Abstract
Look AHEAD is a randomized trial determining whether intensive lifestyle intervention (ILI) aimed at long-term weight loss and increased physical fitness reduces cardiovascular morbidity and mortality in overweight and obese individuals with type 2 diabetes compared to control (diabetes support and education, DSE). We investigated the correlates of NT-proBNP, a biomarker associated with heart failure risk, in a subsample from 15 of 16 participating centers and tested the hypothesis that ILI decreased NT-proBNP levels. Baseline and one-year blood samples were assayed for NT-proBNP in a random sample of 1500 without, and all 628 with, self-reported baseline CVD (N=2128). Linear models were used to assess relationships that logtransformed NT-proBNP had with CVD risk factors at baseline and that 1-year changes in NT-proBNP had with intervention assignment. At baseline, the mean (SD) age, BMI, and hemoglobin A1c were 59.6 (6.8) years, 36.0 kg/m2 (5.8), and 7.2% (1.1), respectively. Baseline geometric mean NT-proBNP was not different by condition (ILI 53.3 vs DSE 51.5, p=0.45), was not associated with BMI, and was inversely associated with hemoglobin A1c. At 1 year, ILI participants achieved an average weight loss of 8.3% compared to 0.7% in DSE. At 1 year, NT-proBNP levels increased to a greater extent in the intervention arm (ILI +21.3% vs. DSE +14.2%, p=0.046). The increased NT-proBNP associated with ILI was correlated with changes in A1c, BMI, and body composition. In conclusion, among overweight and obese persons with diabetes, an intensive lifestyle intervention that reduced weight was associated with an increased NT-proBNP.
Keywords: natriuretic peptides, diabetes mellitus, obesity
Introduction
Heart failure (HF) has become a frequent manifestation of cardiovascular disease (CVD) among persons with T2DM. (1,2) Epidemiologic evidence suggests higher hemoglobin A1c (HbA1c), increased obesity, and elevated blood pressure are risk factors for HF among those with diabetes. (3) Measuring natriuretic peptides as part of risk stratification in persons with diabetes is potentially attractive. Cardiac muscle cells release pro-brain-natriuretic peptide (proBNP), which is cleaved into the metabolically active BNP and inactive NT-pro-BNP. NT-proBNP is more stable in blood and highly correlated with BNP (4). NT-proBNP has been demonstrated to be a fairly sensitive and specific biomarker for HF (5-7). NT-proBNP has also been shown to be predictive of future HF, other cardiovascular events, and mortality (8,9), although a recent analysis of an elderly cohort suggests a non-linear relationship between NT-proBNP levels and incident HF, with increasing risk occuring above 190pg/ml (10). A number of studies have demonstrated that increased body mass index (BMI) is associated with lower BNP and/or NT-proBNP levels (11-15), including studies of persons with diabetes (12, 16). Studies have suggested that the inverse relationship between BMI and BNP is mediated by lean body mass (17,18). The data regarding BNP and HbA1c are mixed, with reports of no association (19, 20) as well as a negative correlation (21). These associations are counterintuitive, given the suggested relationship between HbA1c or BMI and HF. Furthermore, in the non-heart failure setting, data regarding the effect of medical or lifestyle interventions on BNP from randomized trials and/or among persons with diabetes are very limited. Clarification of the relationship between risk factors for HF and NT-proBNP in those who are overweight or obese and have T2DM is required before it could be useful to target HF risk stratification. We investigated the correlates of NT-proBNP in a large sample of adults with type 2 diabetes participating in the Look AHEAD clinical trial, and determined if a lifestyle intervention shown to promote weight loss and improve glycemic control and blood pressure at one year impacted NT-proBNP level at follow-up.
