Abstract
Introduction: Maple Syrup Urine Disease (MSUD) is an autosomal recessive disorder caused by defects in the branched-chain α-ketoacid dehydrogenase complex resulting in accumulation of branched-chain amino acids (BCAAs) and corresponding branched-chain ketoacids (BCKAs) in tissues and plasma, which are neurotoxic. Early diagnosis and subsequent nutritional modification management can reduce the morbidity and mortality. Prior to 1990s, the diagnosis of MSUD and other inborn errors of metabolism (IEM) in Malaysia were merely based on clinical suspicion and qualitative one-dimensional thin layer chromatography technique. We have successfully established specific laboratory diagnostic techniques to diagnose MSUD and other IEM. We described here our experience in performing high-risk screening for IEM in Malaysia from 1999 to 2006. We analysed the clinical and biochemical profiles of 25 patients with MSUD.
Methods: A total of 12,728 plasma and urine samples from patients suspected of having IEM were received from physicians all over Malaysia. Plasma amino acids quantitation using fully automated amino acid analyzer and identification of urinary organic acids using Gas Chromatography Mass Spectrometry (GCMS). Patients’ clinical information were obtained from the request forms and case records
Results: Twenty-five patients were diagnosed MSUD. Nineteen patients (76%) were affected by classical MSUD, whereas six patients had non-classical MSUD. Delayed diagnosis was common among our case series, and 80% of patients had survived with treatment with mild-to-moderate learning difficulties.
Conclusion: Our findings suggested that MSUD is not uncommon in Malaysia especially among the Malay and early laboratory diagnosis is crucial.
Introduction
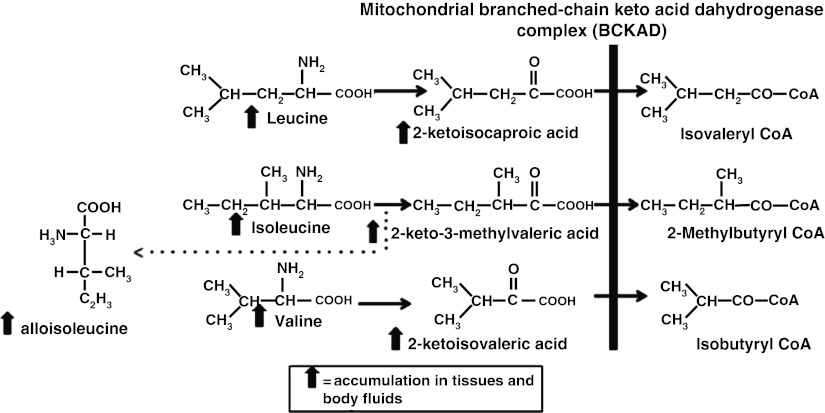
Maple Syrup Urine Disease (MSUD) is an autosomal recessive disorder caused by defects in the mitochondrial branched-chain α-ketoacid dehydrogenase complex (BCKAD). The BCKAD is a large complex with four subunits (E1α, E1β, E2 and E3) and is necessary for decarboxylation of branched-chain ketoacids (BCKAs), which is the second step in the degradation pathway of branched-chain amino acids (BCAAs) leucine, isoleucine and valine. Mutations in both alleles encoding any subunit can result in the accumulation of BCAAs, alloisoleucine and their corresponding BCKAs measured in blood, urine and cerebrospinal fluid (Fig. 1) (Chuang et al. 2001; Wappner and Gibson 2002).
Fig. 1.
Metabolic pathway of branched-chain amino acids degradation. In MSUD, the degradation of the essential branched-chain amino acids leucine, valine and isoleucine and their derived 2-ketoacids is impaired because of deficiency of BCKAD. Alloisoleucine which is a by-product of isoleucine transaminatione significantly elevated
The clinical manifestation of MSUD depends on the severity of BCKAD deficiency. Patients with classic MSUD typically have enzyme activity less than 2%. They present with poor feeding, lethargy, irritability, maple syrup-like or burnt sugar smell and ketonuria in first week of life. Without treatment, they develop progressive neurological deterioration due to cerebral edema, culminating in coma, central respiratory failure and death. In patients with non-classical MSUD, residual enzyme activity varies from 2% to 30%, resulting in delayed clinical presentation to infancy or childhood as feeding problems, poor growth, developmental delay and behavioural problems. Non-classical patients may also experience severe metabolic intoxication and encephalopathy if sufficiently stressed by infectious illness, dehydration or prolonged fasting (Chuang et al. 2001). Early diagnosis and subsequent dietary modification by restricting BCAAs can rescue patients with MSUD from the devastating neurological complications (Chuang et al. 2001; Wappner and Gibson 2002).
