Abstract
Mucopolysaccharidoses (MPS) are severe, inherited metabolic disorders caused by storage of glycosaminoglycans (GAGs). Sanfilippo disease (mucopolysaccharidosis type III, MPS III) is described as severe neurological type of MPS, characterized by rapid deterioration of brain functions. No therapy for Sanfilippo disease is approved to date, however, a specific substrate reduction therapy (SRT), called gene expression-targeted isoflavone therapy (GET IT), has been used as an experimental therapy. In this report, we describe effects of treatment of six Sanfilippo disease patients with GET IT, in which the dose of genistein (5,7-dihydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one), an active compound of GET IT present in the soy isoflavone extract, has been increased to 10, and then to 15 mg/kg/day, contrary to the previously reported dose of 5 mg/kg/day. By measuring levels of urinary GAGs and assessing hair dysmorphology as biomarkers, and by considering clinical symptoms of patients, we obtained results suggesting that elevated doses of genistein may improve efficacy of GET IT for Sanfilippo disease.
Introduction
A large fraction of metabolic brain diseases consists of inherited disorders. Mucopolysaccharidoses (MPS) are a group of inherited metabolic disorders, caused by genetic defects resulting in accumulation of undegraded glycosaminoglycans (GAGs) in lysosomes of patients’ cells (Beck 2007; Neufeld and Muenzer 2001). If heparan sulfate (HS) is one of the accumulated GAGs, severe symptoms occur in the central nervous system (CNS), including rapid deterioration of brain functions (Wegrzyn et al. 2010a). Sanfilippo disease (mucopolysaccharidosis type III or MPS III) is characterized by the sole accumulation of HS, and the brain dysfunction-related symptoms are especially severe in this MPS type (reviewed by Valstar et al. 2008).
Until now, no therapy has been approved for treatment of Sanfilippo disease patients. However, a specific kind of substrate reduction therapy (SRT), called gene expression-targeted isoflavone therapy (GET IT), has been proposed to be used in treatment of MPS patients, especially those suffering from brain dysfunctions. Genistein (5,7-dihydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one) was demonstrated to be an inhibitor of GAG synthesis in fibroblasts of patients suffering from various MPS types, including MPS III (Piotrowska et al. 2006). Subsequent studies indicated that genistein may be effective in treatment of animal models of MPS II (Friso et al. 2010) and MPS IIIB (one of four subtypes of MPS III) (Malinowska et al. 2009). Importantly, a complete correction of behavior of MPS IIIB mice was observed in long-term treatment of animals with a relatively high dose of genistein (Malinowska et al. 2010).
The encouraging results of experiments performed in vitro and on animal models led to the proposal that GET IT may be a hopeful option for treatment of neuronopathic forms of MPS (for see Piotrowska et al. (2006), for discussions see Wegrzyn et al. (2010b) and de Ruijter et al. (2011)). Subsequent, more detailed in vitro studies confirmed that genistein and other flavonoids are potent inhibitors of GAG synthesis, and their low cytotoxicity and a potential to cross the blood–brain barrier confirmed that GET IT should be tested as a putative therapy (Arfi et al. 2010; Piotrowska et al. 2010; Kloska et al. 2011). In fact, a pilot (open-label) clinical study with the use of a genistein-rich isoflavone extract for treatment of Sanfilippo patients has been performed and the results were encouraging, including statistically significant reduction of urinary GAG levels (though the reduction occurred in seven out of ten investigated patients), improvement in hair morphology (in eight patients), and an increase in the score achieved in a psychological test (in eight patients) (Piotrowska et al. 2008). In that study, the genistein dose was 5 mg/kg/day and the duration of the treatment was 1 year. Then, a 2-year follow-up study was performed, indicating that at this particular dose of genistein, after the initial improvement, the patients’ state stabilized (in some patients) or slowly deteriorated (in other patients) (Piotrowska et al. 2011). Another recent study showed that GET IT may be useful in treatment of patients suffering from MPS II (Hunter disease), another type of MPS in which a large fraction of patients suffers from deterioration of brain functions (Marucha et al. 2011).
