Abstract
Sorghum (Sorghum bicolor L. Moench) accumulates the anthocyanin cyanidin 3-dimalonyl glucoside in etiolated mesocotyls in response to light. Inoculation with the nonpathogenic fungus Cochliobolus heterostrophus drastically reduced the light-induced accumulation of anthocyanin by repressing the transcription of the anthocyanin biosynthesis genes encoding flavanone 3-hydroxylase, dihydroflavonol 4-reductase, and anthocyanidin synthase. In contrast to these repression effects, fungal inoculation resulted in the synthesis of the four known 3-deoxyanthocyanidin phytoalexins and a corresponding activation of genes encoding the key branch-point enzymes in the phenylpropanoid pathway, phenylalanine ammonia-lyase and chalcone synthase. In addition, a gene encoding the pathogenesis-related protein PR-10 was strongly induced in response to inoculation. The accumulation of phytoalexins leveled off by 48 h after inoculation and was accompanied by a more rapid increase in the rate of anthocyanin accumulation. The results suggest that the plant represses less essential metabolic activities such as anthocyanin synthesis as a means of compensating for the immediate biochemical and physiological needs for the defense response.
Plants are able to make different physiological adjustments in response to a wide range of stimuli in their environment. In response to light, etiolated seedlings of some sorghum (Sorghum bicolor) cultivars accumulate anthocyanin pigments in epidermal tissue of the mesocotyl (Orczyk et al., 1996; Weiergang et al., 1996). Anthocyanins constitute a large class of flavonoids that account for pigmentation, serve to attract animals for pollination and seed dispersal, and are also believed to be important as protectants against UV irradiation (Holton and Cornish, 1995). In response to attempted fungal infection, sorghum synthesizes a group of structurally related compounds, the 3-deoxyanthocyanidins, which serve as phytoalexins. Phytoalexins are low-molecular-weight antimicrobial compounds produced by plants in response to infection or stress (Nicholson and Hammerschmidt, 1992; Smith, 1996). The 3-deoxyanthocyanidins differ from other anthocyanidins in that they are not hydroxylated at the number 3 carbon of the flavonoid oxygen heterocycle (Fig. 1). They were shown to accumulate within inclusions in epidermal cells of sorghum leaves under pathogen attack (Snyder and Nicholson, 1990; Synder et al., 1991). The inclusions become pigmented, indicating the presence of the phytoalexins, and then move within the cell toward the site of attempted penetration. The inclusions eventually burst and release their contents, which kills both the fungus and the cell that synthesized them.
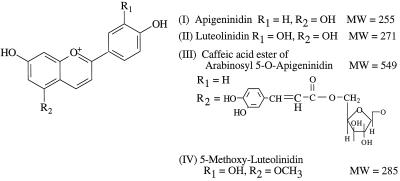
Figure 1.
Chemical structures of the 3-deoxyanthocyanidin phytoalexins. MW, Molecular weight.
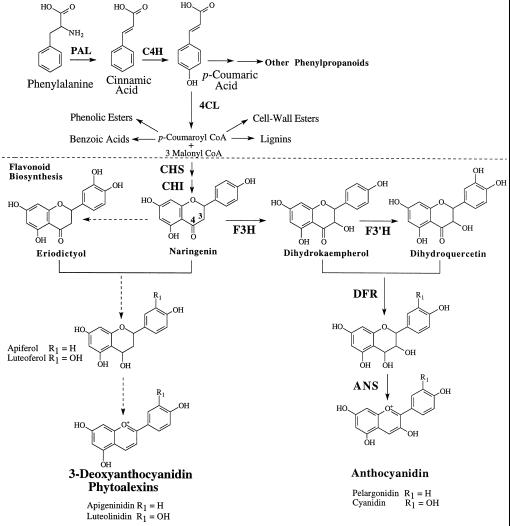
The biosynthesis of anthocyanidins and 3-deoxyanthocyanidins represents two partially overlapping, competing pathways in sorghum. Both compounds are derived from the phenylpropanoid and flavonoid pathways. Activities of the key phenylpropanoid branch-point enzymes PAL and CHS, and expression of their respective genes, are induced by light as well as by fungal infection (Lue et al., 1989; Orczyk et al., 1996). The compounds originate from the condensation of p-coumaroyl CoA and malonyl CoA to form naringenin chalcone, which is converted to the flavanone naringenin. It is from naringenin that both the anthocyanidins and the 3-deoxyanthocyanidins are derived (Dixon and Paiva, 1995; Holton and Cornish, 1995; Hipskind et al., 1996b) (Fig. 2).
Figure 2.
Biosynthetic pathways for 3-deoxyanthocyanidins and anthocyanidins. The proposed route of 3-deoxyanthocyanidin synthesis originates with the first committed step in phenylpropanoid biosynthesis. Phe is deaminated by PAL to form cinnamic acid, which is hydroxylated by C4H to form p-coumaric acid. Subsequently, p-coumaric acid is activated to a high-energy CoA thiol-ester by 4CL, which then serves as one of two substrates for flavonoid biosynthesis mediated by CHS. The biosynthetic routes for normal anthocyanidins and for 3-deoxyanthocyanidins are proposed to diverge from one another after the synthesis of the flavanone naringenin. C4H, Cinnamic acid 4-hydroxylase; 4CL, 4-hydroxycinnamic acid:CoA ligase; CHI, chalcone isomerase; F3′H, flavonoid 3′-hydroxylase. Solid arrows indicate established pathways; dashed arrows indicate proposed metabolic steps in 3-deoxyanthocyanidin synthesis.
The attempted infection of plants by pathogens often evokes extensive and multicomponent defense mechanisms that might include activation of phenylpropanoid biosynthesis (Dixon and Paiva, 1995), modification of cell wall structures by deposition of Hyp-rich proteins (Brisson et al., 1994) and lignin (Bruce and West, 1988), production of PR proteins (van Loon et al., 1994), production of hydrolytic enzymes such as chitinase and glucanases (Kombrink et al., 1991), generation of active oxygen species (Mehdy, 1994), and synthesis of inhibitors of cell wall-degrading enzymes (Degra et al., 1988). Thus, induction of defense mechanisms would inevitably consume considerable amounts of available cellular resources, including substrates and energy. It has been suggested that repression of some cellular functions is likely to occur to ensure a metabolic balance during the host's responses to the stress of disease (Kombrink and Hahlbrock, 1990). Consistent with this, we have observed that anthocyanin accumulation in sorghum is altered after inoculation with fungal pathogens. This observation suggests that infected plants produce the 3-deoxyanthocyanidin phytoalexins at the expense of the light-induced accumulation of anthocyanins. In the present study we demonstrate that inoculation with the fungus Cochliobolus heterostrophus, a nonpathogen of sorghum, results in the synthesis of the deoxyanthocyanidin phytoalexins and the activation of genes encoding for PAL, CHS, and a PR protein, PR-10. In contrast to these stimulatory effects, the accumulation of anthocyanins and the expression of structural genes for anthocyanidin biosynthesis were repressed.
