Abstract
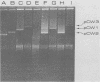
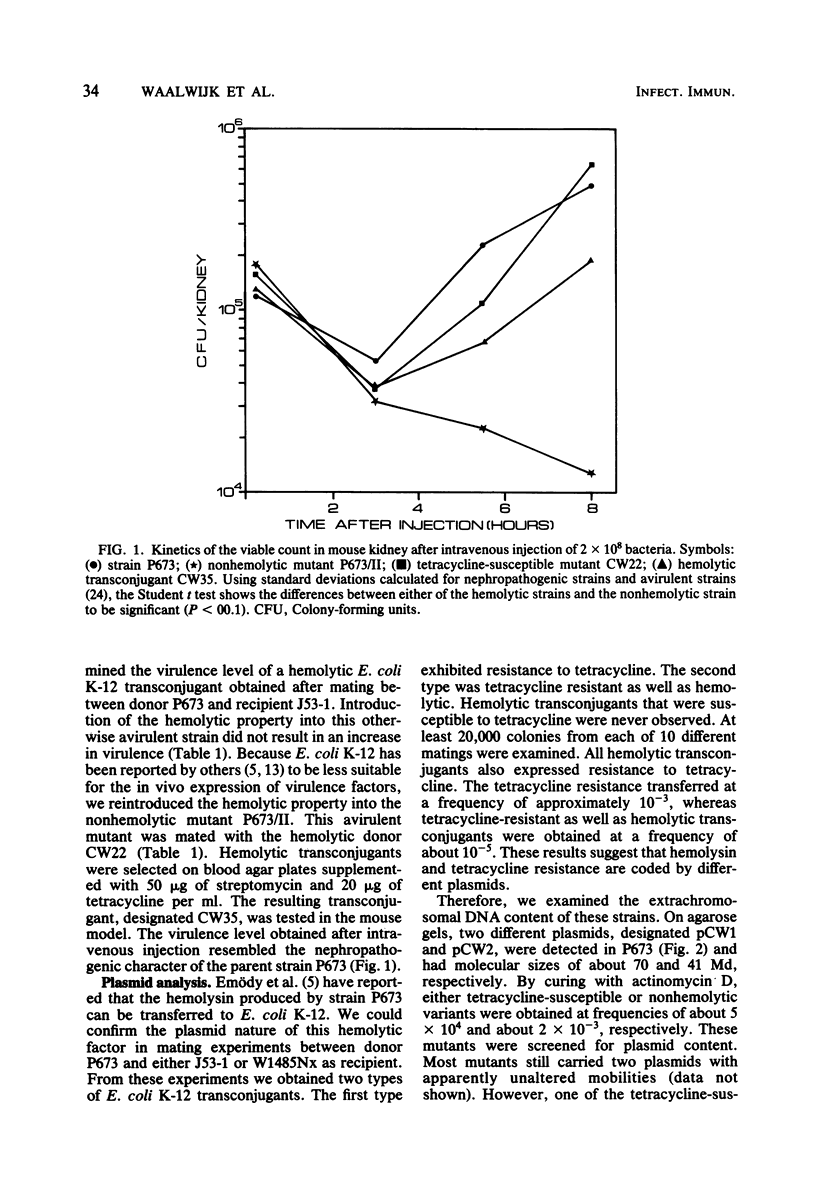
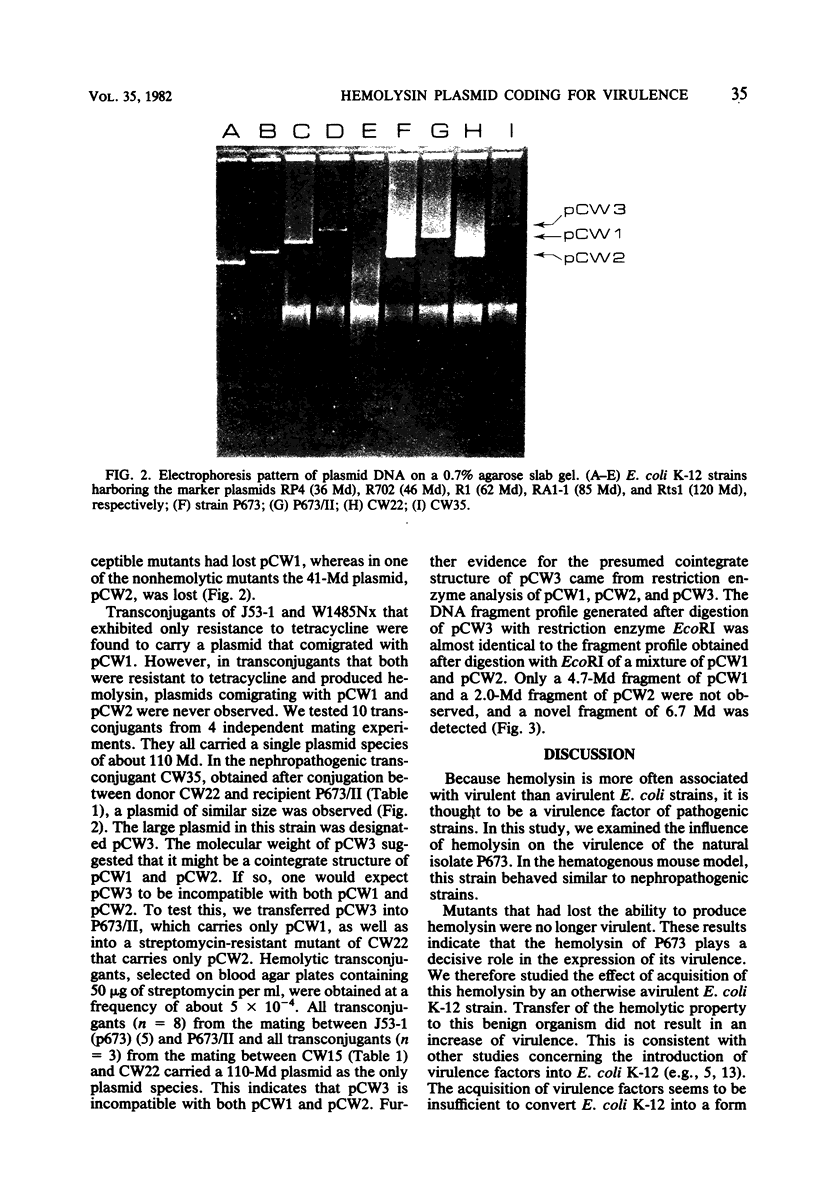
The nephropathogenic Escherichia coli strain P673 was shown to harbor two plasmids with molecular sizes of 70 and 41 megadaltons, respectively. The 70-megadalton plasmid, pCW1, coded for tetracycline resistance, whereas hemolysin production was coded by the 41-megadalton plasmid, pCW2. Plasmid pCW1 proved to be self-transmissible, in contrast to pCW2. Transfer of the hemolysin character was associated with the appearance of a 110-megadalton plasmid, pCW3. The incompatibility of pCW3 with both native plasmids and restriction enzyme analysis led to the conclusion that pCW3 is a cointegrate of pCW1 and pCW2, pCW2, carrying the hemolytic determinant, is involved in the nephropathogenic character of strain P673, because (i) elimination of pCW2 from P673 was associated with a loss of virulence and (ii) the nephropathogenicity of the avirulent mutant could be restored by reintroduction of pCW2 DNA as part of a cointegrate structure.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke E. M., Ewins S. P. Properties of strains of Escherichia coli isolated from a variety of sources. J Med Microbiol. 1975 Feb;8(1):107–111. doi: 10.1099/00222615-8-1-107. [DOI] [PubMed] [Google Scholar]
- Emödy L., Pál T., Safonova N. V., Kuch B., Golutva N. K. alpha-Haemolysin: an additive virulence factor in Escherichia coli. Acta Microbiol Acad Sci Hung. 1980;27(4):333–342. [PubMed] [Google Scholar]
- Fried F. A., Vermeulen C. W., Ginsburg M. J., Cone C. M. Etiology of pyelonephritis: further evidence associating the production of experimental pyelonephritis with hemolysis in Escherichia coli. J Urol. 1971 Sep;106(3):351–354. doi: 10.1016/s0022-5347(17)61286-2. [DOI] [PubMed] [Google Scholar]
- Goebel W., Royer-Pokora B., Lindenmaier W., Bujard H. Plasmids controlling synthesis of hemolysin in Escherichia coli: molecular properties. J Bacteriol. 1974 Jun;118(3):964–973. doi: 10.1128/jb.118.3.964-973.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel W., Schrempf H. Isolation and characterization of supercoiled circular deoxyribonucleic acid from beta-hemolytic strains of Escherichia coli. J Bacteriol. 