The worldwide frequency of MSUD based on the data of newborn screening programme from 26.8 million newborns is approximately 1 in 185,000 live births. In countries where consanguineous marriage is common like Saudi Arabia, Turkey, Spain and India, the frequency is higher (Chuang et al. 2001). In South East Asia, the frequency is not known (Lee et al. 2008; Pangkanon et al. 2008). In Malaysia, MSUD was previously thought to be a rare disease.
Prior to 1990s, the diagnosis of MSUD and other inborn errors of metabolism (IEM) in Malaysia were merely based on clinical suspicion and detection of increased BCAAs and other amino acids in urine by one-dimensional thin layer chromatography (TLC). This approach was undesirable because TLC is only a qualitative method, laborious, time-consuming and interpretation of the results are operator-dependent and often inconclusive due to interference from a lot of substances in patient’s urine such as drugs and bilirubin. Most importantly, alloisoleucine, the diagnostic marker for MSUD, cannot be detected using TLC. In late 1990s, we have successfully established an ion-exchange chromatography method for quantitative analysis of amino acids, as well as a qualitative analysis of urinary organic acid using Gas Chromatograpy Mass Spectrometry (GCMS) method. From 1999 to 2006, we have performed amino acids and/or organic acids analyses in 12,728 patients’ samples using this new diagnostic approach. We described here our experience in performing high-risk screening for IEM in Malaysia. We analysed the clinical and biochemical profiles of 25 patients with MSUD, which was one of the commonest IEM diagnosed during this period.
Materials and Methods
Specimen
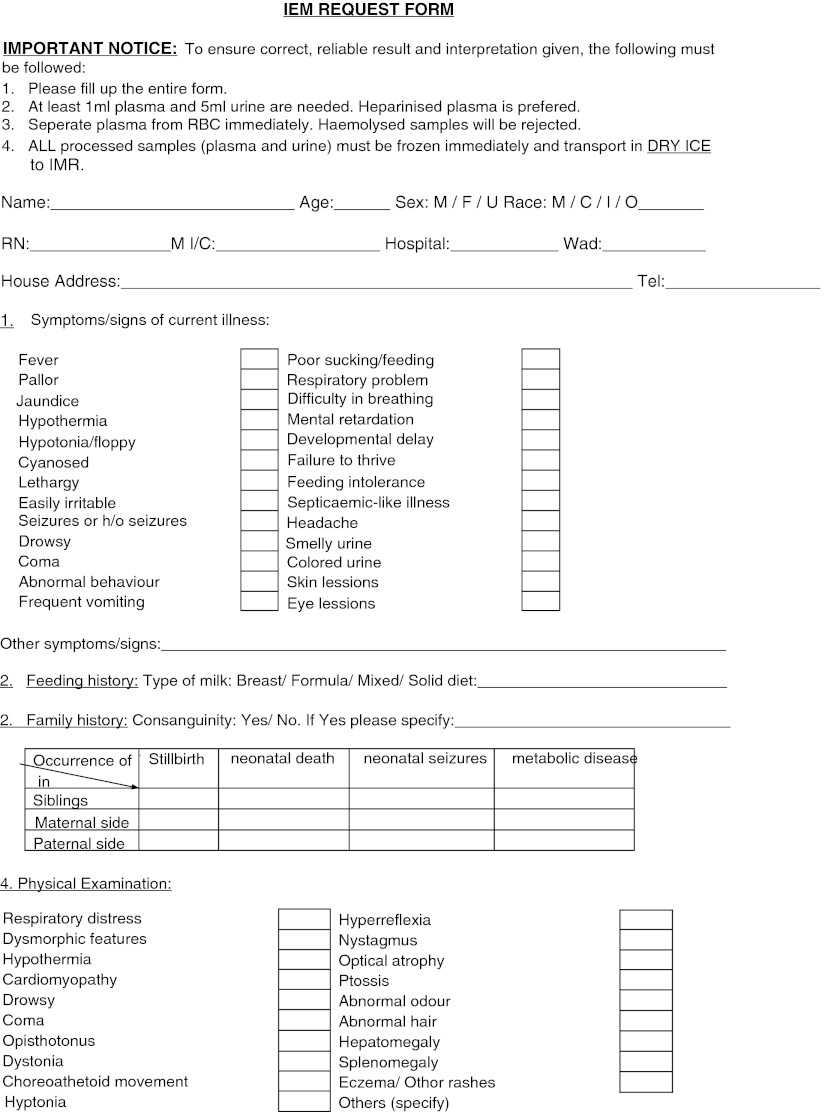
Physicians who wished to request IEM screening for their patients filled in a special request form in which patient’s clinical signs and symptoms and basic laboratory results are documented (Fig. 2). Blood from suspected patients was collected in heparin centrifuged immediately and the plasma was separated. Random urine was collected in a sterile bottle and immediately frozen after collection. Both specimens were transported to the laboratory in ice to prevent degradation of amino acids and organic acids at higher temperature. Plasma and urine from normal control were also analysed for reference.
