Abstract
Exposure to indoor radon has been determined to be the second leading cause of lung cancer after tobacco smoking. Canadian population risk of radon induced lung cancer was assessed in 2005 with the radon distribution characteristics determined from a radon survey carried out in the late 1970s in 19 cities. In that survey, a grab sampling method was used to measure radon levels. The observed radon concentration in 14 000 Canadian homes surveyed followed a log–normal distribution with a geometric mean (GM) of 11.2 Bq m–3 and a geometric standard deviation (GSD) of 3.9. Based on the information from that survey, it was estimated that ∼10 % of lung cancers in Canada resulted from indoor radon exposure. To gain a better understanding of radon concentrations in homes across the country, a national residential radon survey was launched in April 2009. In the recent survey, long-term (3 month or longer) indoor radon measurements were made in roughly 14 000 homes in 121 health regions across Canada. The observed radon concentrations follow, as expected, a log–normal distribution with a GM of 41.9 Bq m–3 and a GSD of 2.8. Based on the more accurate radon distribution characteristics obtained from the recent cross-Canada radon survey, a re-assessment of Canadian population risk for radon induced lung cancer was undertaken. The theoretical estimates show that 16 % of lung cancer deaths among Canadians are attributable to indoor radon exposure. These results strongly suggest the ongoing need for the Canadian National Radon Program. In particular, there is a need for a focus on education and awareness by all levels of government, and in partnership with key stakeholders, to encourage Canadians to take action to reduce the risk from indoor radon exposure.
INTRODUCTION
Radon is a radioactive gas produced by the decay of natural uranium in rocks and soils throughout the earth's crust. A certain fraction of the radon escapes into the air. Radon is quickly diluted outdoors by atmospheric mixing and is of no further concern. However, in confined spaces such as residential homes, radon can accumulate to harmful levels. Long-term exposure to elevated indoor radon concentrations has been determined to be the second leading cause of lung cancer after tobacco smoking(1). In 2005, the Canadian population risk for radon induced lung cancer was assessed with the radon distribution characteristics determined from a radon survey carried out in the late 1970s in 19 cities(2, 3). In that survey, a grab sampling method was used to measure radon levels. The observed radon concentrations in 14 000 Canadian homes surveyed followed a log–normal distribution with a geometric mean (GM) of 11.2 Bq m–3 and a geometric standard deviation (GSD) of 3.9. Based on this radon distribution, the theoretical estimates showed that ∼10 % of lung cancers in Canada resulted from indoor radon exposure(2).
Following the launch of the National Radon Program in 2007, Health Canada started a national residential radon survey in April 2009(4) to gain a better understanding of radon concentrations in homes across the country. In this survey, long-term (3-months or longer) radon measurements were initiated in roughly 14 000 homes in 121 health regions (administrative areas defined by the provincial ministries of health) across Canada. Long-term radon measurements were used in this survey instead of the previous grab sampling method to provide more accurate results, since short-term measurements can be highly variable. Based on the more accurate radon distribution characteristics obtained from the recent cross-Canada radon survey, a re-assessment of the Canadian population risk for radon induced lung cancer was undertaken. The results are reported here.
METHODS
The population risk of radon-induced lung cancer is assessed by an attributable risk (AR). The AR of lung cancer due to ionising radiation is defined as the proportion of lung-cancer deaths attributable to indoor radon exposure(5). This risk indicates the proportion of lung cancer deaths that could be theoretically prevented by reducing indoor radon concentrations to outdoor levels. Given the exposure–response relationship for radon and lung cancer risk, the mortality rates of lung cancers and all cases and the distribution of indoor radon concentrations, the AR can be estimated:
| (1) |
where 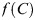 is the probability density of radon concentration and
is the probability density of radon concentration and 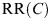 is the lifetime relative risk of lung cancer for a lifetime exposure to radon at a constant concentration of C in the presence of competing risks, i.e. the ratio of lifetime lung cancer risk for exposure rate C to lifetime lung cancer risk for ‘zero’ exposure.
is the lifetime relative risk of lung cancer for a lifetime exposure to radon at a constant concentration of C in the presence of competing risks, i.e. the ratio of lifetime lung cancer risk for exposure rate C to lifetime lung cancer risk for ‘zero’ exposure.