1971 May;106(2):311–317. doi: 10.1128/jb.106.2.311-317.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmuth R., Stephan R., Bulling E., van Leeuwen W. J., van Embden J. D., Guinée P. A., Portnoy D., Falkow S. R-factor cointegrate formation in Salmonella typhimurium bacteriophage type 201 strains. J Bacteriol. 1981 May;146(2):444–452. doi: 10.1128/jb.146.2.444-452.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooykaas P. J., den Dulk-Ras H., Schilperoort R. A. Molecular mechanism of Ti plasmid mobilization by R plasmids: isolation of Ti plasmids with transposon-insertions in Agrobacterium tumefaciens. Plasmid. 1980 Jul;4(1):64–75. doi: 10.1016/0147-619x(80)90083-9. [DOI] [PubMed] [Google Scholar]
- Kétyi I., Emödy L., Kontrohr T., Vertényi A., Pácsa S., Avdeeva T. A., Safonova N. V., Golutova N. K. Mouse lung oedema caused by a toxic substance of Escherichia coli strains. Acta Microbiol Acad Sci Hung. 1978;25(4):307–317. [PubMed] [Google Scholar]
- Minshew B. H., Jorgensen J., Counts G. W., Falkow S. Association of hemolysin production, hemagglutination of human erythrocytes, and virulence for chicken embryos of extraintestinal Escherichia coli isolates. Infect Immun. 1978 Apr;20(1):50–54. doi: 10.1128/iai.20.1.50-54.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshew B. H., Jorgensen J., Swanstrum M., Grootes-Reuvecamp G. A., Falkow S. Some characteristics of Escherichia coli strains isolated from extraintestinal infections of humans. J Infect Dis. 1978 May;137(5):648–654. doi: 10.1093/infdis/137.5.648. [DOI] [PubMed] [Google Scholar]
- Mitchell I., Kenworthy R. Attempted elimination of plasmid-determined haemolysin, K88 antigen and enterotoxin from Escherichia coli pathogenic for pigs. J Appl Bacteriol. 1977 Apr;42(2):207–212. doi: 10.1111/j.1365-2672.1977.tb00686.x. [DOI] [PubMed] [Google Scholar]
