Abstract
Expanded newborn screening (NBS) leads to an increased number of false positive results, causing parental anxiety, greater follow-up costs, and the need for further metabolic investigations. We developed and validated a second-tier approach for NBS of homocystinurias by measuring the total homocysteine (tHcy) on the initial dried blood spot (DBS) samples to reduce the need for further investigation, and investigated newborn DBS homocysteine values in patients with homocystinuria. Total DBS homocysteine was measured in normal newborns, and retrospectively in newborns with established disorders, using liquid chromatography tandem mass spectrometry (LC-MS/MS) with stable isotope-labelled internal standards for homocysteine. Analytes were separated using reverse phase chromatography with a total run time of 3 min. The method was linear over the range of 10–100 μmol/L of tHcy and showed excellent precision; intra-batch CV was 4% and inter-batch precision 6.5%. Comparison of 59 plasma values with DBS for tHcy taken at the same time showed excellent correlation, (r2>0.97). The reference range for current neonatal samples was 5.4–10.7 μmol/L (n=99), and for the stored neonatal samples (stored dry, sealed in plastic at room temperature for 10 years) was 1.7–5.5 μmol/L, (n=50), both being normally distributed. The clinical utility of this method was checked by retrospective analysis of stored NBS samples from patients with different forms of homocystinuria, including four different remethylating disorders. All had clear elevations of tHcy.
Introduction
The homocystinurias are a diverse group of disorders comprising one defect in trans-sulphuration, cystathionine β-synthase (CBS) deficiency, and defects in remethylation, principally disorders of cobalamin (cbl) metabolism, cbl C, D, E, F, G defects, defects of folate metabolism, and methylene tetrahydrofolate reductase (MTHFR) deficiency (Fowler 1997).
The clinical presentations are diverse. Homocystinuria due to CBS deficiency is associated with thrombo-embolic events, ectopia lentis, mental retardation, psychiatric disorders, and skeletal abnormalities (Refsum et al. 2004; Yap and Naughten 1998). The remethylation disorders share a somewhat similar clinical presentation with failure to thrive, acute or chronic neurological deterioration, developmental delay, and sometimes seizures, hypotonia, microcephaly, feeding difficulties, stomatitis, and microcephaly. The cbl defects also present with megaloblastic anaemia, although measured serum vitamin B12 may be normal (Digest 2007; Schiff et al. 2011). In the cbl and folate deficiencies, megaloblastic anaemia is a classical clinical feature, and there are different neuropsychiatric abnormalities which can appear in cbl deficiency due to demyelination of peripheral nerves, the spinal cord, cranial nerves, and the brain (Whitehead 2006). Treatment is available for all these disorders, and there seems likely to be a clinical advantage in starting treatment in the newborn period.
Newborn screening (NBS) by tandem mass spectrometry routinely detects pyridoxine non-responsive CBS deficiency and cblC defect, and may be able to detect the other disorders. NBS for the homocystinurias involves initial measurement of methionine (Met) to detect CBS deficiency, in which Met levels are elevated, and propionyl and acetyl carnitines (C3, C2) to detect the cblC defect, and possibly other remethylation defects. Using this first-tier approach sensitivity is poor for CBS deficiency as a whole, and specificity somewhat poor for both (Wilcken et al. 2003).
Measurement of total homocysteine (tHcy) in DBS samples has been used as a second-tier test in screening for CBS deficiency (Matern et al. 2007), and recently as an initial test (Gan-Schreier et al. 2009). Measurement of methionine and methionine:phenylalanine ratios to detect low levels has been thought useful for the detection of remethylation defects (Tortorelli et al. 2010; Turgeon et al. 2010).
In this chapter, we report our findings from measuring tHcy in DBS in normal newborns and, retrospectively, in newborns with established disorders, using an LC-MS/MS method.
Materials and Methods
Reagents
DL-Homocysteine (95% pure) was purchased from Aldrich Chemical Company (Sydney, NSW, Australia). DL-Dithiothreitol was purchased from Sigma Chemical Company (Sydney, NSW, Australia). HPLC-grade methanol was purchased from BDH chemicals (Minto, NSW, Australia). Formic acid (FA) was obtained from Ajax Finechem (Taren Point, NSW, Australia). The isotopically labelled internal standard (Homocystine −d8) for Hcy was purchased from Cambridge Isotope Laboratories (Andover, MA, USA). The filter paper used for the sample collection was grade 903 (Whatman, Kent, UK). Ultrapure water was generated using a Millipore-MilliQ system (Millipore, Kilsyth, VIC, Australia).