In all studies on experimental GET IT for MPS patients published to date, a genistein-rich isoflavone extract was used with the genistein dose of 5 mg/kg/day (Piotrowska et al. 2008, 2011; Delgadillo et al. 2011; Marucha et al. 2011). However, authors of the most recent studies suggested that an increase in the genistein dose might result in higher efficacy of GET IT (Delgadillo et al. 2011; Piotrowska et al. 2011). Therefore, we aimed to study effects of this experimental therapy when the genistein dose is initially doubled (10 mg/kg/day) and then, after several months, increased up to 15 mg/kg/day. Assessment of previously established biomarkers, urinary GAG levels, and hair morphology, as well as results of clinical observations, were employed to evaluate effects of the treatment.
Materials and Methods
Patients
The patients were diagnosed for Sanfilippo disease (either MPS IIIA, McKusick’s OMIM no. 252900, or MPS IIIB, McKusick’s OMIM no. 252920) by estimation of urinary GAG levels and measurement of activities of particular lysosomal hydrolases in leukocytes, according to the optimized methods described previously (Piotrowska et al. 2008). Deficiency in activity of heparan N-sulfatase (control value: 4.1 ± 1.4 nmol/mg of protein/18 h) or α-N-acetyl glucosaminidase (control value: 90 ± 34 nmol/mg of protein/42 h) was considered as a diagnosis for MPS IIIA or MPS IIIB, respectively. All patients were of Caucasian origin and came from the area of Czech Republic. Among them, there were three males and three females. Information about all patients enrolled into this study is summarized in Table 1.
Table 1.
Characteristics of patients
| Patient no. | MPS type | Gender | Age at diagnosisa | Age at the therapy onseta |
|---|---|---|---|---|
| 1 | MPS IIIA | Female | 0 y 10 m | 7 y 0 m |
| 2 | MPS IIIA | Male | 7 y 10 m | 11 y 1 m |
| 3 | MPS IIIB | Female | 5 y 0 m | 6 y 6 m |
| 4 | MPS IIIA | Female | 3 y 6 m | 3 y 6 m |
| 5 | MPS IIIA | Male | 1 y 8 m | 2 y 0 m |
| 6 | MPS IIIA | Male | 3 y 0 m | 4 y 7 m |
aAge is provided in years (y) and months (m)
Treatment and Assessment of Its Effects
The MPS III patients, characterized in Table 1, were treated for the period of over 1 year, in the range between 15 and 24 months (the differences arose from various times of the enrollment of patients into the study, which resulted from the rarity of the disease and the attempt to include each patient to the study as soon as possible due to ethical issues, namely a lack of an alternative treatment of this severe disease). For the treatment, a genistein-rich soy isoflavone extract (called SE-2000 or Soyfem), provided by the manufacturer (Biofarm, Poznań, Poland) in the form of tablets (the product named Soyfem), was used. The extract consists of genistin and genistein (26.90%), daidzin and daidzein (13.37%), glycitin and glycitein (1.98%), and soy proteins, carbohydrates, and lipids (remaining amount). This extract was administered orally (in the form of whole tablets or tablets crushed into powder) at the initial dose corresponding to 10 mg of genistin and genistein (genistin is a glycan that can be converted to genistein by either acid environment or intestinal bacteriological flora) per 1 kg of body weight daily, which was then (after several months of the treatment) increased to 15 mg/kg/day. The extract was administered four times a day, with equal amounts each time. To assess the effects of the treatment, two biomarkers were measured: (1) urinary GAG levels and (2) hair dysmorphology (the investigator was blinded in the process of sample analysis), which were measured according to the previously published methods (Malinowska et al. 2008; Piotrowska et al. 2008). Clinical symptoms of patients were assessed by their basic investigation and observation during visits in the clinic, as well as by analysis of interviews with patients’ parents. The monitoring of adverse effects was based on reports of parents, who were appropriately instructed to signal any such effects immediately (by either phone or electronic mail, with confirmation of receipt of the information), and who provided an assessment of such effects in a written form every 3 months (even if no adverse effects were observed). This experimental treatment has been approved by the State Institute for Drug Control (Prague, Czech Republic). Parents of the children involved in this study have signed informed consent form.