Fig. 2.
Special request form for investigation of Inborn Errors of Metabolism
Equipments
Plasma amino acids were analysed by ion-exchange chromatography technique using a fully automated amino acids analyzer, Biochrom 30+ which used EZChrom Elite V2B software for quantitation, manufactured by Biochrom Ltd., Cambridge, UK. Organic acids were analysed using GCMS system and Chemstation software manufactured by Agilent Technologies Inc, California, USA. All standards, solvents and chemicals required for the analyses were purchased either from Sigma Aldrich, Missouri, USA or Merck KGaA Darmstadt. Germany. Physiological amino acids kits were purchased from Biochrom Ltd, Cambridge UK. All organic solvents and other reagents used were analytical grade.
Sample Processing
For the plasma amino acid analysis, 100 μL of plasma in eppendorf tube was deproteinised with 100 μL of 10% sulphosalicylic acids. The tube was capped and vortexed for a few seconds and kept for 1 h at 4°C. After 1 h, the sample was centrifuged for 5 min. The supernatant was filtered through 0.2 μm membrane filter to remove any remaining particulate materials prior to analysis. About 20 μL of this supernatant was then loaded by autosampler into a column of cation-exchange resin. Buffers of varying pH and ionic strength were then pumped through the column to separate the various amino acids. The column temperature was accurately controlled and varied as necessary to produce the required separation of all 40 amino acids. The column eluent was mixed with the ninhydrin reagent, and passed through the high temperature reaction coil. In the reaction coil, ninhydrin reacted with the amino acids present in the eluate to form coloured compounds. The amount of coloured compound produced is directly proportional to the quantity of amino acid present in the eluate and detected using UV detector at two wavelengths, 570 nm and 440 nm, depending on the type of the amino acids concern (Biochrom 30 Instruction manual. Issue 6. Sect. 1, pp 1.2–1.3). A running time of 3 h was required for one complete run of amino acids profile. Mixed standards containing basic, acidic, and neutral amino acids were used to quantitate the amount of amino acids in the patient’s sample.
Random urine for organic acids analysis was subjected to organic solvent extraction and derivatisation using method described by Gates et al. (1978), Tanaka et al. (1980), and Majors (1998) with minor modification in our laboratory (Pertiwi et al. 1999). In this method, urine sample was oximated with hydroxyl-amine. An internal standard, pentadecanoic acid, was added and later the organic acids content in the urine was extracted using ethyl-acetate and diethyl-ether. It was later derivatised with BSTFA [N,O-bis(trimethylsily)trifluoroacetamide] and injected into GCMS.
Clinical Information
Patients’ clinical information was obtained from the request forms and case records. The following data were obtained: sex, ethnic group, consanguinity, age of onset of symptoms, clinical manifestations at initial diagnosis and outcome.