MATERIALS AND METHODS
Plant Material and Fungal Inoculation
Seeds of sorghum (Sorghum bicolor L. Moench cvs DK46 and DK18; Dekalb Pfizer Genetics, Lubbock, TX) were allowed to imbibe in water at 28°C overnight. The seeds were then planted in rolls of germination paper and incubated in darkness at the same temperature for 4 d. This technique provides seedlings with uniformly elongated mesocotyls. The fungus used was Cochiiobolus heterostrophus Drechs. (anamorphic stage Bipolaris maydis [Nisikado and Miyake] Shoemaker), which is nonpathogenic to sorghum. Etiolated seedlings were inoculated with spore suspensions (1.0 × 105 conidia mL−1) of C. heterostrophus. Tween 20 served as a wetting agent (100 μL 100 mL−1) in the inoculum suspension. The resulting suspension was misted onto seedlings with an atomizer, and the plants were incubated at 100% RH under constant light (60 μE m−2 s−1) at room temperature.
Extraction of Anthocyanins and Phytoalexins
Triplicate samples of mesocotyl tissue (approximately 200 mg each) were collected at different time intervals after inoculation. The mesocotyls were excised from the seedlings, and 1-cm segments were removed from the base and the apex of each mesocotyl. The remaining tissue was cut into segments, weighed, and placed in 1 mL of HPLC-grade methanol. The anthocyanins and phytoalexins that had accumulated were allowed to leach from the tissue at 4°C for 24 h, after which time the extracts were analyzed.
HPLC Analysis
The composition of plant extracts was determined by HPLC. Separation was carried out on two reverse-phase C-18 Ultrasphere columns (Beckman) connected in tandem. The dimensions of the two columns were 250 × 4.6 mm and 150 × 4.6 mm. Solvent A was 0.6% perchloric acid and solvent B was 100% HPLC-grade methanol. Samples (20 μL) were injected and eluted isocratically with 40% solvent B at a flow rate of 0.8 mL min−1. Compounds were detected at 480 and 535 nm for the presence of phytoalexins and anthocyanins, respectively, with an absorbance detector (model 190, Beckman) at a sensitivity of 0.001 absorbance units full scale. The anthocyanin concentrations were expressed in terms of cyanidin equivalents with a commercially available cyanidin chloride standard (ICN). Phytoalexin standards included luteolinidin, apigeninidin, and the apigeninidin acyl ester, each of which had been isolated previously in this laboratory (Hipskind et al., 1990). The phytoalexin concentrations were determined based on extinction coefficients of 13,800 m−1 cm−1 for luteolinidin, 18,000 m−1 cm−1 for apigeninidin (Stafford, 1966), and 12,700 m−1 cm−1 for the apigeninidin acyl ester (Hipskind et al., 1990). The concentration of 5-methoxyluteolinidin was estimated from the extinction coefficient for luteolinidin (Lo et al., 1996). The standards were chromatographed and their retention times and peak areas were determined with an integrator (model 3396A, Hewlett-Packard). Retention times were used to identify the flavonoid compounds in the test samples, and the amount of each compound in a sample was determined by comparing the compound's peak area with that of a known concentration for an individual standard.
MALDI-TOF MS Analysis
Plant extracts were analyzed at the Purdue Mass Spectrometry Center with a PerSeptive Biosystems (Framingham, MA) Voyager MALDI-TOF instrument operating in a positive-ion linear mode with a nitrogen laser (387 nm) at an accelerating voltage of 28 kV. The matrix used was α-cyano-4-hydroxycinnamic acid (10 mg mL−1) dissolved in H2O:1% trifluoroacetic acid:acetonitrile (4:1:5). Samples were diluted 10-fold in methanol and then mixed with the matrix solution in a ratio of 1:12.
Northern-Blot Analysis
Total RNA was isolated from liquid nitrogen-frozen mesocotyl tissues by the procedure of Hipskind et al. (1996b). Aliquots of 10 μg were denatured and fractionated on a formaldehyde-1.0% agarose gel in 0.2 M Mops buffer, 0.5 m sodium acetate, 0.1 m EDTA, pH 7.0. That the amount of RNA was approximately the same in each lane was determined with ethidium bromide-stained gels. After electrophoresis, RNA was transferred by capillary action to a ZetaProbe Membrane (Bio-Rad) for at least 16 h, then covalently cross-linked to the membrane with a UV cross-linker (Fisher Scientific). Individual membranes were prehybridized for 15 min at 65°C in 0.5 m sodium phosphate buffer (pH 7.0) containing 7% (w/v) SDS. Membranes were then hybridized for 18 h in the same buffer with the addition of denatured 32P-labeled DNA probes. The hybridized membranes were washed briefly in 40 mm sodium phosphate buffer containing 5% SDS and then washed for an additional 45 min at 65°C with a fresh buffer solution. This wash was replaced with the same buffer containing 1% SDS and the membranes were washed twice for 45 minutes at 65°C. The membranes were then exposed to x-ray film (X-Omat AR, Kodak) with intensifying screens at −70°C.
DNA Probes
The cDNA probes were A1 and A2 from maize encoding DFR and ANS, respectively, F3H from barley, and CHS and PAL from sorghum. A1 and A2 were obtained from U. Wienand and A. Gierl (Max Planck Institute, Köln, Germany). F3H was obtained from M. Meldgaard (Carlsberg Laboratory, Copenhagen, Denmark). PAL and CHS were obtained from C. Magill (Texas A&M University, College Station). A full-length sorghum cDNA clone isolated in this laboratory and corresponding to a member of the PR-10 gene family was also used as a probe. Radioactive DNA probes were prepared by random-primer labeling with a labeling kit (Deca-Prime, Ambion, Austin, TX).
RESULTS
Symptoms of Infection in Mesocotyls of DK46 and DK18 Plants
Sorghum cvs DK46 and DK18 differ in their responses to light (Hipskind et al., 1996b). When exposed to light, etiolated mesocotyls of cv DK46 gradually turned pink, indicating the presence of anthocyanin pigments (Fig. 3, A–C). In contrast, etiolated mesocotyls of cv DK18 did not accumulate anthocyanin pigments in response to light (Fig. 3, G–I). Seedlings of both cultivars were inoculated with C. heterostrophus conidia and placed under constant light, as were uninoculated control plants. At 24 h after inoculation, lesions with reddish-brown pigmentation characteristic of the phytoalexins (Nicholson et al., 1987) were found along the mesocotyl surface of infected cv DK46 plants (Fig. 3D). At this time, anthocyanin pigmentation was often absent from the mesocotyl surface. At 48 h after inoculation anthocyanin pigments started to accumulate; however, tissue lacking anthocyanin pigments could still be found surrounding lesions where the phytoalexins were produced in response to attempted fungal infection (Fig. 3E). At 72 h after inoculation anthocyanin pigments had accumulated in all of the epidermal tissue in which the phytoalexins had not been synthesized (Fig. 3F). The anthocyanin pigmentation was still less intense than that of control plants kept under light for the same period of time (Fig. 3C). Inoculated mesocotyls of cv DK18 exhibited lesions with intense phytoalexin accumulation but did not accumulate anthocyanin pigments (Fig. 3, J–L). By 48 h after inoculation, lesions had coalesced (Fig. 3K), and by 72 h lesions with phytoalexins covered much of the mesocotyl surface (Fig. 3L). The extent of lesion development and phytoalexin accumulation in cv DK18 mesocotyls appeared to be much greater than that which occurred in cv DK46 plants at each time interval. It should be noted that lesion development does not reflect the extent of fungal growth; rather, it reflects the extent of deoxyanthocyanidin phytoalexin synthesis around the sites of attempted infection. We have shown previously that neither pathogens nor nonpathogens of sorghum grow in the plant tissue that is responding by the production of phytoalexins (Nicholson et al., 1987, 1988; Snyder and Nicholson, 1990).