The proportions of Canadian homes with different ranges of radon concentration are based on Health Canada's national residential radon survey in homes roughly uniformly distributed in 121 health regions across Canada(4). The survey was conducted over 2 y during the heating seasons of 2009/10 and 2010/11. Long-term radon measurements were performed in all the homes surveyed. The observed radon concentration in Canadian homes follows a log–normal distribution(4) with a population-weighted GM of 41.9 Bq m–3 and a GSD of 2.8. It was assumed that males and females were distributed equally in the various radon concentration ranges.
The lifetime relative risk, 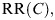 at a given radon concentration, C, can be calculated from the exposure–response relationship for radon and lung cancer risk. Based on worldwide epidemiological studies, the BEIR VI committee recommended two models for estimating radon risks: the exposure–age–duration model and the exposure–age–concentration model(6). The two models are equally preferred and it is not easy to decide which one to use in practice. The United States Environmental Protection Agency devised a single model(7) that gives risk values midway between the two BEIR VI preferred models. The Environmental Protection Agency model was used in the calculation of lifetime RRs for Canadians(8). The computation of lifetime risks depends on the choice of the background age-specific lung-cancer and overall mortality rates. In the calculation, Canadian age-specific mortality rates averaged over 5 y from 1996 to 2000(9) were used. In the adjustment of age-specific lung cancer mortality rates to reflect smoking status, Canadian age-specific smoking prevalence data for males and females in 2002 were used(10). The average age of commencement of smoking is 18 among Canadians. According to the BEIR VI report, never-smokers are defined as those persons who have not yet smoked 100 cigarettes, and ever-smokers are those who have smoked at least 100 cigarettes in their lifetime. It is accepted that smoking and radon exposure combine in a fashion that is sub-multiplicative on the RR scale. It is assumed that smoking-induced lung cancer has a 10-y latency period and the RR for ever-smokers compared with that for never-smokers is approximately 14 for males and 12 for females. Details of the lifetime RR calculation can be found in a previous publication(8). It is further assumed that the lifetime
at a given radon concentration, C, can be calculated from the exposure–response relationship for radon and lung cancer risk. Based on worldwide epidemiological studies, the BEIR VI committee recommended two models for estimating radon risks: the exposure–age–duration model and the exposure–age–concentration model(6). The two models are equally preferred and it is not easy to decide which one to use in practice. The United States Environmental Protection Agency devised a single model(7) that gives risk values midway between the two BEIR VI preferred models. The Environmental Protection Agency model was used in the calculation of lifetime RRs for Canadians(8). The computation of lifetime risks depends on the choice of the background age-specific lung-cancer and overall mortality rates. In the calculation, Canadian age-specific mortality rates averaged over 5 y from 1996 to 2000(9) were used. In the adjustment of age-specific lung cancer mortality rates to reflect smoking status, Canadian age-specific smoking prevalence data for males and females in 2002 were used(10). The average age of commencement of smoking is 18 among Canadians. According to the BEIR VI report, never-smokers are defined as those persons who have not yet smoked 100 cigarettes, and ever-smokers are those who have smoked at least 100 cigarettes in their lifetime. It is accepted that smoking and radon exposure combine in a fashion that is sub-multiplicative on the RR scale. It is assumed that smoking-induced lung cancer has a 10-y latency period and the RR for ever-smokers compared with that for never-smokers is approximately 14 for males and 12 for females. Details of the lifetime RR calculation can be found in a previous publication(8). It is further assumed that the lifetime 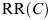 at a given radon concentration C remains unchanged from the previous assessment. Those lifetime RRs are used in the current re-assessment of radon-induced lung cancer with more accurate radon distribution characteristics obtained from the recent cross-Canada residential radon survey.
at a given radon concentration C remains unchanged from the previous assessment. Those lifetime RRs are used in the current re-assessment of radon-induced lung cancer with more accurate radon distribution characteristics obtained from the recent cross-Canada residential radon survey.