- Noegel A., Rdest U., Goebel W. Determination of the functions of hemolytic plasmid pHly152 of Escherichia coli. J Bacteriol. 1981 Jan;145(1):233–247. doi: 10.1128/jb.145.1.233-247.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Moseley S. L., Falkow S. Characterization of plasmids and plasmid-associated determinants of Yersinia enterocolitica pathogenesis. Infect Immun. 1981 Feb;31(2):775–782. doi: 10.1128/iai.31.2.775-782.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer-Pokora B., Goebel W. Plasmids controlling synthesis of hemolysin in Escherichia coli. II. Polynucleotide sequence relationship among hemolytic plasmids. Mol Gen Genet. 1976 Mar 22;144(2):177–183. doi: 10.1007/BF02428106. [DOI] [PubMed] [Google Scholar]
- Shapiro J. A. Molecular model for the transposition and replication of bacteriophage Mu and other transposable elements. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1933–1937. doi: 10.1073/pnas.76.4.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. W., Halls S. The transmissible nature of the genetic factor in Escherichia coli that controls haemolysin production. J Gen Microbiol. 1967 Apr;47(1):153–161. doi: 10.1099/00221287-47-1-153. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Linggood M. A. Observations on the pathogenic properties of the K88, Hly and Ent plasmids of Escherichia coli with particular reference to porcine diarrhoea. J Med Microbiol. 1971 Nov;4(4):467–485. doi: 10.1099/00222615-4-4-467. [DOI] [PubMed] [Google Scholar]
- Thomas M., Davis R. W. Studies on the cleavage of bacteriophage lambda DNA with EcoRI Restriction endonuclease. J Mol Biol. 1975 Jan 25;91(3):315–328. doi: 10.1016/0022-2836(75)90383-6. [DOI] [PubMed] [Google Scholar]
- de la Cruz F., Müller D., Ortiz J. M., Goebel W. Hemolysis determinant common to Escherichia coli hemolytic plasmids of different incompatibility groups. J Bacteriol. 1980 Aug;143(2):825–833. doi: 10.1128/jb.143.2.825-833.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bosch J. F., Postma P., de Graaff J., MacLaren D. M. Haemolysis by urinary Escherichia coli and virulence in mice. J Med Microbiol. 1981 Aug;14(3):321–331. doi: 10.1099/00222615-14-3-321. [DOI] [PubMed] [Google Scholar]
- van den Bosch J. F., de Graaff J., MacLaren D. M. Virulence of Escherichia coli in experimental hematogenous pyelonephritis in mice. Infect Immun. 1979 Jul;25(1):68–74. doi: 10.1128/iai.25.1.68-74.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]