Figure 3.
Comparison of symptoms on etiolated mesocotyls of sorghum cvs DK46 and DK18 after inoculation with conidia of C. heterostrophus. Four-day-old etiolated seedlings were taken from the dark, inoculated, and placed under constant light. Symptom morphology was observed under a dissecting microscope at the indicated time intervals (24, 48, and 72 h). A to C, Uninoculated cv DK46 mesocotyls showing an increase in pink anthocyanin pigmentation over time. D to F, Inoculated cv DK46 mesocotyls. Note the development of reddish-brown lesions (long arrows) and reduction of anthocyanin accumulation (arrowheads) on the plant surface (D). The reddish-brown pigment indicates the presence of 3-deoxyanthocyanidin phytoalexins. The pink coloration characteristic of anthocyanins (small arrows) became darker over time (E and F), but was less intense compared with that of uninoculated plants, which had been placed under light for the same period of time (B and C). G to I, Uninoculated cv DK18 mesocotyls. Note the absence of anthocyanin accumulation. J to L, Inoculated cv DK18 mesocotyls. Note the development of reddish-brown lesions (long arrows) and the lack of anthocyanin pigmentation in the surrounding tissue (arrowheads). Bar = 1 mm.
HPLC Analysis of Anthocyanin and Phytoalexin Accumulation
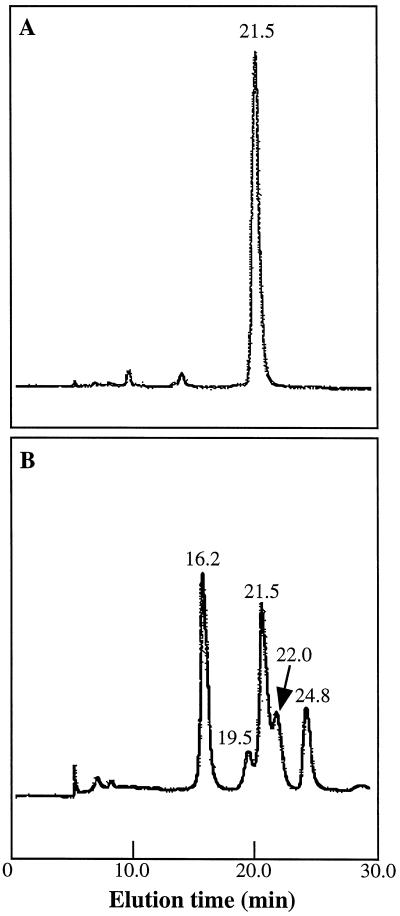
HPLC was used to follow the accumulation of individual 3-deoxyanthocyanidin and anthocyanin pigments in sorghum mesocotyls. The HPLC profile of the pigments extracted from uninoculated control mesocotyls of cv DK46 revealed a major peak with a retention time of 21.5 min (Fig. 4A). Acid hydrolysis (boiling in 2% HCl for 2 h) of the extract resulted in a single peak that co-chromatographed with a cyanidin chloride standard (24.0 min) when separated by HPLC (data not shown). Thus, the anthocyanin that accumulated was a derivative of cyanidin. In addition to the accumulation of the anthocyanin pigment (21.5 min), inoculated mesocotyls of cv DK46 also accumulated each of the known 3-deoxyanthocyanidin phytoalexins (Fig. 4B). The phytoalexin components were luteolinidin (16.2 min), 5-methoxyluteolinidin (19.5 min), apigeninidin (22.0 min), and the caffeic acid ester of arabinosyl 5-O-apigeninidin (24.8 min). Each of these compounds was also produced by inoculated mesocotyls of cv DK18 (Lo et al., 1996).
Figure 4.
HPLC analysis of anthocyanin and 3-deoxyanthocyanidin phytoalexins in cv DK46 mesocotyls. A, Anthocyanin extracted from uninoculated plants. Separation by HPLC revealed a major peak that eluted at 21.5 min. B, Pigments extracted from inoculated plants. Peaks corresponding to luteolinidin (16.2 min), 5-methoxyluteolinidin (19.5 min), apigeninidin (22.0 min), and a caffeic acid ester of arabinosyl-5-O-apigeninidin (24.8 min) were detected in addition to the anthocyanin peak (21.5 min).
MALDI-TOF MS Analysis of Anthocyanin and Phytoalexin Compounds
MALDI coupled with TOF has been used for routine mass-spectrometric analysis of a wide range of biomolecules, including peptides and proteins, oligonucleotides, oligosaccharides, and glycoproteins (Edmondson and Russell, 1996). It is a soft desorption-ionization technique that determines the Mr of both fragile and nonvolatile high-mass molecules. In the past we used plasma desorption MS to identify anthocyanin and 3-deoxyanthocyanidin compounds in plant extracts (Wood et al., 1994). Recently, we have demonstrated that MALDI-TOF is more efficient for the analysis of these flavonoids, especially for the anthocyanins that have higher Mrs because of the presence of sugar residues in the anthocyanidin glycoside. (J.A. Sugui, C. Bonham, S.-C. Lo, K. Wood, and R.L. Nicholson, unpublished data).
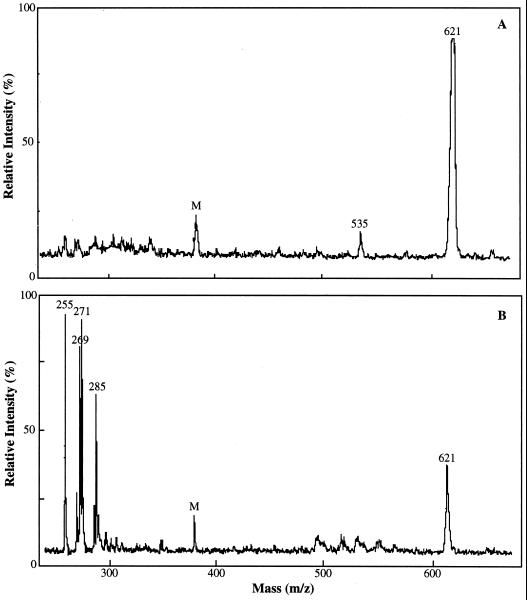
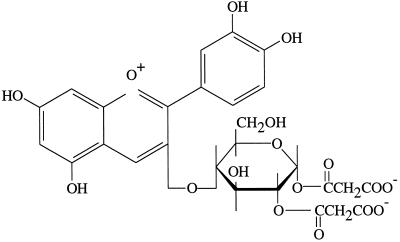
MALDI-TOF analysis of the anthocyanin pigment extracted from uninoculated control cv DK46 mesocotyls exhibited a major ion of 621 m/z and a minor ion of 535 m/z (Fig. 5A). The 621 and 535 Mr are consistent with those of the dimalonyl and monomalonyl derivatives of cyanidin 3-glucoside, respectively, previously identified in maize (Hipskind et al., 1996a) and in members of the Asteraceae (Takeda et al., 1986). The structure of cyanidin 3-dimalonyl glucoside is shown in Figure 6. The aglycone cyanidin is structurally similar to luteolinidin except for the hydroxylation at the number 3 carbon of the flavonoid oxygen heterocycle where glycosylation occurs. The 535 m/z ion probably represents the fragmentation of a malonyl group from the 621 m/z ion during MS analysis. MALDI-TOF data for pigment extracts from inoculated cv DK46 mesocotyls revealed the anthocyanin ion of 621 m/z, and the 3-deoxyanthocyanidin ions of 255 m/z (apigeninidin), 271 m/z (luteolinidin), 285 m/z (5-methoxyluteolinidin), as expected (Fig. 5B). The ion at 269 m/z has also been reported to occur in the inoculated cv DK18 plants (Hipskind et al., 1996b). This Mr is consistent with that of a methyl ether of apigeninidin, a compound that was recently found in grain of Sorghum caudatum and identified as 7-O-methylapigeninidin, Mr 269 (Pale et al., 1997). In our HPLC system this compound probably co-chromatographed with apigeninidin (Fig. 4) and was not detected. Although HPLC analysis revealed the presence of the acyl ester of apigeninidin, it was not detected by MALDI-TOF analysis.