RESULTS
Attributable risks
Based on Equation (1), ARs are calculated for Canadian men and women. Results are given in Tables 1 and 2, respectively. The total ARs for Canadian males are 0.153 for ever-smokers and 0.294 for never-smokers. For Canadian women, the total ARs are 0.143 for ever-smokers and 0.278 for never-smokers, respectively. The total AR is the sum of the ARs for each radon range. The detailed breakdown of AR in different radon ranges is listed in Tables 1 and 2. For easy comparison, the same radon ranges are used here as in the previous assessment(2).
Table 1.
Lifetime RRs at different radon exposures, the distribution of ARs for Canadian men from indoor radon exposure and estimated lung-cancer deaths attributable to radon for the year 2011.
| Radon range |
Proportion of homes |
Relative risk, RR(C) |
Contribution to AR |
Lung-cancer deaths in 2011 attributable to radon |
|||
|---|---|---|---|---|---|---|---|
| Bq m–3 | (%) | Ever-smoker | Never-smoker | Ever-smoker | Never-smoker | Ever-smoker | Never-smoker |
| 0–18 | 21.12 | 1.0414 | 1.0937 | 0.0074 | 0.0138 | 79 | 8 |
| 19–37 | 24.55 | 1.0847 | 1.1924 | 0.0175 | 0.0330 | 188 | 19 |
| 38–56 | 15.88 | 1.1277 | 1.2911 | 0.0171 | 0.0322 | 184 | 18 |
| 57–74 | 9.85 | 1.1682 | 1.3844 | 0.0140 | 0.0264 | 150 | 15 |
| 75–92 | 6.76 | 1.2084 | 1.4777 | 0.0119 | 0.0225 | 128 | 13 |
| 93–111 | 5.01 | 1.2505 | 1.5760 | 0.0106 | 0.0201 | 114 | 11 |
| 112–130 | 3.60 | 1.2924 | 1.6742 | 0.0089 | 0.0169 | 95 | 9 |
| 131–148 | 2.53 | 1.3317 | 1.7672 | 0.0071 | 0.0135 | 76 | 8 |
| 149–166 | 1.93 | 1.3708 | 1.8600 | 0.0060 | 0.0116 | 65 | 6 |
| 167–185 | 1.57 | 1.4118 | 1.9580 | 0.0055 | 0.0105 | 59 | 6 |
| 186–200 | 0.99 | 1.4439 | 2.0352 | 0.0037 | 0.0072 | 40 | 4 |
| 201–250 | 2.26 | 1.5498 | 2.2921 | 0.0105 | 0.0204 | 112 | 11 |
| 251–300 | 1.30 | 1.6536 | 2.5482 | 0.0072 | 0.0141 | 77 | 8 |
| 301–400 | 1.32 | 1.8555 | 3.0583 | 0.0095 | 0.0190 | 102 | 11 |
| 401–500 | 0.59 | 2.0500 | 3.5655 | 0.0052 | 0.0106 | 56 | 6 |
| 501–600 | 0.30 | 2.2373 | 4.0696 | 0.0031 | 0.0064 | 33 | 4 |
| 601–800 | 0.26 | 2.5916 | 5.0692 | 0.0035 | 0.0074 | 37 | 4 |
| >800 | 0.25 | 2.9206 | 6.0573 | 0.0041 | 0.0089 | 44 | 5 |
| Total | 100.00 | — | — | 0.1527 | 0.2945 | 1639 | 166 |
The same radon ranges are used here as in the previous assessment(2).
A radon concentration range of 0–18 in Bq m–3 corresponds to 0–0.5 in pCi l–1.
Table 2.