Samples
The reference range of tHcy in normal newborn DBS was established using 99 current neonatal samples. A separate range for samples stored dry, sealed in plastic at room temperature was obtained using 50 samples stored for 10 years and analysed by LC-MS/MS. As part of the method validation, 59 DBS samples were obtained from blood samples of homocystinuric patients submitted for analysis of plasma homocysteine. The tHcy determined in these DBS on LC-MS/MS was then correlated with simultaneous plasma sample results. Plasma tHcy was analysed by LC-MS/MS method developed in house, based on the method of Magera et al. (1999). In addition, we measured DBS tHcy in six patients with confirmed CBS deficiency, ten cblC deficient patients, two cblG patients, and one MTHFR patient. These samples were stored at room temperature under dry conditions.
Experimental Conditions
The HPLC analysis of tHcy in DBS was based on the method of McCann et al. (2003) with some modifications. A Waters 1,525 μ Binary HPLC Pump system (Waters Corporation, Rydalmere, NSW, Australia) was used, and separation from the bulk of the sample matrix performed on an Altima C18 (150 × 2.1 mm, 3 μm) column (Alltech Associates Australia Pty Ltd, Baulkham Hills, NSW, Australia) equipped with a cartridge guard column. The chromatographic separation was performed using isocratic elution with a mobile phase of methanol:water (30:70, v/v) containing 0.1% formic acid, injection volume 10 μL, flow rate of 200 μL/min, and total run time of 3 min. A Quattro Micro tandem mass spectrometer (Waters Corporation, Rydalmere, NSW, Australia) was used for the detection and was operated in a positive ion multiple reaction monitoring (MRM) mode. The ion source was operated at 3.5 kV and the temperature of source gas was 110°C. The optimised MRM transitions used for the measurement of tHcy were m/z 136 → 90 for the native species and m/z 140 → 94 for the d4 homocysteine internal standard. The data were analysed using QuanLynx software.
Standards
A stock solution of DL-homocysteine (7.4 mmol/L) was prepared by dissolving 100 mg of DL-homocysteine in 100 mL of 0.02 mol/L HCl. For preparation of standards, the working dried blood spot (DBS) standards were prepared from a healthy control blood sample with added Hcy. The added amounts were 0, 10, 50, and 100 μmol/L of Hcy. Standards were spotted onto NBS cards and left to dry overnight at room temperature. After drying, DBS standards were placed in plastic bags separately and stored at −20°C. Endogenous homocysteine in the sample was estimated by back calculation using least squares regression following analysis of enriched standards.
Sample Preparation
A single 3 mm disc was punched out of the DBS samples and standards in a 1.5 mL microcentrifuge tube Eppendorf (Hamburg, Germany), followed by addition of 20 μL of 10 μmol/L internal standard. Tubes were vortex-mixed gently for 1 min and then 20 μL of 500 mmol/L Dithiothreitol and 100 μL of M-Q water containing 0.1% (w/v) formic acid were added to the tubes. Each tube was mixed for 2 min and then centrifuged at 2,150×g, 40°C for 5 min to remove any particulate matter. Eighty microlitres of supernatant was transferred to the 96-well polypropylene microtitre plate for automated injection.
Results
Linearity was tested using the prepared standard curve over the range of 10–100 μmol/L of tHcy. The minimum correlation coefficient for this standard curve was 0.997 over 21 batches with negligible intercepts observed. The inter- and intra-assay precision expressed as the coefficient of variation was evaluated using a quality control sample of blood spiked with 10 μmol/L Hcy and was 4.0% and 6.5%, respectively (n = 96 and 16).
Limits of detection were not formally tested; however, signal to noise was >100:1 at the lowest levels of homocysteine observed (1.7 μmol/L). The results for DBS samples analysed by this method were compared with the plasma samples collected at the same time. The linear regression analysis of the results (n = 59) showed excellent correlation (r2 = 0.97) (Fig. 1). It is important to note that no attempt was made to create a directly comparable result with plasma since this assay is purely for second-tier NBS testing, and not used for monitoring.