Figure 5.
MALDI-TOF analysis of anthocyanin and 3-deoxyanthocyanidin phytoalexins in cv DK46 mesocotyls. A, Anthocyanin extracted from uninoculated plants 48 h after exposure to light. A major ion at 621 m/z and a minor ion at 535 m/z were detected; 621 and 535 m/z correspond to the Mr of dimalonyl and monomalonyl derivatives of cyanidin 3-glucoside, respectively. B, Pigment extracted from plants 48 h after inoculation and exposure to light. Major ions corresponding to the molecular masses of luteolinidin (271 m/z), 5-methoxyluteolinidin (285 m/z), and apigeninidin (255 m/z) were detected in addition to the anthocyanin ion (621 m/z). The 269 m/z ion is believed to be a methylated derivative of apigeninidin. M, Matrix peak.
Figure 6.
Chemical structure of cyanidin-3-dimalonyl glucoside.
Quantification of Anthocyanin and 3-Deoxyanthocyanidins in Plant Extracts
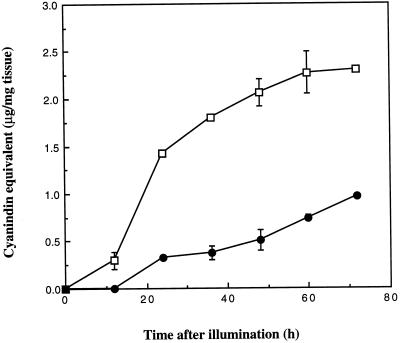
In most studies total anthocyanin contents in plant extracts are measured quantitatively by their absorption between 500 and 550 nm (Strack and Wray, 1989). Because the presence of pigmented 3-deoxyanthocyanidin compounds in extracts from infected tissue would have interfered with the spectrophotometric measurement of anthocyanins, we quantified anthocyanin contents by HPLC and expressed them in terms of cyanidin equivalents. The time course of accumulation of the anthocyanin cyanidin 3-dimalonyl glucoside in uninoculated and inoculated cv DK46 mesocotyls is shown in Figure 7. The anthocyanin was first detected in uninoculated tissues 12 h after exposure to light and continued to accumulate through 60 h. Anthocyanin production was appreciably lower in inoculated tissues and was first detected at 24 h after inoculation (Fig. 7). The total anthocyanin that accumulated was 2.5 times less than that in uninoculated control plants by 72 h after inoculation (Fig. 7).
Figure 7.
Accumulation of the anthocyanin cyanidin 3-dimalonyl glucoside in uninoculated (□) and inoculated (•) cv DK46 mesocotyls after exposure to light. Anthocyanin concentration is expressed as cyanidin equivalents with cyanidin chloride as a standard.
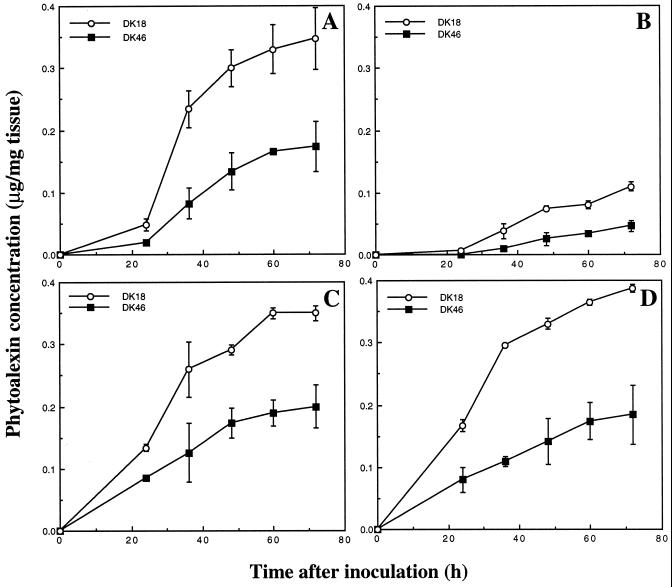
Production of the 3-deoxyanthocyanidin phytoalexins in cvs DK46 and DK18 mesocotyls in response to fungal inoculation is shown in Figure 8. Cultivar DK18 is a sorghum cultivar that does not accumulate anthocyanin pigments upon illumination (Fig. 3, G–I). The patterns of accumulation of each of the 3-deoxyanthocyanidins were similar in each cultivar. However, inoculated cv DK46 plants synthesized significantly less phytoalexins than were synthesized by inoculated cv DK18 plants. The results suggest that in the absence of anthocyanin synthesis, cv DK18 plants are able to synthesize a significantly greater amount of the phytoalexins in response to pathogen attack.
Figure 8.
Accumulation of 3-deoxyanthocyanidin phytoalexins in inoculated cvs DK46 and DK18 mesocotyls. A, Luteolinidin; B, 5-methoxyluteolinidin; C, apigeninidin; and D, caffeic acid ester of arabinosyl-5-O-apigeninidin. The concentration of each phytoalexin was determined based on known extinction coefficients. The concentration of the 5-methoxyluteolinidin was estimated using the extinction coefficient (13,800 m−1 cm−1) for luteolinidin.
Expression of Anthocyanin Biosynthesis Genes F3H, DFR,and ANS
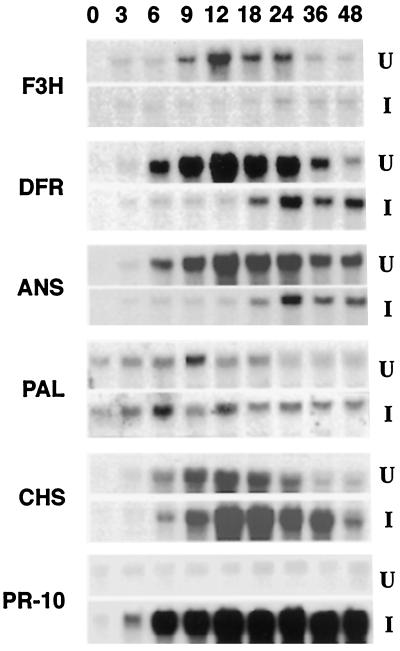
To examine whether the reduction of anthocyanin accumulation in cv DK46 mesocotyls in response to fungal inoculation was attributable to a decrease in mRNA levels for genes encoding anthocyanidin-biosynthesis enzymes, we extracted total RNA from uninoculated and inoculated mesocotyls and carried out gel-blot hybridization experiments. Experiments were performed with various cDNAs corresponding to genes encoding F3H, DFR, and ANS. Figure 9 shows that F3H, DFR, and ANS mRNA levels were all induced in uninoculated tissues accumulating anthocyanin upon exposure to light. DFR and ANS transcripts were detected as early as 3 h after exposure to light. The reduction in transcript levels 24 h after illumination (Fig. 9) preceded the leveling off of anthocyanin accumulation at 48 h after exposure to light (Fig. 7). Similar experiments with RNA isolated from inoculated tissues revealed that expression of the F3H, DFR, and ANS genes was strongly repressed for the first 18 h after inoculation (Fig. 9). The level of these transcripts was enhanced beginning 24 h after inoculation (Fig. 9), a period of time that preceded a more rapid increase in the accumulation of the anthocyanin pigment in inoculated plants (Fig. 7).