Methods and Procedures
The Look AHEAD (Action for Health in Diabetes) study is a multicenter, randomized trial to determine, in overweight and obese volunteers with type 2 diabetes, the long-term effects of two study conditions: an intensive lifestyle intervention designed to achieve and maintain weight loss by decreased caloric intake and increased physical activity versus a control condition of diabetes support and education on the combined incidence of serious cardiovascular events (cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke). Details regarding the design and rationale for the study have been published (22). Participants included persons with type 2 diabetes aged 45-76 years of age with a body-mass index (BMI) ≥ 25 kg/m2 (≥27 if on insulin) recruited at 16 clinical centers across the U.S. between August 2001 and April 2004. Major exclusion criteria were HbA1c >11%, BP ≥160/100 mmHg, triglycerides ≥600 mg/dl, weight >350 pounds, CVD events in the prior 3 months, or recent weight loss. Persons meeting these inclusion criteria were additionally screened with an exercise stress test; those with abnormal results were still potentially eligible for randomization after evaluation by their physician. This research was conducted with the approval of each center’s Institutional Review Board and participants provided informed consent. The one-year results of the study have also been published; briefly, ILI resulted in clinically significant weight loss in people with type 2 diabetes which was associated with improved diabetes control and CVD risk factors. (23)
Participant socio-demographic and medical history characteristics were obtained by self-report, including age, gender, race/ethnicity, education, income, source of medical care, medications, and prior cardiovascular disease (CVD). Prior CVD included history of myocardial infarction, coronary artery bypass, angioplasty/stent procedures, peripheral vascular disease, stroke, stable angina, and class I/II heart failure. Trained and certified clinic staff measured weight and height with the participants wearing light clothing and without shoes. Systolic and diastolic blood pressures were measured as the average of two seated blood pressures, obtained 30 seconds apart after an initial 5 minute rest period, using a calibrated device (Dinamap Pro 100). Hypertension was defined as BP≥ 140/90 mmHg or use of antihypertensive medication. Phlebotomy was performed on fasting participants; specimens were assayed at a central laboratory. HbA1c was measured by a dedicated ion-exchange high-performance liquid chromatography instrument. Measurements of fasting lipids were performed enzymatically using methods standardized to the Centers for Disease Control and Prevention reference.
This manuscript is based on a subset of the baseline data set. Participants were from 15 of the 16 clinical sites; those from the Southwest American Indian Center are not included. The complete data set has been described previously. (24) For this ancillary study, we selected a random sample of 1500 participants without prior CVD at baseline and all 628 participants with prior CVD who met the following criteria: provided consent for future testing of blood and had baseline and one year serum samples stored at −70 degrees Celsius. NT-proBNP was measured using a sandwich electrochemiluminescence immunoassay performed on a Roche Cobas E411 instrument. To assess the coefficient of variation (CV%), high and low quality controls were run on two consecutive days, 10 replicates in each run. The CV% for the high quality control was 1.1%, and for the low quality control was 1.2%. For a given participant, both baseline and one-year NT-proBNP was measured simultaneously in the same batch.
An analysis was also performed on participants from clinics participating in a body composition substudy and had NT-proBNP measured (N=581). Body composition was measured by dual energy x-ray absorptiometry (DXA) at baseline and one year; the methods utilized by Look AHEAD have been previously published (25). DXA provides a measurement of fat and fat-free mass, and the fat-free component can be subdivided into bone mineral and lean soft tissue. We utilized the total body fat and total body lean tissue measures in our analyses.
Statistical Analyses
The distribution of NT-proBNP was right-skewed; thus we performed a log-transformation. To assess pair-wise cross-sectional relationships that baseline NT-proBNP had with continuous variables, linear regression analyses and graphical displays were utilized; for categorical variables, analysis of variance was utilized. Based on prior literature we expected NT-proBNP to be associated with age, gender, and prior CVD status, and additionally added race/ethnicity as variables for adjustment (Model 1). We selected additional variables for full covariate adjustment as follows. Candidate variables included randomization arm, date of randomization, clinical center, diabetes duration, BMI, hemoglobin HbA1c, systolic and diastolic BP, anti-hypertensive, lipid lowering, and diabetes medications, smoking status, and renal function Continuous variables were standardized. Each term was entered into a linear regression with model 1 variables; those found to be associated with NT-proBNP (p<0.2) were retained for the final model (Model 2). We assessed for interactions between predictor variables and intervention condition, CVD status, gender, and race/ethnicity. We also assessed whether baseline predictors of NT-proBNP were balanced by condition. To determine the impact of ILI on NT-proBNP level compared to DSE, we examined the distribution of changes between baseline to 1-year in log transformed NT-proBNP using analysis of covariance A linear regression model was run including change in NT-proBNP as the outcome, and study arm, demographic characteristics and covariates found to be imbalanced as independent variables. . We also examined pair-wise correlations between change in NT-proBNP and change in the main variables affected by the intervention (weight, glucose control, CVD risk factors) in both ILI and DSE arms. To investigate possible mediation we added change in these variables to our regression models and determined if the intervention effect (i.e. study arm beta coefficient) remained significant. A two-tailed p-value of <0.05 was considered significant. All analyses were performed using STATA 11 (StataCorp, College Station TX).