Results
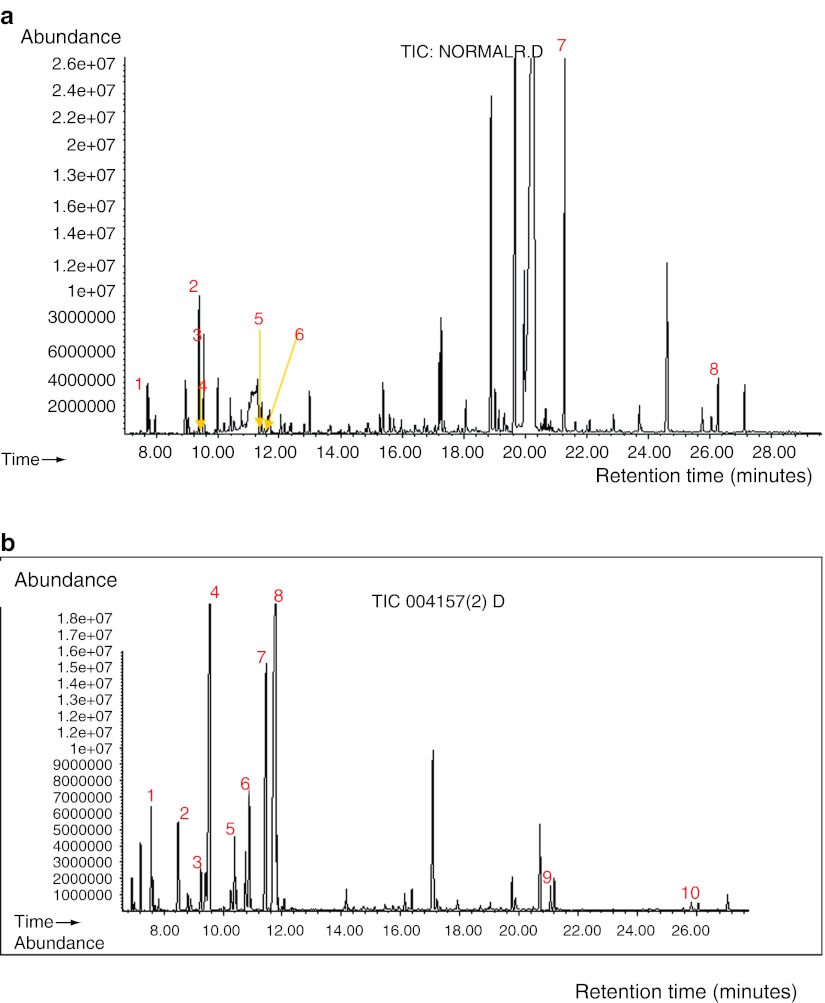
From 12,728 patients’ samples that were analysed, approximately 2% of the samples yielded abnormal results. Twenty-five patients (13 males and 12 females) were diagnosed with MSUD based on elevated BCAAs with the presence of alloisoleucine in plasma and/or increased BCKAs in urine organic acids analysis. Figure 3a, b illustrates the plasma amino acids chromatogram from a normal control and one of the patients with MSUD, respectively. Their corresponding urine organic acids chromatogram is shown in Fig. 4a, b.
Fig. 3.
Plasma amino acid chromatograms of (a) a normal control and (b) a patient with MSUD. (a) Plasma amino acid chromatogram from a normal control. Peak 1, valine; 2, isoleucine; 3, leucine; 4, internal standard (norleucine). Alloisoleucine is not detected. (b) Plasma amino acid chromatogram from a patient with MSUD showing markedly increased leucine. Plasma isoleucine and valine were also elevated. Alloisoleucine was detected. Peak 1, valine; 2, alloisoleucine; 3, isoleucine; 4, leucine; 5, internal standard (norleucine)
Fig. 4.
Urinary organic acids chromatogram of (a) a normal control (b) a patient with MSUD. (a) Urine organic acids chromatogram from a normal control. Peak 1, lactate; 2, pyruvate; 3, 3-hydroxybutyrate; 4, 2-hydroxyisovalerate; 5, 2-keto-3-methylvalerate; 6, 2-keto-isocaproate; 7 and 8 are internal standard and external standard respectively. (b) Urine organic acids chromatogram from a patient with MSUD showing branched-chain ketoacids were excreted in large quantity in urine. Peak 1, lactate; 2 and 8, 2-keto-isocaproate (2 peaks); 3, 3-OH butyrate; 4, 2-hydroxyisovalerate; 5, 2-ketoisovalerate; 6, 2-hydroxy-isocaproate; 7, 2-keto-3-methylvalerate; 9 and 10, internal standard and external standard, respectively
Among these 25 patients, 19 patients (76%) fall under the classical MSUD group, whereas six patients have non-classical MSUD based on the age of onset of initial symptoms. Parental consanguinity was found in one-third of the cases. Twenty-one cases (84%) were Malay, two cases were Chinese, and two cases were Bidayuh (an indigenous subpopulation in Sarawak). Case #7 and #8 are identical twin.