Figure 9.
Temporal expression of different genes in mesocotyls of uninoculated (U) and inoculated (I) cv DK46 plants. Northern blots are of total RNA (10 μg) from mesocotyls taken at different time intervals, probed with cDNA clones for F3H, DFR, ANS, PAL, CHS, and PR-10. Numbers indicate hours after inoculation of plants with C. heterostrophus conidia.
Expression of PAL and CHS Genes
The effects of fungal inoculation on the expression of the PAL and CHS genes was also determined by northern-blot analysis with cDNAs isolated from sorghum. Expression of the PAL gene was induced in the uninoculated control cv DK46 plants as early as 3 h after exposure to light (Fig. 9). The level of PAL transcripts increased through 9 h and gradually declined thereafter. After inoculation, the level of PAL transcripts increased and remained relatively constant over the same period of 48 h. A basal level of the PAL transcript was detected in plants when they were removed from the dark (time 0). The pattern of CHS transcript accumulation in uninoculated seedlings resembled that of inoculated seedlings, except that the apparent level of transcript was greater in RNA isolated from inoculated tissue (Fig. 9). Maximum expression of CHS occurred at 12 h after illumination in both uninoculated and inoculated tissues. That PAL transcripts began to accumulate before CHS transcripts is consistent with the fact that flavonoid synthesis is dependent on phenylpropanoid synthesis. PAL and CHS are the first committed enzymes of the phenylpropanoid and flavonoid pathways, respectively, and the pathways themselves are coordinately regulated (Schröder et al., 1979; Heller and Forkman, 1988). Unlike the anthocyanin-biosynthesis genes F3H, DFR, and ANS, accumulation of the PAL and CHS transcripts was stimulated when the plants were simultaneously inoculated and exposed to light.
Expression of PR-10 Gene
A number of cDNAs had been isolated that appeared to correspond to mRNAs that increased in abundance after inoculation. One of these clones was identified as a member of the PR-10 family of PR proteins by the similarity of the deduced amino acid sequence of the translation product to that of other PR-10 proteins (J. Hipskind, S.-C. Lo, and R.L. Nicholson, unpublished data).
RNA gel-blot hybridization experiments were subsequently performed with total RNA isolated from uninoculated and inoculated mesocotyls of cv DK46 with the sorghum PR-10 cDNA as a probe. Figure 9 shows a significant and sustained accumulation of PR-10 transcripts in mesocotyls by 6 h after inoculation with the fungus. The temporal increase in the level of PR-10 transcripts was also consistent with increases in the levels of PAL and CHS transcripts in inoculated tissue (Fig. 9).
Temporal Relationship between the Production of Anthocyanins and Phytoalexins
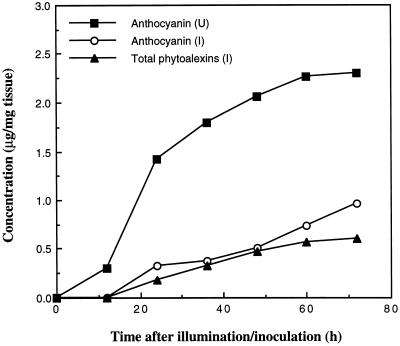
A close temporal relationship was demonstrated between the accumulation of anthocyanin pigments and the synthesis of 3-deoxyanthocyanidin phytoalexins in inoculated cv DK46 plants (Fig. 10). Anthocyanin accumulated at a slow rate after its first detection at 24 h after illumination/inoculation and accumulated more rapidly at 48 h after illumination/inoculation. In contrast, the rate of accumulation of the total 3-deoxyanthocyanidin phytoalexins was observed to decrease over the same time period, indicating a shift back to the synthesis of the light-induced anthocyanin pigment. It is also important to note that elevated accumulation of transcripts of the anthocyanin biosynthesis genes F3H, DFR, and ANS preceded these changes in inoculated tissues (Fig. 9).
Figure 10.
Accumulation of the anthocyanin cyanidin 3-dimalonyl glucoside in uninoculated cv DK46 mesocotyls (U) and the accumulation of total 3-deoxyanthocyanidin phytoalexins and the anthocyanin cyanidin 3-dimalonyl glucoside in inoculated mesocotyls (I). The values for total phytoalexins are the sum of the mean values for individual phytoalexins at each time.
DISCUSSION
Accumulation of anthocyanins is induced by various environmental stimuli, including UV and red irradiation (Shichijo et al., 1993; Reddy et al., 1994), low temperature (Christie et al., 1994), pathogen attack (Harrison and Strictland, 1980; Heim et al., 1983; Hipskind et al., 1996a), and several plant-growth regulators, such as cytokinins (Deikman and Hammer, 1995), GA (Mealem-Beno et al., 1997), and ethylene (Woltering and Somhorst, 1990). Stimulation of anthocyanin accumulation has often been correlated with the activation of genes involved in the anthocyanin biosynthetic pathway. Using MALDI-TOF in conjunction with HPLC techniques, we showed that sorghum cv DK46 accumulates cyanidin 3-dimalonyl glucoside as the major anthocyanin. Synthesis of red-light-induced anthocyanin in sorghum has been shown to be enhanced by low temperature during the preirradiation period (Shichijo et al., 1993). In contrast to the inductive effects of most other environmental stresses, we demonstrated that fungal inoculation drastically reduced anthocyanin accumulation in sorghum mesocotyls. Northern-blot analysis of the expression of the genes encoding F3H, DFR, and ANS in inoculated mesocotyls indicated that anthocyanin synthesis was inhibited at the transcriptional level and could thus be correlated with a reduced expression of these genes.
F3H, DFR, and ANS are involved in the anthocyanin branch pathways in flavonoid metabolism (Fig. 2). F3H catalyzes the hydroxylation of the number 3 carbon in the flavonoid oxygen heterocycle (C-ring) of naringenin (Meldgaard, 1992) and directs the flow of carbon to the synthesis of cyanidin and pelargonidin-based anthocyanins. Subsequent reactions involve an NADPH-dependent reduction of the carbonyl group at the number 4 carbon of the C-ring by DFR, which is encoded in maize by the A1 gene (Reddy et al., 1987; Holton and Cornish, 1995). It is believed that the removal of the subsequent hydroxyl group occurs via the enzyme ANS, which is the putative product of the maize A2 gene (Weiss et al., 1993). A coordinate repression of the F3H, DFR, and ANS genes after inoculation suggests a decrease in the activities of the enzymes they encode, thereby reducing the flow of carbon to the anthocyanin-synthesis pathway. Disease-induced reduction of anthocyanin accumulation in plant tissues has been investigated only to a limited extent. Inoculation with tungro virus reduced the contents of both anthocyanins and flavanols in rice plants, and the reduction was markedly greater in the susceptible cultivar than in either a cultivar with intermediate resistance or a tolerant cultivar (Mohanty and Sridhar, 1989). Similarly, susceptible cultivars of maize inoculated with C. heterostrophus (B. maydis) accumulated significantly lower levels of anthocyanins before lesion development (Heim et al., 1983). It is not clear, however, if these effects are also the result of transcriptional repression.