Lifetime RRs at different radon exposures, the distribution of ARs for Canadian women from indoor radon exposure and estimated lung-cancer deaths attributable to radon for the year 2011.
| Radon range |
Proportion of homes |
Relative risk, RR(C) |
Contribution to AR |
Lung-cancer deaths in 2011 attributable to radon |
|||
|---|---|---|---|---|---|---|---|
| Bq m–3 | (%) | Ever-smoker | Never-smoker | Ever-smoker | Never-smoker | Ever-smoker | Never-smoker |
| 0–18 | 21.12 | 1.0382 | 1.0862 | 0.0069 | 0.0130 | 58 | 12 |
| 19–37 | 24.55 | 1.0782 | 1.1770 | 0.0164 | 0.0311 | 137 | 29 |
| 38–56 | 15.88 | 1.1180 | 1.2677 | 0.0160 | 0.0304 | 134 | 28 |
| 57–74 | 9.85 | 1.1554 | 1.3536 | 0.0131 | 0.0249 | 109 | 23 |
| 75–92 | 6.76 | 1.1926 | 1.4394 | 0.0111 | 0.0212 | 93 | 20 |
| 93–111 | 5.01 | 1.2317 | 1.5298 | 0.0099 | 0.0190 | 83 | 18 |
| 112–130 | 3.60 | 1.2705 | 1.6201 | 0.0083 | 0.0159 | 69 | 15 |
| 131–148 | 2.53 | 1.3070 | 1.7056 | 0.0066 | 0.0128 | 55 | 12 |
| 149–166 | 1.93 | 1.3433 | 1.7910 | 0.0057 | 0.0109 | 47 | 10 |
| 167–185 | 1.57 | 1.3814 | 1.8811 | 0.0051 | 0.0099 | 43 | 9 |
| 186–200 | 0.99 | 1.4113 | 1.9521 | 0.0035 | 0.0067 | 29 | 6 |
| 201–250 | 2.26 | 1.5099 | 2.1883 | 0.0098 | 0.0192 | 82 | 18 |
| 251–300 | 1.30 | 1.6069 | 2.4239 | 0.0068 | 0.0133 | 57 | 12 |
| 301–400 | 1.32 | 1.7961 | 2.8928 | 0.0090 | 0.0179 | 75 | 17 |
| 401–500 | 0.59 | 1.9790 | 3.3590 | 0.0049 | 0.0100 | 41 | 9 |
| 501–600 | 0.30 | 2.1561 | 3.8224 | 0.0029 | 0.0060 | 25 | 6 |
| 601–800 | 0.26 | 2.4930 | 4.7409 | 0.0033 | 0.0069 | 28 | 6 |
| >800 | 0.25 | 2.8084 | 5.6486 | 0.0039 | 0.0084 | 33 | 8 |
| Total | 100.00 | — | — | 0.1431 | 0.2776 | 1198 | 258 |
Attributable lung cancer deaths
The estimated lung cancer deaths attributable to indoor radon exposure were based on Canadian Cancer Statistics 2011(11). In 2011, there were 20 600 deaths from lung cancer in Canada (11 300 in men and 9300 in women). As in BEIR VI, it was assumed that 95 % of lung cancer deaths in men and 90 % of lung cancer deaths in women are ever-smokers.
The ARs for Canadian males are 0.153 for ever-smokers and 0.294 for never-smokers. This means that, of the 11 300 lung cancers in men reported for the year 2011, 1805 were attributable to radon exposure. This is the sum of 1639 (11 300 × 0.95 × 0.1527) in ever-smokers and 166 (11 300 × 0.05 × 0.2945) in never-smokers. Details are given in column 5 of Table 1. The theoretical estimates show that 16 % of lung cancer deaths among Canadian males are attributable to indoor radon exposure.