Results and Discussion
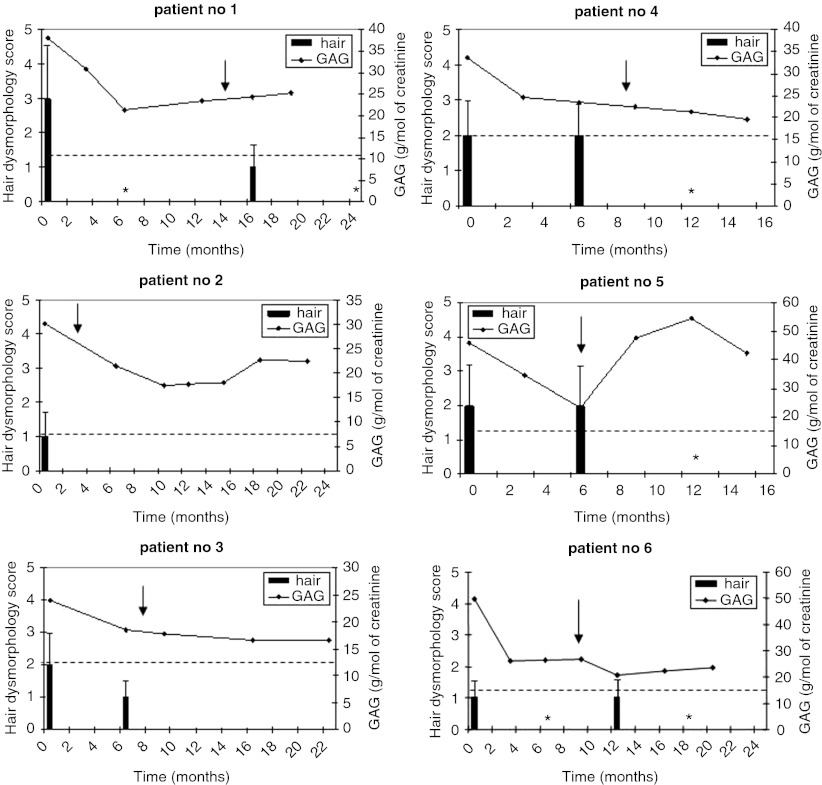
The results of the treatment of six patients suffering from Sanfilippo disease with GET IT, assessed by monitoring two biomarkers, urinary GAG levels, and hair dysmorphology, are presented in Fig. 1. Initially, patients were treated with the genistein dose of 10 mg/kg/day (from time 0), and this treatment resulted in a decrease in the urinary GAG level in all patients (this decrease was statistically significant, p < 0.05, when the analysis of results was performed considering all patients as an investigated population; data not shown), though in none of them the level dropped to the norm value (Fig. 1). However, at this dose, an improvement in hair dysmorphology was observed only in three patients (patients no. 1, 3, and 6). After various times of such a treatment, the dose of genistein has been increased to 15 mg/kg/day (the time of this increase is marked by arrow in Fig. 1). The exact time of the dose change was chosen on the basis of the clinical status of particular patient, and represented either at the end of the period of the plateau of clinical symptoms observed between two subsequent visits in the clinic after initial improvement or after a lack of improvement seen between the first and the second visit. In most patients, this change had either minor or not significant effects on the further decrease of urinary GAG levels, however, after the dose increase, the level of hair dysmorphology decreased significantly and reached the norm value in all patients (Fig. 1). Unexpected results of urinary GAG levels and hair morphology were obtained in patient no. 5, and patients no. 1 and 6, respectively, after the dose increase. Namely, the GAG level increased and hair morphology deteriorated transiently, despite significant initial improvements in the values of these biomarkers. The reason(s) for these phenomena remain(s) unknown, but we cannot exclude an unspecific reaction to any unidentified conditions, other than the primary disease.
