Abstract
Congenital Disorders of Glycosylation (CDG) are a group of recently described inborn errors of metabolism affecting glycosylation. CDG are disorders that have been reported with a great variability in the clinical presentation, especially for the most common PMM2-CDG. The classical form is neurologic but severe forms with multisystem disorders and hydrops fetalis have been described. Here, we extend on the opposite end the clinical spectrum to an asymptomatic PMM2-CDG case. The case was the father of a child who died of neonatal galactosemia few days after birth. He presented without any clinical or biological signs, except a typical CDG 1 pattern in Western blot of glycoproteins associated with a deficient phosphomannomutase activity in blood leukocytes and compound heterozygosity in PMM2 gene. The sister of the father, who was also affected by PMM deficiency, presented with infertility and premature ovarian failure. Finally, the absence of any abnormal clinical or biological signs as for the case completes the clinical spectrum of PMM2-CDG at its extreme end, at the opposite of the supposed total lethality of the R141H homozygous status.
Congenital Disorders of Glycosylation (CDG) are a group of recently described inborn errors of metabolism affecting glycosylation. Clinically, CDG patients share many symptoms such as psychomotor retardation, ataxia, failure to thrive, dysmorphic features, and coagulopathy, but none of these are pathognomonic. Even more, a great variability in the clinical presentation has been reported, especially for PMM2-CDG (OMIM#212065), the most common up to now CDG type (Jaeken and Carchon 2004).
PMM2-CDG is associated with deficiency in enzymatic activity of cellular phosphomannomutase (PMM), the enzyme responsible for conversion of Man 6-phosphate to Man 1-phosphate, and defective PMM2 gene. PMM2-CDG patients present with inverted nipples, subcutaneous fat pads, psychomotor retardation, ataxia due to cerebellar hypoplasia, retinis pigmentosa, this phenotype still considered the classical presentation of PMM2-CDG (Grunewald 2009). However, this presentation may vary in terms of severity and age of the patient. Here, we report on a PMM2-CDG case without any apparent clinical symptoms.
The case was a married 38-year-old man, without any reported health problems and normal serum transaminases, prothrombine time, and fibrinogen values. He had a superior education level and worked ever after graduation in informatics. With his nonconsanguineous wife, he had two children, first a healthy boy and then a girl. The familial history was unremarkable until the birth of his daughter at 37 weeks gestation after an uneventful pregnancy. Respiratory distress appeared secondary and the child was discharged from an intensive care unit. Then weight loss was occurred secondary to a severe secretory diarrhea and polyuria. Neurological exam showed central and peripheral hypotonia. At day 9, cardiac arrest occurred without any previous sign of cardiac failure. The only available biological sample for this child before she died was filter paper whole blood spots sent to our laboratory in order to exclude CDG, given the fact that she presented with unrelated symptoms from at least two organs and no evident diagnosis (Jaeken and Carchon 2004).
Western blotting assay of different serum glycoproteins from the daughter identified an abnormal CDG 1 glycosylation pattern. PMM activity could not be measured because of the absence of any cellular sample; therefore, we asked for parental cells. Leukocyte PMM activity for parents was not heterozygote as expected: the mother showed a normal activity and the father (case) showed a decreased one, overlapping with low heterozygote and high residual activity. As a consequence, serum glycoprotein western blot of the father was performed and also showed a typical CDG 1 glycosylation pattern.
Direct sequencing of the father’s genomic DNA extracted from whole blood sample identified two mutations: c.367C>T p.R123X already described as pathogenic (Matthijs et al. 2000) and c.614A > G p.Y205C. The Y205C mutation was not found on 100 control chromosomes and after transgenesis in E. coli the mutated protein showed a diminished PMM activity of 25% to wild type (data not shown). His daughter was found heterozygote R123X/-.