The ARs for Canadian females are 0.143 for ever-smokers and 0.278 for never-smokers, which gave 1456 radon-induced lung cancer deaths (1198 in ever-smokers and 258 in never-smokers) for the year 2011. Details are given in column 5 of Table 2. As in the case of Canadian males, the theoretical estimates show that 16 % of lung cancer deaths among Canadian females are attributable to indoor radon exposure.
Effect of radon mitigation on attributable risk
The overall AR describes the anticipated consequences of the virtual elimination of indoor radon exposure, which is not practical. A more realistic assessment of the reduction of radon AR can be made on exposure–reduction scenarios that are realisable in practice. A scenario is considered in which all homes above a specified radon concentration are remediated to the outdoor radon level. Table 3 provides estimates of the number of Canadian lives that could be saved every year in such a scenario. In spite of the high individual risk at 800 Bq m–3, the number of lives saved is only 90 per year, because of the very small proportion of homes above this level. At the current Canadian action level of 200 Bq m–3, the number of lives saved rises to 927, out of a total of 3261 estimated radon-induced lung cancers. The current Canadian action level was set on the basis of a balance between health risks regarded as too high to ignore and a practical value. Below 200 Bq m–3, it was not clear whether there would be an increase in benefits to Canadians due to technological difficulties in remediating below this level and a reduced success rate and costs. If more protective action at 100 Bq m–3 were taken, as recommended by the World Health Organization(1), the long-term result would be an additional seven hundred Canadian lives saved every year.
Table 3.
Estimated lung cancer deaths that could have been prevented in 2011 if all the homes with radon concentrations above given levels had taken effective remedical actions to outdoor levels.
| Radon level Bq m–3 | Lung cancer deaths prevented |
||
|---|---|---|---|
| Males | Females | Total | |
| 100 | 941 | 763 | 1704 |
| 200 | 510 | 417 | 927 |
| 400 | 189 | 156 | 345 |
| 600 | 90 | 75 | 165 |
| 800 | 49 | 41 | 90 |
DISCUSSION
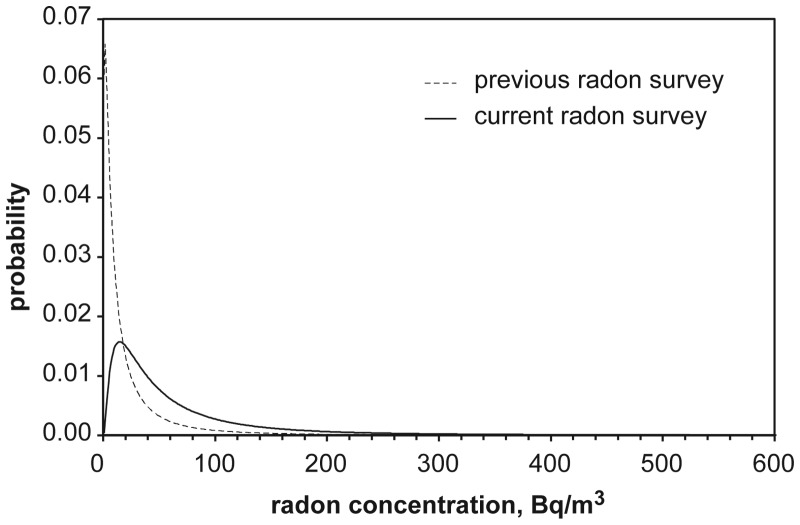
Based on radon distribution characteristics determined from a radon survey carried out in the late 1970s using a grab sampling technique, it was estimated that ∼10 % of lung cancers in Canada result from indoor radon exposure. However, recently radon distribution characteristics for Canada were more accurately determined through a second cross-country radon survey using long-term alpha track detectors. A comparison of radon concentrations in Canadian homes from the first and the second radon surveys is given in Figure 1. One can see that the current radon survey revealed that more Canadians are exposed to radon concentrations above 20 Bq m–3 than previously estimated.
Figure 1.
Distributions of radon concentrations in Canadian homes obtained from the previous cross-Canada radon survey(3) and the current radon survey(4).