Fig. 1.
Regression analysis of DBS results for homocysteine analysed by HPLC-MS/MS and plasma samples taken simultaneously analysed by HPLC-MS/MS. Squared correlation coefficient (r2)
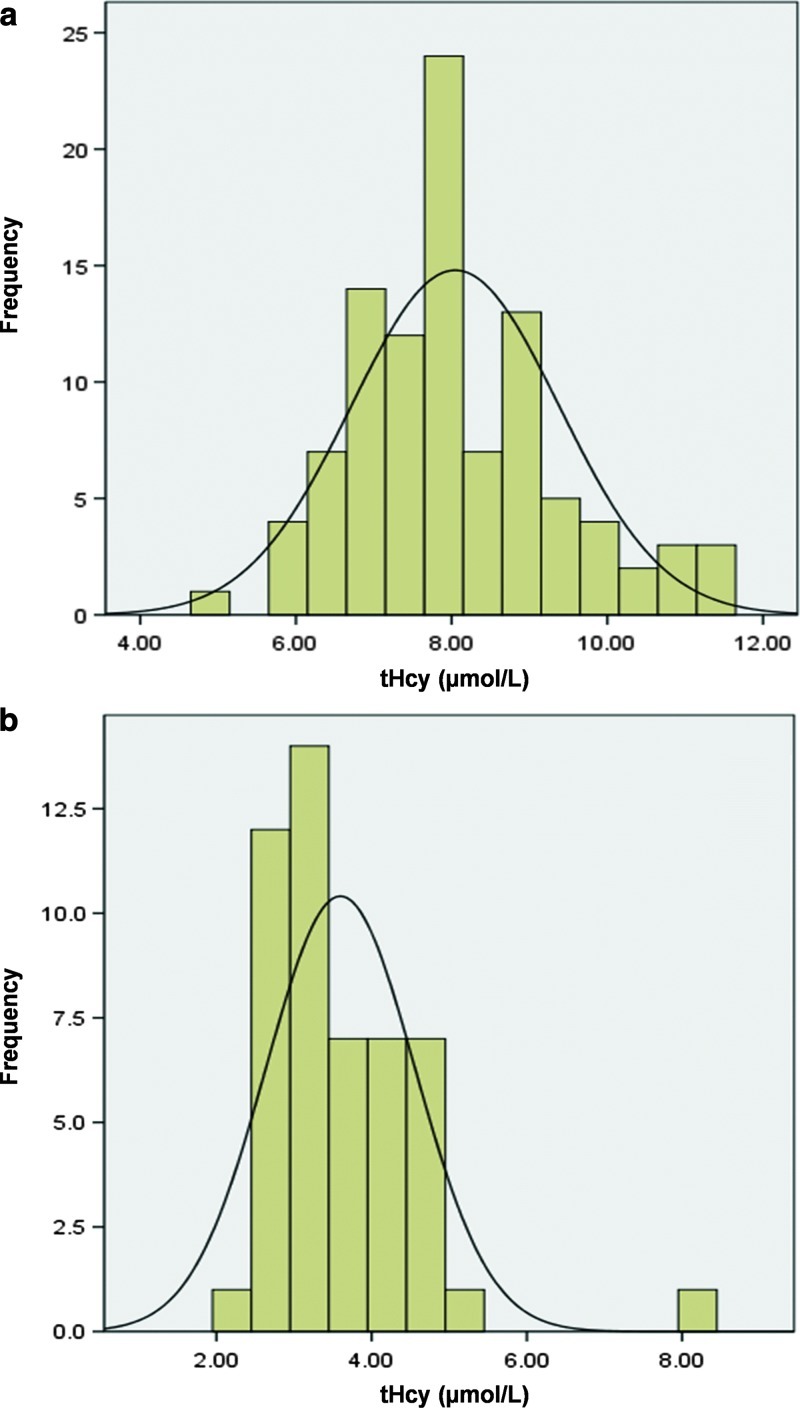
Reference ranges were established for both the current neonatal DBS samples and the stored neonatal DBS samples to evaluate the effect of the storage time. Reference ranges were calculated as (mean ± 1.96 SD) in both stored and current samples. The reference range obtained for current neonatal samples was 5.4–10.7 μmol/L (n = 99), and for samples stored for 10 years was 1.7–5.5 μmol/L (n = 50). Figure 2 shows the distribution of tHcy concentrations from both the current and the stored normal DBS.