Fig. 1.
Effects of GET IT in six Sanfilippo disease patients as assessed by measuring urinary GAG levels and hair dysmorphology as biomarkers. Numbers of patients correspond to those provided in Table 1. The patients were treated with genistein-rich soy isoflavone extract from time = 0 with the dose corresponding to the amount of genistein equal to 10 mg/kg/day, and the dose was elevated to 15 mg/kg/day at the time indicated by arrow. Urinary GAG levels (in g/mol of creatinine) are shown as diagrams, with the age-specific upper norm value represented by a dashed horizontal line. Level of hair dysmorphology is presented in the form of histograms, with average values from at least ten independent estimations and error bars representing standard deviation (SD); asterisks represent investigated samples for which the value was 0 with 0 SD; the scale is from 0 (normal hair morphology) to 5 (the most abnormal hair morphology), according to Malinowska et al. (2008)
Clinical improvement was observed in all patients, but to various extent (Table 2). In patients no. 2, 3, 4, and 5, the clinical effects were noted only after the dose increase up to 15 mg/kg/day (Table 2).
Table 2.
Clinical changes in patients during GET IT as assessed by: (a) basic investigation and observation of patients during their visits in the clinic, and (b) interviews with patients’ parents
| Patient no. | Clinical observations | |
|---|---|---|
| Major symptoms at baseline | Changes from baseline to the endpoint | |
| 1 | Facial dysmorphology; hepatosplenomegaly; frequent infections; frequent falls; epileptic attacks; severe mental retardation with regression of development; autistic features of behavior; sleep problems; hyperactivity | Ability to walk without support; no epileptic attacks since the therapy onset; improved reactions to surrounding stimulation; improved sleep; decreased hyperactivity |
| 2 | Facial dysmorphology; hepatosplenomegaly; mental retardation with regression of development | Mild improvement in communication skills; improved response to surrounding signs (these changes were observed only after the dose increase) |
| 3 | Facial dysmorphology; frequent falls; epileptic attacks; mental retardation with regression of development; attacks of aggression and self-injuries | Inhibition of developmental regression (observed only after the dose increase) |
| 4 | Hepatomegaly; delay in motor skills; slow regression in speech and social adaptation; sleep problems; increased agitation | Inhibition of developmental regression; improvement in speech (more words used and better pronunciation); decreased agitation (these changes were observed only after the dose increase) |
| 5 | Hepatosplenomegaly; frequent falls; developmental regression; impaired speech; sleep problems; hyperactivity | Inhibition of developmental regression; improved sleep; decreased hyperactivity (these changes were observed only after the dose increase) |
| 6 | Hepatosplenomegaly; developmental regression; sleep problems; hyperactivity | Improved sleep; decreased hyperactivity |
Generally, the results presented in Fig. 1 and Table 2 indicate that GET IT with the use of elevated doses of genistein (10 and then 15 mg/kg/day) relative to that administered in previous studies (5 mg/kg/day, Piotrowska et al. 2008, 2011; Delgadillo et al. 2011; Marucha et al. 2011) improved both the values of biomarkers and clinical parameters used for assessment of the efficacy of this treatment. Contrary to the use of the lower dose of genistein (5 mg/kg/day), in this study, we observed a decrease in urinary GAG levels in all (not only in some) patients treated with the dose of 10 mg/kg/day, and a further increase in the dose, up to 15 mg/kg/day, correlated with normalization of hair morphology in all patients. This may suggest an improved efficacy after the dose increase, however, we cannot exclude that the hair morphology normalization was caused by a long exposure to genistein rather than to the use of the increased dose. Although some clinical improvement was observed in all patients, in four out of six patients, the positive changes could be noted only during the treatment with genistein at 15 mg/kg/day. Therefore, we suggest that the proposals to increase the dose of genistein in GET IT for Sanfilippo disease, published recently (Delgadillo et al. 2011; Piotrowska et al. 2011), were substantiated, though further studies on efficacy of GET IT are undoubtedly required.