In the classical MSUD group, all patients developed initial symptoms within first week. Clinical manifestations were mostly non-specific and included poor feeding, breathing abnormalities, seizures and coma. Burnt sugar smell was present in nearly all patients by retrospective questioning of their parents. In 15 patients, the diagnosis was made within 1 month; whereas four other patients had their diagnosis made after neonatal period, ranged from 3 months to 12 months. Four patients died during the initial presentation. Learning difficulties were common among the survivors. The clinical and biochemical details of this group are summarised in Table 1. Plasma amino acids were done for case #5 and #9, but the results were not traceable. Clinical presentations showed that these two patients belong to the classical type.
Table 1.
Clinical and biochemical characteristics of patients with classical MSUD
| Case | Age at diagnosis | Ethnic | Sex | Consanguinity | Clinical presentation | Leucine | Isoleucine | Valine | Elevated BCKA | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| #1 | 13d | Malay | M | No | Coma, poor feeding, difficulty in breathing, FTT, sepsis | 3,675 | 244 | 406 | NA | Moderate MR |
| #2 | 31d | Malay | M | No | Poor feeding, FTT, sepsis | 4,770 | 504 | 1,532 | NA | Severe MR |
| #3 | 12m | Malay | M | Yes | Seizure at day 11, spastic | 5,093 | 1,053 | 1,870 | NA | Severe MR |
| #4 | 28d | Malay | F | No | Seizure, vomiting, difficulty in breathing, FTT | 3,218 | 646 | 806 | Yes | Severe MR |
| #5 | 3m | Malay | F | No | Sepsis, poor feeding, developmental delay | NA | NA | NA | Yes | Died |
| #6 | 1m | Bidayuh | F | No | Seizure, coma, poor feeding | 3,390 | 646 | 806 | Yes | Died during crises in neonatal period |
| #7 | 14d | Malay | M | Yes | Seizure, poor feeding, FTT, difficulty breathing | 4,126 | 794 | 1,185 | Yes | Well, normal IQ |
| #8 | 15d | Malay | F | No | Seizure, poor feeding, difficulty breathing | 3,908 | 534 | 798 | Yes | Died during crises in neonatal period |
| #9 | 26d | Malay | M | No | Floppy, poor feeding | NA | NA | NA | Yes | Moderate MR |
| #10 | 19d | Chinese | F | No | Difficulty breathing, sepsis | 3,476 | 497 | 691 | Yes | Moderate MR |
| #11 | 9d | Bidayuh | F | No | Poor feeding, difficulty breathing | 3,572 | 419 | 386 | Yes | Severe MR |
| #12 | 11d | Malay | F | No | Seizure, poor feeding | 1,115 | 187 | 486 | Yes | NA |
| #13 | 11d | Malay | M | No | Hypotonia, poor feeding, difficulty breathing, sepsis | 3,305 | 405 | 577 | Yes | NA |
| #14 | 12m | Malay | F | Yes | Seizure, coma, developmental delay, FTT | 1,812 | 349 | 718 | Yes | moderate MR |
| #15 | 22d | Malay | M | No | Poor feeding, coma, floppy, developmental delay | 902 | 237 | 622 | Yes | mild MR |
| #16 | 1m | Malay | M | Yes | Poor feeding, coma, developmental delay, FTT | 2,473 | 728 | 1,128 | Yes | mild MR |
| #17 | 1m | Malay | M | Yes | Poor feeding, coma, developmental delay, FTT | 1,287 | 191 | 457 | Yes | mild MR |
| #18 | 4.7m | Malay | F | Yes | Seizure, poor feeding, developmental delay, FTT | 2,463 | 455 | 765 | Yes | died |
| #19 | 6m | Malay | M | No | Poor feeding, seizure, developmental delay | NA | NA | NA | Yes | NA |
d day, m month, F female, M male, NA not available, FTT failure to thrive, MR mental retardation
Six patients have non-classical MSUD and presented with chronic neurological symptoms included developmental delay and seizure. Four of them have acute metabolic intoxication episode precipitated by febrile illness and one of them succumbed during the acute episode. Table 2 shows summary of clinical and biochemical details of these six patients.
Table 2.