Fig. 2.
The distribution of tHcy concentrations from (a) current neonatal DBS and (b) stored neonatal DBS
We retrospectively analysed 20 NBS samples from patients with homocystinuria of different aetiologies. They included six patients with confirmed CBS deficiency (one patient had two NBS samples taken), ten with cblC, two with cblG, and one with MTHFR deficiency. This method clearly identified the affected patients in DBS, as all patients had very clear elevations of tHcy. Combined results of those patients are shown in Table 1, and the distribution of tHcy in those patients is shown in Fig. 3.
Table 1.
Metabolic data of 19 patients diagnosed with homocystinurias
| Disorder (number of cases) |
NBS results (assayed in newborn period) |
tHcy in DBS (assayed after storage) |
Sample storage | |
|---|---|---|---|---|
| Met (cutoff <5 or >75) μmol/L |
C3 (cutoff >8.5) μmol/L |
Reference range 5.4–10.7 (current) 1.7–5.5 (stored) μmol/L |
||
| CBS (6 cases, 7 samples)a | 120–470 | 0.6–3.2 | 21.2–46.6 | 4 mc to 10 yd |
| cblC (10)b | 6.3–19.3 | 6.6–16 | 11.1–186.3 | 1 m to 15 y |
| cblG (2) | 16 and 21 | 2.1 and 5.6 | 13.5 and 25.8 | 4 and 16 y |
| MTHFR (1) | 16 | 2.1 | 93.4 | 2 y |
aThe actual Met levels of CBS patients are 120, 150, 190, 190, 250, 350, and 470 μmol/L
bThe actual Met levels of cblC patients are 6.3, 7.5, 8.1, 10.9, 16.6, 19.3 μmol/L. Four samples were quantified prior to tandem mass spectrometry (MS/MS) use in NBS
cm month
dy years
Fig. 3.
Distribution of tHcy (μmol/L) in DBS from 19 individuals with a metabolic disorder associated with homocystinuria. Dotted line denotes upper limit of normal in samples stored 10 years
Methionine:phenylalanine ratios were calculated retrospectively in NBS samples from patients with remethylation defects and ranged from 0.10 to 0.38 with all but one being below the cutoff of 0.22 suggested by Tortorelli et al. (2010).
Discussion
Several LC-MS/MS methods have been reported to determine tHcy in DBS, plasma, and urine (Gempel et al. 2000; Magera et al. 1999). In this report, a stable isotope dilution LC-MS/MS method based on the method of McCann et al. (2003) was developed and validated for the quantification of tHcy in DBS samples as a second-tier NBS test for the diagnosis of suspected homocystinuria patients. This method is also suitable for adult population screening for mild hyperhomocystinaemia, taking advantage of its fast separation and detection within 3 min, easy sample collection and delivery to the testing laboratory, and low cost per assay.
This method was validated by evaluating its linearity, precision, and between methods comparison. This method was linear over the range of 10–100 μmol/L of tHcy. The method also showed excellent precision; intra-batch CV was 4%, while inter-batch precision was 6.5%. Our results of comparison of 59 plasma values with DBS for tHcy taken at the same time showed an excellent correlation (r2 > 0.97), but with the previously noted lower values in DBS (Fig. 1). However, no attempt was made to create a directly comparable result with plasma in this assay since this assay is purely for second-tier NBS testing, and not used for monitoring.
Our results showed that the tHcy concentrations in DBS are clearly lower than in plasma, which is in accord with the previous report of McCann et al. (2003). They suggested that it is likely the concentration of homocysteine in plasma is higher than red blood cells, thus explaining the lower results obtained from whole blood samples.