Results
We obtained NT-proBNP measurements in a subsample (n=2128) of the entire Look AHEAD trial (1079 in ILI and 1049 in DSE). The characteristics of the subsample did not differ from the characteristics of the overall Look AHEAD cohort on age, gender, ethnicity, or BMI (data not shown). By design, this sample was enriched with persons with prior CVD (29.5% in this subsample vs 14.1% in the overall cohort). The distribution of baseline characteristics did not differ by intervention condition (Table 1) with the exception of a marginal difference in HbA1c. Baseline geometric mean NT-proBNP was 52.4 pg/mL (SD=2.9) and levels did not differ between conditions. Overall 10.9% had NT-proBNP >190 (10.0% in DSE and 11.7% in ILI, p=0.2).
Table 1.
Baseline Characteristics of the sample with NT-proBNP measured by study arm in the Look AHEAD Trial, 2001-2004
| Characteristic | ILI (N=1,079) | DSE (N=1,049) | p-value |
|---|---|---|---|
| Age | 59.5 (6.8) | 59.7 (6.9) | 0.5 |
|
| |||
| Female | 55.0% | 54.0% | 0.6 |
| Race/Ethnicity | 0.3 | ||
| White | 68.3% | 69.2% | |
| Black | 16.6% | 14.6% | |
| Hispanic | 10.8% | 12.6% | |
| Other | 4.4% | 3.6% | |
|
| |||
| Prior cardiovascular disease | 30.5% | 28.5% | 0.3 |
|
| |||
| Diabetes duration, years | 7.0 (6.9) | 7.2 (6.9) | 0.4 |
|
| |||
| Insulin Use | 19.5% | 19.6% | 0.9 |
|
| |||
| Body-Mass Index, kg/m2 | 36.0 (5.8) | 35.9 (5.8) | 0.7 |
|
| |||
| Hemoglobin HbA1c % | 7.2 (1.1) | 7.3 (1.2) | 0.04 |
|
| |||
| Blood Pressure, mmHG | 129/70 (17/9) | 130/70 (17/10) | 0.3 |
|
| |||
| Antihypertensive use | 78.6% | 75.9% | 0.1 |
|
| |||
| Maximal MET value (at 80% HR) | 5.2 (1.5) | 5.2 (1.6) | 0.3 |
|
| |||
| eGFR, ml/min/1.73m2 | 87.2 (21.8) | 86.3 (21.8) | 0.3 |
|
| |||
| Stage ≥3 Kidney Disease a | 8.2% | 7.3% | 0.6 |
|
| |||
| Urine albumin, mg/dl | 5.4 (20.5) | 5.9 (33.5) | 0.7 |
|
| |||
| LDL-Cholesterol, mmol/l | 2.9 (0.9) | 2.9 (0.8) | 0.5 |
|
| |||
| Lipid Lowering Drug | 56.6% | 53.9% | 0.2 |
|
| |||
| Smoking Status | 0.9 | ||
| Current Smoking | 4.6% | 4.3% | |
| Past Smoking | 47.0% | 47.6% | |
|
| |||
| NT-proBNP, pg/ml | 95.2 (140.2) | 91.5 (134.6) | 0.5 |
| Geometric mean NT-proBNP | 53.3 (2.9) | 51.5 (2.9) | 0.5 |
eGFR= estimated glomerular filtration rate.