Biosynthesis of phytoalexins is an inducible defense mechanism and is a major component of resistance to pathogens in some plants (Nicholson and Hammerschmidt, 1992; Smith, 1996). Sorghum is one of the few monocotyledonous plants known to synthesize phytoalexins. An important feature of the sorghum phytoalexins is that they are produced in a site-specific manner (Snyder and Nicholson, 1990). These compounds are localized at the site of attempted fungal penetration in both mesocotyls and juvenile leaves (Nicholson et al., 1987, 1988; Snyder and Nicholson, 1990). Our results demonstrate that each of the previously identified 3-deoxyanthocyanidin phytoalexins was synthesized in inoculated cv DK46 plants. These plants also synthesized the anthocyanin cyanidin 3-dimalonyl glucoside in response to light, but that synthesis was significantly repressed as a result of inoculation (Fig. 10).
The phytoalexin response generally operates only when plants are under the stress of pathogen attack. Kombrink and Hahlbrock (1990) suggested that if the gene activation and mRNA and protein synthesis required for defense are to be attained, then it is likely that genes for relatively less-important metabolic activities are simultaneously repressed. Anthocyanin accumulation could be considered one of the less-essential metabolic activities that are sacrificed for the extensive and multicomponent defense response. Mutations in anthocyanin genes generally have no deleterious effect on plant growth and development (Holton and Cornish, 1995). Repression of anthocyanin accumulation and the associated expression of anthocyanin genes is likely to play a compensatory role in the defense response of sorghum cv DK46 plants.
3-Deoxyanthocyanidin phytoalexins are structurally related to normal anthocyanidins (compare Figs. 1 and 6). Reduction and dehydroxylation-type reactions similar to those in the anthocyanin branch pathway (Fig. 2) have been proposed for the synthesis of 3-deoxyanthocyanidin phytoalexins (Netzly and Butler, 1986). The carbonyl group at the number 4 carbon of naringenin and/or eriodictyol is reduced to form apiferol and/or luteoferol, respectively. The newly formed hydroxyl group is thought to be subsequently removed by a dehydroxylation and oxidation reaction, resulting in the formation of apigeninidin and luteolinidin from apiferol and luteoferol, respectively. In spite of the apparent structural similarities between the end products and intermediates, synthesis of the 3-deoxyanthocyanidins does not involve the expression of genes similar to those encoding DFR and ANS in the anthocyanin branch pathway (Hipskind et al., 1996b). The repression of these genes in inoculated sorghum mesocotyls would increase the availability of naringenin, which is the last common precursor of the two types of flavonoids for the biosynthesis of the 3-deoxyanthocyanidin phytoalexins.
Compatible with this hypothesis is the observation that inoculated cv DK18 plants, which did not accumulate anthocyanin in response to light, produced appreciably higher levels of 3-deoxyanthocyanidin phytoalexins than did inoculated cv DK46 plants (Fig. 7). In the absence of anthocyanin accumulation, it is likely that more resources, including substrates and energy, were available to cv DK18 plants for the phytoalexin response than to cv DK46 plants, which were actively synthesizing two types of structurally and biosynthetically related compounds at the same time. It should be noted that cv DK46 plants did not show any visible reduced resistance to the invading pathogen (Fig. 3, D–F). Microspectrophotometry has shown that the sorghum phytoalexins accumulate to levels as high as 0.15 m, which is significantly greater than that required for fungi toxicity (Snyder et al., 1991).
PAL is the first committed enzyme in the general phenylpropanoid pathway that converts Phe to 4-coumaroyl-CoA, the activated precursor of flavonoid compounds. CHS, on the other hand, directs the flow of carbon from the phenylpropanoid pathway to flavonoid metabolism. Expression of both PAL and CHS genes was stimulated simultaneously with inactivation of the anthocyanin biosynthesis genes in infected mesocotyls of cv DK46 (Fig. 9). This was logical because both PAL and CHS are involved in the overlapping pathway for biosynthesis of anthocyanins and 3-deoxyanthocyanidins, whereas F3H, DFR, and ANS are only involved in anthocyanin biosynthesis (Hipskind et al., 1996b). We demonstrated here that, upon simultaneous treatment of mesocotyls with light and fungal inoculation, PAL and CHS genes were even more strongly induced than by light alone and, in the case of PAL, gene expression was enhanced over an extended period of time (Fig. 9). In many plant species, key branch enzymes of the phenylpropanoid pathway, such as PAL and CHS, are encoded by multiple genes (Dixon and Paiva, 1995). PAL and CHS genes are always among the anthocyanin biosynthesis genes that are transcriptionally activated by external stimuli (Reddy et al., 1994; Deikman and Hammer, 1995; Boss et al., 1996; Mealem-Beno et al., 1997). The concomitant but opposite effects of fungal inoculation on the PAL and CHS genes and the other anthocyanin biosynthesis genes (F3H, DFR,and ANS) suggest the presence of PAL and CHS multigene families in sorghum, the members of which may be regulated by different environmental stimuli and have specific roles in plant defense. This suggestion is further substantiated by our previous findings that the accumulation of phytoalexin-associated PAL and CHS transcripts occurred in a light-independent manner (Weiergang et al., 1996). Southern-blot analysis with a sorghum CHS partial cDNA fragment (approximately 600 bp) revealed completely different hybridization patterns in cv DK46 total DNA digested with different restriction enzymes (S.-C. Lo, unpublished data). These data were further evidence for a CHS gene family.
Repression of anthocyanin accumulation was not confined to infection sites, where the phytoalexins accumulate. For example, at 24 h after inoculation, large areas of tissue surrounding lesions with phytoalexin pigments failed to exhibit any accumulation of anthocyanins (Fig. 3D). This phenomenon was further substantiated by the quantitative measurement of anthocyanin and phytoalexin content of the tissue by HPLC analysis. The analysis revealed that the amount of the reduction in anthocyanin synthesis was not equivalent to the amount of phytoalexins synthesized in inoculated plants (Fig. 10). Thus, defense responses in addition to phytoalexin synthesis were probably also induced. Such a phenomenon appeared to occur in potato, in which the gene for the small subunit of ribulose-1,5-bisphosphate was repressed systemically, whereas the gene for 1,3-β-glucanase was systemically activated. In contrast, a localized induction of genes for phenylpropanoid synthesis occurred specifically at the infection site (Kombrink and Hahlbrock, 1990). It is possible that in the sorghum mesocotyl, defense genes other than those related to the phytoalexin response are induced in the regions of the tissue that fail to produce anthocyanin pigment. For example, we demonstrated an increase in abundance of mRNA of a gene encoding a PR protein in inoculated plants (Fig. 9). This work was carried out with a probe made from a previously isolated full-length cDNA encoding a member of the PR-10 gene family in sorghum. Production of PR proteins represents another common defense response in addition to the accumulation of phytoalexins (van Loon et al., 1994; Smith, 1996). Several PR proteins in potato are 1,3-β-glucanases and chitinases (Kombrink et al., 1988).
