard M. Electron transfer proteins from the haloalkaliphilic Archaeon Natronobacterium pharaonis: possible components of the respiratory chain include cytochrome bc and a terminal oxidase cytochrome ba3. Biochemistry. 1997;36:4471–4479. doi: 10.1021/bi962312d. [DOI] [PubMed] [Google Scholar]
- Schoepp-Cothenet B, et al. Menaquinone as a pool quinone in a purple bacterium. Proc Natl Acad Sci U S A. 2009;106:8549–8554. doi: 10.1073/pnas.0813173106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröter T, et al. Mutational analysis of residues forming hydrogen bonds in the Rieske [2Fe-2S] cluster of the cytochrome bc1 complex in Paracoccus denitrificans. Eur J Biochem. 1998;255:100–106. doi: 10.1046/j.1432-1327.1998.2550100.x. [DOI] [PubMed] [Google Scholar]
- Schütz M, et al. Early evolution of cytochrome bc-complexes. J Mol Biol. 2000;300:663–676. doi: 10.1006/jmbi.2000.3915. [DOI] [PubMed] [Google Scholar]
- Schütz M, et al. The naphthoquinol oxidizing cytochrome bc1 complex of the hyperthermophilic knallgasbacterium Aquifex aeolicus: properties and phylogenetic relationships. Biochemistry. 2003;42:10800–10808. doi: 10.1021/bi034452a. [DOI] [PubMed] [Google Scholar]
- Shapleigh JP, Payne WJ. Nitric oxide-dependant proton translocation in various denitrifiers. J Bact. 1985;163:837–840. doi: 10.1128/jb.163.3.837-840.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sone N, Fujiwara Y. Effects of aeration during growth of Bacillus stearothermophilus on proton pumping activity and change of terminal oxidases. J Biochem. 1991;110:1016–1021. doi: 10.1093/oxfordjournals.jbchem.a123671. [DOI] [PubMed] [Google Scholar]
- Sone N, Sawa G, Sone T, Noguchi S. Thermophilic bacilli have split cytochrome b genes for cytochrome b6 and subunit IV. First cloning of cytochrome b from a Gram-positive bacterium (Bacillus stearothermophilus) J Biol Chem. 1995;270:10612–10617. doi: 10.1074/jbc.270.18.10612. [DOI] [PubMed] [Google Scholar]
- Sone N, et al. A novel hydrophobic diheme c-type cytochrome. Purification from Corynebacterium glutamicum and analysis of the QcrCBA operon encoding three subunit proteins of a putative cytochrome reductase complex. Biochim Biophys Acta. 2001;1503:279–290. doi: 10.1016/s0005-2728(00)00205-x. [DOI] [PubMed] [Google Scholar]
- Sone N, et al. QcrCAB operon of a nocardia-form actinomycete Rhodococcus rhodochrous encodes cytochrome reductase complex with diheme cytochrome cc subunit. Biochim Biophys Acta. 2003;1557:125–131. doi: 10.1016/s0005-2728(02)00394-8. [DOI] [PubMed] [Google Scholar]
- Sreeramulu K, Schmidt CL, Schäfer G, Anemüller S. Studies on the electron transport chain of the Euryarchaeon Halobacterium salinarum: indications for a type II NADH dehydrogenase and a complex III analog. J Bioenerg Biomemb. 1998;30:447–453. doi: 10.1023/a:1020538129400. [DOI] [PubMed] [Google Scholar]
- Stroebel D, Choquet Y, Popot JL, Picot D. An atypical haem in the cytochrome b(6)f complex. Nature. 2003;426:413–418. doi: 10.1038/nature02155. [DOI] [PubMed] [Google Scholar]
- Suharti, Heering HA, de Vries S. NO reductase from Bacillus azotoformans is a bifunctional enzyme accepting electrons from menaquinol and a specific endogenous membrane-bound cytochrome c551. Biochemistry. 1984;43:13487–13495. doi: 10.1021/bi0488101. [DOI] [PubMed] [Google Scholar]
- Swierczek M, et al. An electronic bus bar lies in the core of cytochrome bc1. Science. 2010;329:451–454. doi: 10.1126/science.1190899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Inoue M, Sakamoto J, Sone N. Intra- and inter-complex cross-linking of subunits in the quinol oxidase super-complex from thermophilic Bacillus PS3. J Biochem. 1996;119:482–486. doi: 10.1093/oxfordjournals.jbchem.a021267. [DOI] [PubMed] [Google Scholar]
- Thauer R, Kaster A-K, Seedorf H, Buckel W, Hedderich R. Methanogenic archaea: ecologically relevant differences in energy conservation. Nat Rev. 2008;6:579–591. doi: 10.1038/nrmicro1931. [DOI] [PubMed] [Google Scholar]
- van Lis R, Ducluzeau A-L, Nitschke W, Schoepp-Cothenet B. The nitrogen cycle in the archaean: an intricate interplay of enzymatic and abiotic reactions. In: Moir JWB, editor. Nitrogen cycling in bacteria: molecular analysis. Norfolk (UK): Castor Academic Press; 2010. pp. 1–12. [Google Scholar]
- Wainø M, Tindall BJ, Ingvorsen K. Halorhabdus utahensis gen. nov., sp. nov., an aerobic, extremely halophilic member of the Archaea from Great Salt Lake, Utah. Int J Sys Evol Biol. 2000;50:183–190. doi: 10.1099/00207713-50-1-183. [DOI] [PubMed] [Google Scholar]
- Widger WR, Cramer WA, Herrmann RG, Trebst A. Sequence homology and structural similarity between cytochrome b of mitochondrial complex III and the chloroplast b6-f complex: position of cytochrome b hemes in the membrane. Proc Natl Acad Sci U S A. 1984;81:674–678. doi: 10.1073/pnas.81.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia D, et al. Crystal structure of the cytochrome bc1 complex from bovine heart mitochondria. Science. 1984;277:60–66. doi: 10.1126/science.277.5322.60. [DOI] [PubMed] [Google Scholar]
- Yarza P, et al. The all-species living tree project: a 16S rRNA-based phylogenetic tree of all sequenced type strains. Syst Appl Microbiol. 2008;31:241–250. doi: 10.1016/j.syapm.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Yoshimatsu K, Iwasaki T, Fujiwara T. Sequence and electron paramagnetic resonance analyses of nitrate reductase NarGH from a denitrifying halophilic euryarchaeote Haloarcula marismortui. FEBS Lett. 2002;516:145–150. doi: 10.1016/s0014-5793(02)02524-3. [DOI] [PubMed] [Google Scholar]
- Yoshimatsu K, Araya O, Fujiwara T. Haloarcula marismortui cytochrome b-561 is encoded by the narC gene in the dissimilatory nitrate reductase operon. Extremophiles. 2007;11:41–47. doi: 10.1007/s00792-006-0016-3. [DOI] [PubMed] [Google Scholar]
- Yu J, Le Brun N. Studies of the cytochrome subunits of menaquinone:cytochrome c reductase (bc-complex) of Bacillus subtilis. Evidence for the covalent attachment of heme to the cytochrome b subunit. J Biol Chem. 1988;273:8860–8866. doi: 10.1074/jbc.273.15.8860. [DOI] [PubMed] [Google Scholar]
- Zhang Z, et al. Electron transfer by domain movement in cytochrome bc1. Nature. 1998;392:677–684. doi: 10.1038/33612. [DOI] [PubMed] [Google Scholar]