Clinical and biochemical characteristics of patients with non-classical MSUD
| Case | Age at diagnosis | Ethnic | Sex | Consanguinity | Presentation | Leu | Ile | Val | Elevated BCKA | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| #20 | 5y | Malay | F | Yes | Developmental delay | 921 | 284 | 386 | NA | Mild MR |
| #21 | 3y | Malay | F | Yes | Mental retardation, developmental delay | 611 | 152 | 264 | Yes | Died during one of the crisis episodes |
| #22 | 1.3y | Malay | F | No | Seizure, developmental delay | 2,658 | 753 | 1,690 | NA | Moderate MR |
| #23 | 10y | Malay | M | No | Coma, mental retardation, developmental delay | 1,713 | 337 | 999 | Yes | Mild MR |
| #24 | 7y | Malay | M | No | Lethargy, drowsy | 1,307 | 493 | 906 | Yes | NA |
| #25 | 1y | Chinese | M | No | Drowsy, developmental delay. Not walking and talking | 776 | 231 | 551 | Yes | Walking and talking soon after treatment started. Now attending normal school, mild learning difficulty |
y year, NA not available, FTT failure to thrive, MR mental retardation
Discussion
Definitive laboratory diagnosis is crucial in confirming clinical suspicion of MSUD since its clinical presentation is non-specific and can mimic common conditions such as infections and other IEMs. With the availability of quantitative plasma amino acid analysis and qualitative urine organic acids analysis in our centre, we have started to see an increasing number of children diagnosed to have MSUD in Malaysia. In our case series, MSUD is found to occur predominantly in a Malay population (84%). Most cases are classical type, comparable to a report from the Philippines where also the majority is in the classical group (Lee et al. 2008). Another noteworthy finding was that parental consanguinity was found in 1/3 of cases. Further genetic study is worthwhile consideration to find out the possible explanation about the higher prevalence of MSUD among Malay.
Delayed diagnosis is common in our case series. We believe that the delayed diagnosis is contributed by the lack of awareness among physicians. IEM was only being suspected when patient did not respond to treatment for common diseases such as infections. Very few physicians are able to identify clinically the special burnt sugar smell associated with MSUD, although this was present in almost all patients in our case series. The problem of delayed diagnosis is compounded by lack of diagnostic facilities. Our fully automated amino acids analyzer can only analyse maximum eight samples in a day. The number of samples we received far exceeded the capacity of our laboratory. The use of a special request form has made it easier for us to identify cases that require urgent attention. Cases with high index of suspicion of IEM such as clinical history of acute encephalopathy after a symptom-free period, positive family history; and abnormal routine biochemistry results such as persistent metabolic acidosis, recurrent hypoglycemia, hyperammonemia and ketosis were prioritised for immediate analysis. The awareness and understanding of physicians about MSUD should be heightened through continuous medical education and publicity.
Other researchers have suggested that leucine and its metabolite, α-ketoisocaproic acids, may be the main neurotoxic metabolites in MSUD since increased plasma concentration of these metabolites are associated with the appearance of neurological symptoms (Chuang et al. 2001; Funchal et al. 2007). Plasma leucine is invariably elevated in symptomatic MSUD patients, typically >1,000 μmol/L when there are acute neurological symptoms. The mean level of plasma leucine at the time of diagnosis in our case series was 3,037 μmol/L and 1,331 μmol/L, respectively, for classical and non-classical group. Plasma isoleucine and valine are also typically elevated, but may be normal or reduced. Plasma alloisoleucine, which is a distinctive metabolite present in all forms of MSUD, was elevated in all patients tested in our case series.
MSUD is a treatable genetic disease. Renal replacement methods such as hemodialysis or hemofiltration can achieve rapid corrections of BCAAs and BCKAs during the acute phase of MSUD crisis. Current long-term therapy for MSUD includes lifelong maintenance of a low BCAAs diet and regular monitoring of plasma BCAAs level. Aggressive treatment of intercurrent illness is necessary to prevent a catabolic state that can rapidly lead to release of BCAAs from muscle protein. With good treatment, patients with MSUD can reach adulthood with normal intelligence. However, mild-to-moderate learning difficulties are common (Chuang et al. 2001; Wappner and Gibson 2002).
Early diagnosis by the expanded newborn screening programme using Tandem Mass Spectrometry (TMS) in the developed countries followed by early intervention during the presymptomatic or early symptomatic period have been shown to improve the outcome of patients with MSUD (Simon et al. 2006; Yoon et al. 2003; Heldt et al. 2005). This should probably be the way forward for our country. If the screening procedure is properly applied (blood sampling at day 2–3 of life, sending the filter paper with dry blood spot without any delay and by overnight mail to a newborn screening laboratory, biochemical testing at the same day – notification of the attending physician and the parents of an abnormal test result) the tentative diagnosis of MSUD should be possible by days 3–5 and thus sufficiently early for adequate intervention.