The opposing effects of fungal inoculation on the stimulation of the phytoalexin response and PR-10 gene expression and transcriptional repression of anthocyanin accumulation in sorghum are unlikely to be coincidental. Instead, they suggest an attempt by the infected plant to make possible resources available for a complete commitment to the defense response. Possible compensatory roles of gene repression in defense responses have been observed in various plant-parasite or plant-elicitor interactions. For example, induction of sesquiterpene cyclase activity and sesquiterpene accumulation was detected simultaneously with a drastic reduction of sterol biosynthesis and squalene synthase activity in tobacco upon elicitor treatment (Vögeli and Chappell, 1988). In cultured parsley cells, fungal elicitor exhibited dual activity by blocking the light-induced accumulation of flavonoids through the repression of the transcription of the CHS gene and by stimulating the secretion of furanocoumarin phytoalexins (Lozoya et al., 1991). Transient repression of cell-cycle-related genes was observed in suspension-cultured parsley cells simultaneously with the activation of PAL and CHS genes in response to treatment with fungal elicitor (Logemann et al., 1995). Therefore, a consequential shutdown of comparatively less-important metabolic activities might represent a general phenomenon in plant defense to achieve “balanced cellular economy” (Kombrink and Hahlbrock, 1990).
The repression of light-induced expression of anthocyanin genes by fungal inoculation supports our previous hypothesis that synthesis of 3-deoxyanthocyanidin phytoalexins does not occur via the same pathway as synthesis of 3-hydroxylated anthocyanidins (Hipskind et al., 1996b). Anthocyanin and 3-deoxyanthocyanidin are two classes of structurally related, but functionally distinct, flavonoid compounds. The metabolic shift from the light-induced accumulation of anthocyanin pigments to the pathogen-stimulated synthesis of 3-deoxyanthcyanidin phytoalexins in sorghum also represents a novel model for the study of metabolic regulation in response to light and pathogen attack. A unique feature of this system is that the consequences of the metabolic changes are clearly observable (Fig. 3). An understanding of the regulation in the diversion of metabolism leading to resistance expression would provide insights for the development of innovative strategies to enhance disease resistance in sorghum and related crop species.
ACKNOWLEDGMENTS
The authors acknowledge Janyce A. Sugui for her capable assistance in MALDI-TOF analysis. We also thank the following people for providing cDNAs used in this study: C. Magill (PAL and CHS), M. Meldgaard (F3H), and U. Wienand (A1 and A2).
Abbreviations:
- ANS
anthocyanidin synthase
- CHS
chalcone synthase
- DFR
dihydroflavanone 4-reductase
- F3H
flavanone 3-hydroxylase
- MALDI-TOF
matrix-assisted laser desorption ionization-time of flight
- PAL
Phe ammonia-lyase
- PR
pathogenesis-related
Footnotes
This research was supported in part by grant no. MCB-9603439 to R.L.N. from the National Science Foundation. This is journal article no. 15,518 of the Purdue University Agricultural Experiment Station.
The accession number for the nucleotide sequence for sorghum PR-10 described in this article is U60764.
LITERATURE CITED
- Boss PK, Davies C, Robinson SP. Analysis of anthocyanin pathway genes in developing Vitis viniferaL. cv Shiraz grapes berries and the implications for pathway regulation. Plant Physiol. 1996;111:1059–1066. doi: 10.1104/pp.111.4.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson LF, Tenhaken R, Lamb C. Functions of oxidative cross-linking of cell wall structural protein in plant disease resistance. Plant Cell. 1994;6:1703–1712. doi: 10.1105/tpc.6.12.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce RJ, West CA. Elicitation of lignin biosynthesis and isoperoxidase activity by pectic fragments in suspension cultures of castor bean. Plant Physiol. 1988;91:889–897. doi: 10.1104/pp.91.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie PJ, Alfenito MR, Walbot V. Impact of low-temperature stress on general phenylpropanoid and anthocyanin pathways: enhancement of transcript abundance and anthocyanin pigmentation in maize seedlings. Planta. 1994;194:541–549. [Google Scholar]
- Degra L, Salvi G, Marrioti D, De Lorenzo D, Cervone F. A poly-galacturonase-inhibiting protein in alfalfa callus cultures. J Plant Physiol. 1988;133:364–371. [Google Scholar]
- Deikman J, Hammer PE. Induction of anthocyanin accumulation by cytokinins in Arabidopsis thaliana. Plant Physiol. 1995;108:47–57. doi: 10.1104/pp.108.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon RA, Paiva NL. Stress-induced phenylpropanoid metabolism. Plant Cell. 1995;7:1085–1097. doi: 10.1105/tpc.7.7.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson RD, Russell DH. Evaluation of matrix-assisted laser desorption ionization-time-of-flight mass measurement accuracy by delayed extraction. J Am Soc Mass Spectrom. 1996;7:995–1001. doi: 10.1016/1044-0305(96)00027-X. [DOI] [PubMed] [Google Scholar]
- Harrison BJ, Strickland RG. Precursors and genetic control of pigmentation. V. Initiation of anthocyanin synthesis in Antirrhinum majus by Botrytis cinerea. Heredity. 1980;44:103–109. [Google Scholar]
- Heim D, Nicholson RL, Pascholati SF, Hagerman AE, Billet W. Etiolated maize mesocotyls: a tool for investigating disease interactions. Phytopathology. 1983;73:424–428. [Google Scholar]
- Heller W, Formann G. Biosynthesis. In: Harborne JB, editor. The Flavonoids. New York: Chapman & Hall; 1988. pp. 399–426. [Google Scholar]
- Hipskind J, Hanau R, Leite B, Nicholson RL. Phytoalexin synthesis in sorghum: identification of an apigeninidin acyl ester. Physiol Mol Plant Pathol. 1990;36:381–396. [Google Scholar]
- Hipskind J, Wood K, Nicholson RL. Localized stimulation of anthocyanin accumulation and delineation of pathogen ingress in maize genetically resistant to Bipolaris maydisrace O. Physiol Mol Plant Pathol. 1996a;49:247–256. [Google Scholar]
- Hipskind JD, Goldsbrough PB, Urmeev F, Nicholson RL. Synthesis of 3-deoxyanthocyanidin phytoalexins in sorghum does not occur via the same pathway as 3-hydroxylated anthocyanidins and phlobaphenes. Maydica. 1996b;41:155–166. [Google Scholar]
- Holton TA, Cornish EC. Genetics and biochemistry of anthocyanin biosynthesis. Plant Cell. 1995;7:1071–1083. doi: 10.1105/tpc.7.7.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kombrink E, Hahlbrock K. Rapid, systemic repression of the synthesis of ribulose 1,5-bisphosphate carboxylase small-subunit mRNA in fungus-infected or elicitor-treated potato leaves. Planta. 1990;181:216–219. doi: 10.1007/BF02411541. [DOI] [PubMed] [Google Scholar]