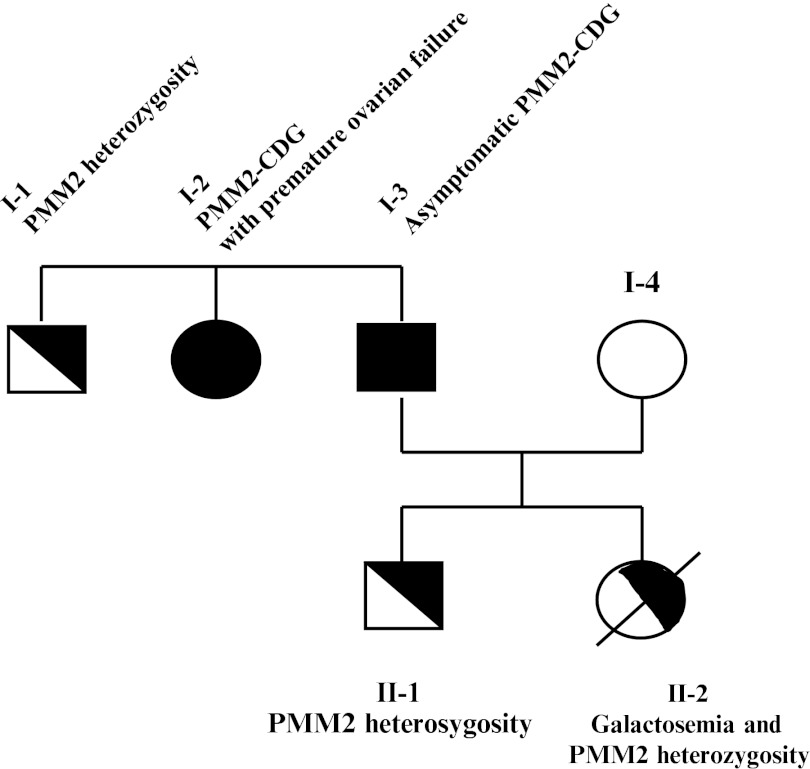
To complete the family study (Fig 1), we obtained blood from the son, the sister, and brother of the father, all of them being apparently healthy, although the sister had been suffering from infertility and premature ovarian failure, at 26 years old. The son and the brother were found, respectively, heterozygote, Y205C/- and R123X/-, with heterozygote PMM activities. The sister was found composite heterozygote R123X/Y205C with a deficient PMM activity and a typical CDG 1 glycosylation pattern, like the father.
Fig. 1.
Family tree for PMM2-CDG-mutated alleles
In order to finalize the diagnosis for the daughter for whom the biochemical screening was positive, we tested the genes involved in secondary CDG and identified a compound heterozygosity for two frequent and severe point mutations on GALT (Galactose-1-P uridyltransferase; galactosemia-OMIM#230400) inherited from her parents : c.563a > G (Q188R) and c.855G>T (K285N).
In this family, as detailed in Table 1, contrarily to what was initially suspected, the daughter did not suffer from PMM2-CDG but galactosemia, and the father and his sister were the ones with PMM2-CDG.
Table 1.
Characteristics of the PMM2-CDG case and his family
| Leukocyte PMM activity (N > 4.2 U/g TP) | PMM2 mutations | GALT mutations | CDG screening pattern | Diagnosis | |
|---|---|---|---|---|---|
| I-3 Father (Case) | 1.2 U/g TP | [c.367C>T (R123X)] + [c.614A>G (Y205C)] | [c.563a>G (Q188R)] + [N] | CDG I | Asymptomatic PMM2-CDG (GALT heterozygosity) |
| I-1 Brother | 3.3 U/g TP | [c.367C>T (R123X)] + [N] | Not tested | Normal | PMM2 heterozygosity |
| I-2 Sister | 1.2 U/g TP | [c.367C>T (R123X)] + [c.614A>G (Y205C)] | [c.563a>G (Q188R)] + [N] | CDG I | PMM2-CDG with premature ovarian failure (GALT heterozygosity) |
| I-4 Wife | 5.1 U/g TP | [N] + [N] | [c.855G>T (K285N)] + [N] | Normal | GALT heterozygosity |
| II-1 Son | 3.2 U/g TP | [c.614A>G (Y205C)] + [N] | Not tested | Normal | PMM2 heterozygosity |
| II-2 Daughter | Not tested | [c.367C>T (R123X)] + [N] | [c.563a>G (Q188R)] + [ c.855G>T (K285N)] | CDG I | Galactosemia (PMM2 heterozygosity) |
The absence of clinical presentation in the father could be related to the mutation combination that was never described before or afterwards. However, leukocyte PMM activity was very low, just as in other PMM2-CDG patients, assessing the inborn error of metabolism. Considering the sister of the father who was also affected, the causality of PMM deficiency in infertility and premature ovarian failure she presented with, remains to be explored in a series of patients with such clinical signs as ovarian failure is a well-known feature of PMM2-CDG (Kristiansson et al. 1995).