Conclusion
MSUD is probably not uncommon in Malaysia especially among the Malay subpopulation. The majority of patients has classical MSUD and presented with acute neurological symptoms within first week of life. Although we are able to diagnose and manage MSUD, we recognise that the clinical outcome remains to be optimised. We should aim towards earlier diagnosis through improving accessibility to diagnostic facilities, increasing awareness among physicians and general public and establishing a newborn screening programme.
Acknowledgements
We thank the Director General of Health Malaysia for permission to publish this paper. We would also like to express our gratitude to all Biochemistry staff for their technical assistance and to Dr Zakiah Ismail for initiating the project. Our special thanks to Dr Shahnaz Murad, Director of IMR, Dr. Rohani Md Yasin, Head of Specialized Diagnostic Centre and Dr Mohd Helmi Ismail for critical reading of the manuscript and valuable comments.
Footnotes
Competing interests: None declared.
References
- Chuang DT, Shih VE (2001) Maple syrup urine disease (branched-chain ketoaciduria). In: Scriver CR Beaudet AL, Sly WS, Valle D (eds), Childs B, Kinzler KW, Vogelstein B (assoc eds). The Metabolic and Molecular Basis of Inerited Disease, 8th edn. Mc Graw-Hill, New York, pp 1971–2006
- Funchal C, Tramontina F, Quincozes dos Santos A, Frage de Souza D, Goncalves CA, Pessoa-Pureur R, Wajner M. Effect of the branched-chain alpha-keto acids accumulating in maple syrup urine disease on S100B release from glial cells. J Neurol Sci. 2007;260(1–2):87–94. doi: 10.1016/j.jns.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Gates SC, Sweely CC, Krivit W, Dewitt D, Blaisdell BE. Automated metabolic profiling of organic acids in human urine, II. Analysis of urine samples from healthy adults, sick children and children with neuroblastoma. Clin Chem. 1978;24(10):1680–1689. [PubMed] [Google Scholar]
- Heldt K, Schwahn B, Marquardt I, Grotzke M, Wendel U. Diagnosis of MSUD by newborn screening allows early intervention without extraneous detoxification. Mol Genet Metab. 2005;84(4):313–316. doi: 10.1016/j.ymgme.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Lee JY, Chiong MA, Estrada SC, Cutingco-De la Paz EM, Silao CLT, Padilla CD (2008) Maple syrup urine disease (MSUD)-Clinical profile of 47 Filipino patients. JIMD Short report #135 Online [DOI] [PubMed]
- Majors RE. Liquid extraction technique for sample preparation. LC.GC Asia Pacific. 1998;1(2):10–15. [Google Scholar]
- Pangkanon S, Charoensiriwatana W, Sangtawesin V. Maple syrup urine disease in Thai infants. J Med Assoc Thai. 2008;91(Suppl 3):S41–S44. [PubMed] [Google Scholar]
- Pertiwi AKD, Suwaji S, Zabedah MY, Zakiah I (1999) Screening for organic acidurias: method development using capillary gas chromatography with flame ionisation detector (FID) identification. Proceedings for 9th Annual Scientific Meeting of Malaysian Association of Clinical Biochemists
- Simon E, Fingerhut R, Baumkötter J, Konstantopoulou V, Ratschmann R, Wendel U. Maple syrup urine disease: favourable effect of early diagnosis by newborn screening on the neonatal course of the disease. J Inherit Metab Dis. 2006;29(4):532–537. doi: 10.1007/s10545-006-0315-y. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Hine DG, West-Dull A, Lynn TB. Gas chromatographic method for analysis of urinary organic acids.1. Retention indices of 155 metabolically important compounds. Clin Chem. 1980;16(13):1839–1846. [PubMed] [Google Scholar]
- Wappner RS, Gibson KM. Disorders of leucine metabolism. Physician’s guide to the laboratory diagnosis of metabolic disease. 2. Berlin: Springer; 2002. pp. 165–189. [Google Scholar]
- Yoon HR, Lee KR, Kim H, Kang S, Ha Y, Lee DH. Tandem mass spectrometric analysis for disorders in amino, organic and fatty acid metabolism: two year experience in South Korea. Southeast Asian J Trop Med Public Health. 2003;34(Suppl 3):115–120. [PubMed] [Google Scholar]