- Kombrink E, Hahlbrock K, Hinze K, Schröder M (1991) Molecular responses of potato to infection by Phytophthora infestans. In CJ Smith, ed, Biochemistry and Molecular Biology of Plant-Pathogen Interactions. Oxford University Press, Oxford, UK, pp 237–254
- Kombrink E, Schröder M, Hahlbrock K. Several “pathogenesis-related” proteins in potato are 1,3-β-glucanases and chitinases. Proc Natl Acad Sci USA. 1988;85:215–224. doi: 10.1073/pnas.85.3.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo S-C, Weiergang I, Bonham C, Hipskind J, Wood K, Nicholson RL. Phytoalexin accumulation in sorghum: identification of a methyl ether of luteolinidin. Physiol Mol Plant Pathol. 1996;49:21–31. [Google Scholar]
- Logemann E, Wu S, Schröder J, Somssich IE, Hahlbrock K. Gene activation by UV light, fungal elicitor or fungal infection in Petroselinum crispumis correlated with repression of cell cycle related genes. Plant J. 1995;8:865–876. doi: 10.1046/j.1365-313x.1995.8060865.x. [DOI] [PubMed] [Google Scholar]
- Lozoya E, Block A, Lois R, Hahlbrock K, Scheel D. Transcriptional activation of light-induced flavonoid synthesis by elicitor treatment of cultured parsley cells. Plant J. 1991;1:227–234. [Google Scholar]
- Lue WL, Kuhn D, Nicholson RL. Chalcone synthase activity in sorghum mesocotyls inoculated with Colletotrichum graminicola. Physiol Mol Plant Pathol. 1989;35:413–422. [Google Scholar]
- Mealem-Beno D, Tamari G, Leitner-Dagan YL, Borochov A, Weiss D. Sugar-dependent gibberellin-induced chalcone synthase gene expression in petunia corollas. Plant Physiol. 1997;113:419–424. doi: 10.1104/pp.113.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehdy MC. Active oxygen species in plant defense against pathogens. Plant Physiol. 1994;105:467–472. doi: 10.1104/pp.105.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldgaard M. Expression of chalcone synthase, dihydroflavonol reductase, and flavanone-3-hydroxylase in mutants of barley deficient in anthocyanin and proanthocyanidin biosynthesis. Theor Appl Genet. 1992;83:695–706. doi: 10.1007/BF00226687. [DOI] [PubMed] [Google Scholar]
- Mohanty SK, Sridhar R. Physiology of rice tungro virus disease: changes in leaf pigments due to infection. Acta Phytopathol Entomol Hung. 1989;24:375–383. [Google Scholar]
- Netzly DH, Butler LG. Roots of sorghum exude hydrophobic droplets containing biologically active components. Crop Sci. 1986;26:775–778. [Google Scholar]
- Nicholson RL, Hammerschmidt R. Phenolic compounds and their role in disease resistance. Annu Rev Phytopathol. 1992;30:369–389. [Google Scholar]
- Nicholson RL, Jamil FF, Snyder BA, Lue WL, Hipskind J. Phytoalexin synthesis in the juvenile sorghum leaf. Physiol Mol Plant Pathol. 1988;33:271–278. [Google Scholar]
- Nicholson RL, Kollipara SS, Vincent JR, Lyons PC, Cadena-Gomez G. Phytoalexin synthesis by the sorghum mesocotyl in response to infection by pathogenic and nonpathogenic fungi. Proc Natl Acad Sci USA. 1987;84:5520–5524. doi: 10.1073/pnas.84.16.5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orczyk W, Hipskind J, deNeergaard E, Goldsbrough P, Nicholson RL. Stimulation of phenylalanine ammonia-lyase in sorghum in response to inoculation with Bipolaris maydis. Physiol Mol Plant Pathol. 1996;48:55–64. [Google Scholar]
- Pale E, Kouda-Bonafos M, Nacro M, Vanhaelen M, Vanhaelen-Fastrét R, Ottinger R. 7-O-Methylapigeninidin from Sorghum caudatum. Phytochemistry. 1997;45:1091–1092. [Google Scholar]
- Reddy AR, Britsch L, Salamini F, Saedler H, Rohds W. The A1 (anthocyanin-1) locus in Zea maysencodes dihydroquercetin reductase. Plant Sci. 1987;52:7–13. [Google Scholar]
- Reddy VS, Goud KV, Sharma R, Reddy AR. Ultraviolet-B-responsive anthocyanin production in rice is associated with a specific phase of phenylalanine ammonia lyase biosynthesis. Plant Physiol. 1994;105:1059–1066. doi: 10.1104/pp.105.4.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder J, Kreuzaler F, Schäfer E, Hahlbrock K. Concomitant induction of phenylalanine ammonia-lyase and flavanone synthase mRNAs in irradiated plant cells. J Biol Chem. 1979;254:57–65. [PubMed] [Google Scholar]
- Shichijo C, Hamada T, Hiraoka M, Johnson CB, Hashimoto T. Enhancement of red-light-induced anthocyanin synthesis in sorghum first internode by low temperature given in pre-irradiation culture period. Planta. 1993;191:238–245. [Google Scholar]
- Smith CJ. Accumulation of phytoalexins: defense mechanism and stimulus response system. New Phytol. 1996;132:1–45. doi: 10.1111/j.1469-8137.1996.tb04506.x. [DOI] [PubMed] [Google Scholar]
- Snyder BA, Leite B, Hipskind J, Butler LG, Nicholson RL. Accumulation of sorghum phytoalexins induced by Colletotrichum graminicolaat the infection site. Physiol Mol Plant Pathol. 1991;39:463–470. [Google Scholar]
- Snyder BA, Nicholson RL. Synthesis of phytoalexins in sorghum as a site specific response to fungal ingress. Science. 1990;248:1637–1639. doi: 10.1126/science.248.4963.1637. [DOI] [PubMed] [Google Scholar]
- Stafford HA. Regulatory mechanisms in anthocyanin biosynthesis in the first internodes of Sorghum vulgare. Effect of presumed inhibitors of protein synthesis. Plant Physiol. 1966;42:953–961. doi: 10.1104/pp.41.6.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack D, Wray V. Flavonoids. In: Harborne JB, editor. Methods in Plant Biochemistry, Vol 1. San Diego, CA: Academic Press; 1989. pp. 283–324. [Google Scholar]
- Takeda K, Harborne JB, Self R. Identification and distribution of malonated anthocyanins in plants of the Compositae. Phytochemistry. 1986;25:1337–1342. [Google Scholar]
- van Loon LC, Pierpoint WS, Boller TH, Conejero V. Recommendations for naming plant pathogenesis-related proteins. Plant Mol Biol Rep. 1994;12:254–264. [Google Scholar]
- Vögeli U, Chappell J. Induction of sesquiterpene cyclase and suppression of squalene activities in plant cell cultures treated with fungal elicitor. Plant Physiol. 1988;88:1291–1296. doi: 10.1104/pp.88.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiergang I, Hipskind JD, Nicholson RL. Synthesis of 3-deoxyanthocyanidin phytoalexins in sorghum occurs independent of light. Physiol Mol Plant Pathol. 1996;49:377–388. [Google Scholar]
- Weiss D, Van der Luit AH, Kroon TM, Mol J, Kooter JM. The petunia homologue of the Antirrhinum majus candi and Zea maysA2 flavonoid genes: homology to flavanone 3-hydroxylase and ethylene-forming enzyme. Plant Mol Biol. 1993;22:893–897. doi: 10.1007/BF00027374. [DOI] [PubMed] [Google Scholar]
- Woltering EJ, Somhorst D. Regulation of anthocyanin synthesis in Cymbidiumflowers: effects of emasculation and ethylene. J Plant Physiol. 1990;136:295–299. [Google Scholar]
- Wood KV, Bonham C, Hipskind J, Nicholson RL. Analysis of anthocyanins and deoxyanthocyanidins by plasma desorption mass spectrometry. Phytochemistry. 1994;37:557–560. [Google Scholar]