This report is the first one about an apparently healthy subject with PMM2-CDG. The father never ever had any signs usually observed in PMM2-CDG patients, no mental delay or neurological defects, and even no other signs as reported in his medical file. His status was discovered only because of his daughter’s illness; otherwise, he would have never been tested for CDG. Some patients with very mild signs have been reported previously, such as mild intellectual delay or coagulopathy. All had gone to the doctor’s because of health problems and finally were screened for CDG. In a previous study, we reported hydrops fetalis in two related fetuses with PMM2-CDG, adding them to the list of other similar cases with CDG (Leticee et al. 2010). This unspecific sign is observed in clinically severe forms of CDG whatever the type, in presumable relation to hepatic failure commonly described in CDG.
The description of this adult PMM2-CDG case is essential. It could help understanding the discrepancy between expected (1/25,000 to 1/50,000) (Schollen et al. 2000) and observed (less than 150 PMM2-CDG patients diagnosed in France) prevalence of PMM2-CDG, which could be explained in part by fetal or neonatal death prior to diagnosis, or under ascertainment of mild and atypical cases at the other end of the spectrum. Indeed, the patients that are screened for CDG whatever their age are only the ones visiting specialized pediatricians, geneticists, neurologists, etc. The severity of the fetal presentation and its absence of specificity can mislead to other diagnoses than CDG, as can the presence of very mild and isolated unspecific signs, in the same way. Finally, the absence of any abnormal clinical or biological signs completes the clinical spectrum of PMM2-CDG at its extreme end, at the opposite of the supposed total lethality of the R141H homozygous status.
Synopsis
Misdiagnosis of neonatal galactosemia expands the phenotype of PMM2-CDG to an asymptomatic adult man and a woman with premature ovarian failure.
Footnotes
Competing interests: None declared.
References
- Grunewald S. The clinical spectrum of phosphomannomutase 2 deficiency (CDG-Ia) Biochim Biophys Acta. 2009;1792:827–834. doi: 10.1016/j.bbadis.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Jaeken J, Carchon H. Congenital disorders of glycosylation: a booming chapter of pediatrics. Curr Opin Pediatr. 2004;16:434–439. doi: 10.1097/01.mop.0000133636.56790.4a. [DOI] [PubMed] [Google Scholar]
- Kristiansson B, Stibler H, Wide L. Gonadal function and glycoprotein hormones in the carbohydrate-deficient glycoprotein (CDG) syndrome. Acta Paediatr. 1995;84:655–659. doi: 10.1111/j.1651-2227.1995.tb13720.x. [DOI] [PubMed] [Google Scholar]
- Leticee N, Bessieres-Grattagliano B, Dupre T, et al. Should PMM2-deficiency (CDG Ia) be searched in every case of unexplained hydrops fetalis? Mol Genet Metab. 2010;101:253–257. doi: 10.1016/j.ymgme.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Matthijs G, Schollen E, Bjursell C, et al. Mutations in PMM2 that cause congenital disorders of glycosylation, type Ia (CDG-Ia) Hum Mutat. 2000;16:386–394. doi: 10.1002/1098-1004(200011)16:5<386::AID-HUMU2>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Schollen E, Kjaergaard S, Legius E, et al. Lack of Hardy-Weinberg equilibrium for the most prevalent PMM2 mutation in CDG-Ia (congenital disorders of glycosylation type Ia) Eur J Hum Genet. 2000;8:367–371. doi: 10.1038/sj.ejhg.5200470. [DOI] [PubMed] [Google Scholar]