Since the best results in correction of the behavior of mice suffering from MPS IIIB have been obtained with the use of the dose of genistein as high as 160 mg/kg/day (Malinowska et al. 2010), it remains to be determined whether further increase in the genistein dose may further improve the efficacy of GET IT in humans. Safety of the treatment should be an important issue in such putative studies. No adverse effects were reported by parents of the patients investigated in this study, which confirmed previous observations (Piotrowska et al. 2008, 2011; Delgadillo et al. 2011; Marucha et al. 2011) that this treatment is safe. However, it is clear that to achieve the genistein dose similar to that used in experiments with mice, it will not be possible to provide a genistein-rich soy isoflavone extract. Rather, pure (either purified or synthetic) genistein should be used to eliminate potential adverse effects caused by the compounds present in the extracts along with genistein.
In conclusion, it appears that the use of increased doses of genistein may have positive effects on efficacy of GET IT in treatment of patients suffering from Sanfilippo disease. However, further studies, including double-blinded placebo-controlled clinical trials with various genistein doses, are required to unambiguously assess efficacy and to optimize the treatment procedures.
Acknowledgments
This research was supported by Ministry of Sciences and Higher Education of Poland (project grant no. N N301 668540 to GW), and was operated within the Foundation for Polish Science Team Programme co-financed by the EU European Regional Development Fund (grant no. TEAM/2008-2/7 to GW). A support from Polish MPS Society is greatly acknowledged.
Conflict of Interest
The authors declare no conflict of interest.
Footnotes
Competing interests: None declared.
References
- Arfi A, Richard M, Gandolphe C, Scherman D. Storage correction in cells of patients suffering from mucopolysaccharidoses types IIIA and VII after treatment with genistein and other isoflavones. J Inherit Metab Dis. 2010;33:61–67. doi: 10.1007/s10545-009-9029-2. [DOI] [PubMed] [Google Scholar]
- Beck M. Mucopolysaccharidoses: clinical features and management. In: vom Dahl S, Wendel U, Strohmeyer G, editors. Genetic metabolic disorders: management, costs and sociomedical aspects. Cologne: Deutscher Arzte-Verlag; 2007. pp. 13–18. [Google Scholar]
- de Ruijter J, Valstar MJ, Wijburg FA. Mucopolysaccharidosis type III (Sanfilippo syndrome): emerging treatment strategies. Curr Pharm Biotechnol. 2011;12(6):923–930. doi: 10.2174/138920111795542651. [DOI] [PubMed] [Google Scholar]
- Delgadillo V, del Mar O’Callaghan M, Artuch R, Montero R, Pineda M (2011) Genistein supplementation in patients affected by Sanfilippo disease. J Inherit Metab Dis 34(5):1039–1044, doi:10.1007/s10545-011-9342-4 [DOI] [PubMed]
- Friso A, Tomanin R, Salvalaio M, Scarpa M. Genistein reduces glycosaminoglycan levels in a mouse model of mucopolysaccharidosis type II. Br J Pharmacol. 2010;159:1082–1091. doi: 10.1111/j.1476-5381.2009.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloska A, Jakóbkiewicz-Banecka J, Narajczyk M, Banecka-Majkutewicz Z, Węgrzyn G. Effects of flavonoids on glycosaminoglycan synthesis: implications for substrate reduction therapy in Sanfilippo disease and other mucopolysaccharidoses. Metab Brain Dis. 2011;26:1–8. doi: 10.1007/s11011-011-9233-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinowska M, Jakóbkiewicz-Banecka J, Kloska A, Tylki-Szymańska A, Czartoryska B, Piotrowska E, Wegrzyn A, Wegrzyn G. Abnormalities in the hair morphology of patients with some but not all types of mucopolysaccharidoses. Eur J Pediatr. 2008;167:203–209. doi: 10.1007/s00431-007-0462-7. [DOI] [PubMed] [Google Scholar]