It is well known that lung cancer risk increases with increased exposure concentration. Based on the more accurate radon distribution characteristics obtained from the recent cross-Canada radon survey, the new estimate of lung cancers in Canada attributable to indoor radon exposure is 16 %, compared with the previous estimate of 10 %.
In 2011, an estimated 25 300 Canadians will be diagnosed with lung cancer and 20 600 will die of it(11). Lung cancer remains the leading cause of cancer death for both men and women. Based on the current distribution of radon in homes, indoor radon exposure can be expected to cause more than three thousand lung cancer deaths each year. If all homes with radon levels above 200 Bq m–3 were remediated to the outdoor level, 927 of the anticipated 3 261 radon-induced lung cancers could potentially be prevented per year.
In 2007 Health Canada committed to the development and implementation of the National Radon Program, a programme designed to reduce lung cancer incidence by increasing public awareness of risk and promoting testing and action to reduce radon exposure. The current radon distribution characteristics and the associated re-assessment of the number of lung cancers attributable to radon exposure strongly support the ongoing need for the programme as well as further action in Canada related to the protection of Canadians from this health risk. In order to see a reduction in lives lost, it will be necessary to continue to raise awareness and to encourage testing and action to reduce radon levels in Canadian homes. To effectively reduce the risks for Canadians from radon exposure, this effort needs to come not only from the Federal government but from all levels of the government, and with the support of key stakeholders in the health care and building/construction industries and not-for-profit organisations.
REFERENCES
- 1.The World Health Organization (WHO) WHO Handbook on Indoor Radon. . 2009 ISBN 978-92-4-154767-3. Available at http://whqlibdoc.who.int/publications/2009/9789241547673_eng.pdf . [Google Scholar]
- 2.Chen J., Tracy B. Canadian population risk of radon induced lung cancer. Can. J. Respir. Ther. 2005;41:19–27. [Google Scholar]
- 3.McGregor R. G., Vasudev P., Letourneau E. G., McCullough R. S., Prantl F. A., Taniguchi H. Background concentration of radon daughters in Canadian homes. Health Phys. 1980;39:285–289. doi: 10.1097/00004032-198008000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Health Canada. Cross-Canada survey of radon concentrations in homes . Available at: http://www.hc-sc.gc.ca/ewh-semt/radiation/radon/survey-sondage-eng.php. (22 accessed December 2011)
- 5.Lubin J. H., Boice J. D. Estimating Rn-induced lung cancer in the United States. Health Phys. 1989;57:417–427. doi: 10.1097/00004032-198909000-00008. [DOI] [PubMed] [Google Scholar]
- 6.National Research Council. National Academy Press; 1999. Biological Effects of Ionizing Radiation (BEIR) VI Report. Health effects of exposure to radon. [Google Scholar]
- 7.Environmental Protection Agency. Office of Radiation and Indoor Air; 2003. EPA assessment of risks from radon in homes. [Google Scholar]
- 8.Chen J. Canadian individual risks of radon induced lung cancer for different exposure profiles. Can. J. Public Health. 2005;6:360–363. doi: 10.1007/BF03404033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9., Health Statistics Division; Data source: Canadian Vital Statistics—Death Database 1996–2000 . Statistics Canada. [Google Scholar]
- 10. The analyses were performed on Health Canada's DAIS|nesstar edition of anonymized microdata from the Canadian Tobacco Use Monitoring Survey, 2002 Annual-Persons File, which contains anonymized microdata from collected by the Special Surveys Sub-division. Labour and Household Surveys Branch, Statistics Canada.
- 11.Canadian Cancer Society. Canadian Cancer Statistics 2011 . Available at: http://www.cancer.ca/~/media/CCS/Canada%20wide/Files%20List/English%20files%20heading/PDF%20-%20Policy%20-%20Canadian%20Cancer%20Statistics%20-%20English/Canadian%20Cancer%20Statistics%202011%20-%20English.ashx .