Since retrospective analysis of stored samples from positive cases was undertaken, reference ranges were established for both the current neonatal DBS samples and the stored neonatal DBS samples to examine the effect of extended storage time. Reference ranges calculated as mean ± 1.96 SD in both stored and current samples were normally distributed (Fig. 2). The reference range determined in the current neonatal samples was 5.4–10.7 μmol/L (n = 99), which is in close agreement with published reports (Gan-Schreier et al. 2009). The reference range in stored neonatal samples was markedly lower (1.7–5.5 μmol/L; n = 50), illustrating the loss of Hcy on long-term storage, which agrees with a previous report by Bowron et al. (2005). They found that the Hcy concentration in DBS is stable at room temperature for 24 h, with a reduction after 28 days of storage.
In order to evaluate the clinical utility of this method, 20 stored NBS samples from patients with various inborn errors of metabolism causing homocystinuria were retrospectively analysed. This method clearly identified those affected patients with homocystinuria with obvious elevations of tHcy, all of which, despite storage, were above the reference range for current samples (Fig. 3 and Table 1). In terms of the primary markers to trigger second-tier testing, it was clear that elevated Met appeared in the classical homocystinuria patients, while either low or normal Met levels were found in the patients with disorders of homocysteine remethylation. Thus, in our experience, although low Met might be a helpful marker for remethylating defects, it has poor sensitivity. This may be improved by the use of ratios to phenylalanine as suggested by others (Tortorelli et al. 2010; Turgeon et al. 2010), and retrospective review of our data where initial NBS was performed by tandem mass spectrometry supports this with five of the six samples showing a methionine:phenylalanine ratio <0.22, the cutoff used by Tortorelli et al.
On the other hand, propionylcarnitine was usually elevated either primarily or as a ratio with acetylcarnitine in all patients with cblC defect (one of the homocysteine remethylation disorders), while this marker was normal in patients with classical homocystinuria and the other two defects of remethylation, MTHFR and cblG. These observed results are consistent with the literature (Tortorelli et al. 2010; Turgeon et al. 2010).
This tHcy method showed the ability to distinguish between normal and affected patients with different forms of homocystinuria. However, using this method as a second-tier test has limitations. Using elevated Met as a primary marker in routine NBS for classical homcystinuria will miss patients with other causes of homocystinuria, who have normal or low levels of Met. Two patients diagnosed with cblG and MTHFR were missed through the NBS programme with normal values of Met and C3-carnitine (Table 1). Both these patients had borderline low ratios of methionine:phenylalanine (0.20 and 0.21) which could have triggered further testing if ratios were included as a secondary marker, depending on the cutoff used. These results agree with previous reports, where the NBS results of Met were normal or low, with low methionine:phenylalanine ratios in the remethylation disorders of homocysteine (Tortorelli et al. 2010; Turgeon et al. 2010).
In summary, we have developed a method for the reliable quantitative analysis of tHcy using the initial DBS of NBS samples, and have demonstrated the utility of this method as a second-tier test for NBS programmes. This method clearly distinguished between normal and affected infants in stored DBSs from 19 patients previously diagnosed as CBS deficient (six patients), cblC deficient (ten patients), cblG deficient (two patients), and one patient with MTHFR deficiency.
All cases were or would have been detected using a second-tier tHcy assay triggered by increased methionine, or increased C3 or C3/C2 ratio, and/or low methionine:phenylalanine ratio on initial NBS.
Abbreviations
- AC
Acylcarnitine
- C3
Propionylcarnitine
- cbl
Cobalamin
- CBS
Cystathionine β-synthase
- DBS
Dried blood spot
- ESI-MS/MS
Electrospray tandem mass spectrometry
- LC-MS/MS
Liquid chromatography tandem mass spectrometry
- Met
Methionine
- MS/MS
Tandem mass spectrometry
- MTHFR
Methylene tetrahydrofolate reductase
- NBS
Newborn screening
- tHcy
Total homocysteine
Competing Interests
Nil.
Funding
This research was funded by NSW Biochemical Genetics Service.
Ethical Approval
This research was carried out within the guidelines of The Children’s Hospital at Westmead Research Ethics Committee.
Guarantor
K. Carpenter.
Contributorship
A. Alodaib conducted the method development and wrote the first draft of the manuscript. K. Carpenter supervised the project. V. Wiley and T. Wotton provided newborn screening samples and results. J. Christodoulou and B. Wilcken directed the research. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
Footnotes
Competing interests: None declared.