- Malinowska M, Wilkinson FL, Bennett W, et al. Genistein reduces lysosomal storage in peripheral tissues of mucopolysaccharide IIIB mice. Mol Genet Metab. 2009;98:235–242. doi: 10.1016/j.ymgme.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Malinowska M, Wilkinson FL, Langford-Smith KJ, et al. Genistein improves neuropathology and corrects behaviour in a mouse model of neurodegenerative metabolic disease. PLoS One. 2010;5:e14192. doi: 10.1371/journal.pone.0014192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marucha J, Tylki-Szymańska A, Jakóbkiewicz-Banecka J, Piotrowska E, Kloska A, Czartoryska B, Węgrzyn G (2011) Improvement in the range of joint motion in seven patients with mucopolysaccharidosis type II during experimental gene expression-targeted isoflavone therapy (GET IT). Am J Med Genet A 155A(9):2257–2262 [DOI] [PubMed]
- Neufeld EF, Muenzer J. The mucopolysaccharidoses. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited disease. New York: McGraw-Hill Co.; 2001. pp. 3421–3452. [Google Scholar]
- Piotrowska E, Jakobkiewicz-Banecka J, Baranska S, et al. Genistein-mediated inhibition of glycosaminoglycan synthesis as a basis for gene expression-targeted isoflavone therapy for mucopolysaccharidoses. Eur J Hum Genet. 2006;14:846–852. doi: 10.1038/sj.ejhg.5201623. [DOI] [PubMed] [Google Scholar]
- Piotrowska E, Jakobkiewicz-Banecka J, Tylki-Szymanska A, et al. Genistin-rich soy isoflavone extract in substrate reduction therapy for Sanfilippo syndrome: An open-label, pilot study in 10 pediatric patients. Curr Ther Res Clin Exp. 2008;63:166–179. doi: 10.1016/j.curtheres.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrowska E, Jakóbkiewicz-Banecka J, Wegrzyn G. Different amounts of isoflavones in various commercially available soy extracts in the light of gene expression-targeted isoflavone therapy. Phytother Res. 2010;24(Suppl 1):S109–S113. doi: 10.1002/ptr.2944. [DOI] [PubMed] [Google Scholar]
- Piotrowska E, Jakobkiewicz-Banecka J, Maryniak A, Tylki-Szymanska A, Puk E, Liberek A, Wegrzyn A, Czartoryska B, Slominska-Wojewodzka M, Wegrzyn G. Two-year follow-up of Sanfilippo Disease patients treated with a genistein-rich isoflavone extract: Assessment of effects on cognitive functions and general status of patients. Med Sci Monit. 2011;17:CR196–CR202. doi: 10.12659/MSM.881715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valstar MJ, Ruijter GJ, van Diggelen OP, Poorthuis BJ, Wijburg FA. Sanfilippo syndrome: a mini-review. J Inherit Metab Dis. 2008;31:240–252. doi: 10.1007/s10545-008-0838-5. [DOI] [PubMed] [Google Scholar]
- Wegrzyn G, Jakóbkiewicz-Banecka J, Narajczyk M, Wiśniewski A, Piotrowska E, Gabig-Cimińska M, Kloska A, Słomińska-Wojewódzka M, Korzon-Burakowska A, Węgrzyn A. Why are behaviors of children suffering from various neuronopathic types of mucopolysaccharidoses different. Med Hypoth. 2010;75:605–609. doi: 10.1016/j.mehy.2010.07.044. [DOI] [PubMed] [Google Scholar]
- Wegrzyn G, Jakóbkiewicz-Banecka J, Gabig-Cimińska M, Piotrowska E, Narajczyk M, Kloska A, Malinowska M, Dziedzic D, Gołebiewska I, Moskot M, Wegrzyn A. Genistein: a natural isoflavone with a potential for treatment of genetic diseases. Biochem Soc Trans. 2010;38:695–701. doi: 10.1042/BST0380695. [DOI] [PubMed] [Google Scholar]