References
- Bowron A, Barton A, Scott J, Stansbie D. Blood spot homocysteine: a feasibility and stability study. Clin Chem. 2005;51:257–258. doi: 10.1373/clinchem.2004.041640. [DOI] [PubMed] [Google Scholar]
- Digest I. Hyperhomocysteinemia and cobalamin disorders. Mol Genet Metab. 2007;90:113–121. doi: 10.1016/j.ymgme.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Fowler B. Disorders of homocysteine metabolism. J Inherit Metab Dis. 1997;20:270–285. doi: 10.1023/A:1005369109055. [DOI] [PubMed] [Google Scholar]
- Gan-Schreier H, Kebbewar M, Fang-Hoffmann J, et al. Newborn population screening for classic homocystinuria by determination of total homocysteine from guthrie cards. J Pediatr. 2009;156:427–432. doi: 10.1016/j.jpeds.2009.09.054. [DOI] [PubMed] [Google Scholar]
- Gempel K, Gerbitz K-D, Casetta B, Bauer MF. Rapid determination of total homocysteine in blood spots by liquid chromatography-electrospray ionization-tandem mass spectrometry. Clin Chem. 2000;46:122–123. [PubMed] [Google Scholar]
- Magera MJ, Lacey JM, Casetta B, Rinaldo P. Method for the determination of total homocysteine in plasma and urine by stable isotope dilution and electrospray tandem mass spectrometry. Clin Chem. 1999;45:1517–1522. [PubMed] [Google Scholar]
- Matern D, Tortorelli S, Oglesbee D, Gavrilov D, Rinaldo P. Reduction of the false-positive rate in newborn screening by implementation of MS/MS-based second-tier tests: the Mayo Clinic experience (2004–2007) J Inherit Metab Dis. 2007;30:585–592. doi: 10.1007/s10545-007-0691-y. [DOI] [PubMed] [Google Scholar]
- McCann SJ, Gillingwater S, Keevil BG, Cooper DP, Morris MR. Measurement of total homocysteine in plasma and blood spots using liquid chromatography-tandem mass spectrometry: comparison with the plasma Abbott IMx method. Ann Clin Biochem. 2003;40:161–165. doi: 10.1258/000456303763046094. [DOI] [PubMed] [Google Scholar]
- Refsum H, Fredriksen Å, Meyer K, Ueland PM, Kase BF. Birth prevalence of homocystinuria. J Pediatr. 2004;144:830–832. doi: 10.1016/j.jpeds.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Schiff M, Benoist J-F, Tilea B, Royer N, Giraudier S, Ogier de Baulny H (2011) Isolated remethylation disorders: do our treatments benefit patients? J Inherit Metab Dis 34:137–45 [DOI] [PubMed]
- Tortorelli S, Turgeon CT, Lim JS, et al. Two-tier approach to the newborn screening of methylenetetrahydrofolate reductase deficiency and other remethylation disorders with tandem mass spectrometry. J Pediatr. 2010;157:271–275. doi: 10.1016/j.jpeds.2010.02.027. [DOI] [PubMed] [Google Scholar]
- Turgeon CT, Magera MJ, Cuthbert CD, et al. Determination of total homocysteine, methylmalonic acid, and 2-methylcitric acid in dried blood spots by tandem mass spectrometry. Clin Chem. 2010;56:1686–1695. doi: 10.1373/clinchem.2010.148957. [DOI] [PubMed] [Google Scholar]
- Whitehead VM. Acquired and inherited disorders of cobalamin and folate in children. Br J Haematol. 2006;134:125–136. doi: 10.1111/j.1365-2141.2006.06133.x. [DOI] [PubMed] [Google Scholar]
- Wilcken B, Wiley V, Hammond J, Carpenter K. Screening newborns for inborn errors of metabolism by tandem mass spectrometry. N Engl J Med. 2003;348:2304–2312. doi: 10.1056/NEJMoa025225. [DOI] [PubMed] [Google Scholar]
- Yap S, Naughten E. Homocystinuria due to cystathionine β-synthase deficiency in Ireland: 25 years' experience of a newborn screened and treated population with reference to clinical outcome and biochemical control. J Inherit Metab Dis. 1998;21:738–747. doi: 10.1023/A:1005445132327. [DOI] [PubMed] [Google Scholar]