Stage 3 or greater kidney disease defined as eGFR<60 ml/min/1.73m2
Cross sectional analyses
NT-proBNP level was positively associated older age, female gender, systolic BP, pulse pressure (SBP-DBP), duration of diabetes, use of TZDs and antihypertensive drugs, urine albumin, and BMI. Participants with prior CVD had a higher geometric mean NT-proBNP (86.6 pg/ml) compared to those without CVD (42.5 pg.ml) (difference p<0.001). We observed a negative association between estimated GFR and African American race/ethnicity and NTproBNP. No statistically significant association with diastolic BP, HbA1c, lipid lowering drugs, fitness level, or smoking was observed. We found no association between date of randomization or clinic and NT-proBNP (data not shown). Model 2 included all variables that had a p-value for the association of ≤0.2; thus, we included all the variables considered above except for fitness and smoking. In this model, a one standard deviation increment of HbA1c was associated with a 5% lower NT-proBNP level (p=0.02). The relationships that SBP and DBP had with NT-proBNP were strong, but in opposite directions. . Higher albumin excretion associated with higher NT-proBNP; higher estimated GFR (indicating better renal function) was associated with lower NT-proBNP. When substituting pulse pressure (PP) for SBP and DBP, a standard deviation increment of PP was associated with +13% relative difference in NT-proBNP (95%CI 9%, 17%); the estimates associated with other variables did not appreciably change.
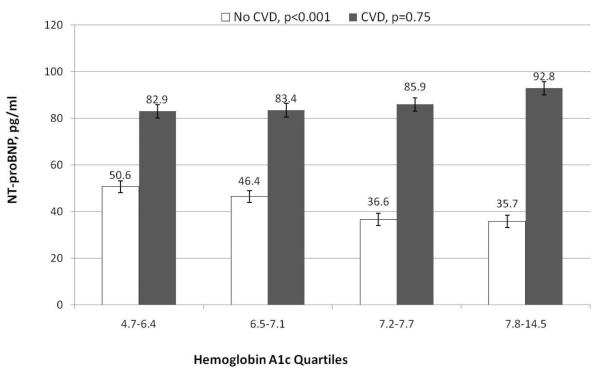
There was a significant interaction between HbA1c and prior CVD (p value for CVD* HbA1c =0.008). After adjusting for Model 2 variables, among those without CVD, a standard deviation increment of baseline HbA1c was associated with a lower level of NT-proBNP (relative difference −8.6%; 95%CI −13%,−4%, p<0.001). In contrast, among those with CVD, there was no association present between HbA1c and NT-proBNP (relative difference +2.8%; 95%CI −6, +11%, p=0.52). At a given level of HbA1c, however, NTproBNP was substantially higher among those with prior CVD (Figure 1).The other significant interaction was between prior CVD and gender (p=0.002); among those with CVD there was no difference in NT-proBNP by gender (data not shown).
Figure 1.
Geometric mean NT-proBNP (pg/ml) at baseline by quartiles of hemoglobin A1c among partipants enrolled in the Look AHEAD trial, stratified by presence or absence of prior cardiovascular disease. P values noted on graphic are for trend across quartiles.
Longitudinal analyses
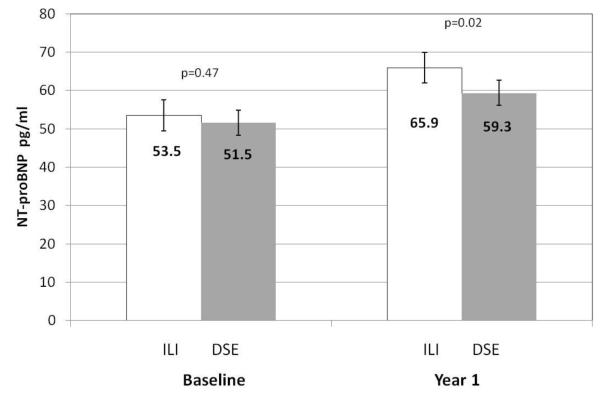
At one year, participants in the ILI group lost more weight (−8.5%) compared to DSE (−0.7%) This was accompanied by improved mean HbA1c (−0.6% vs −0.1%), SBP (−7mmHg vs −3mm Hg) and DBP (−3mmHg vs. −2mmHg). NT-proBNP level increased significantly in both groups between baseline and one year (Figure 2). The mean increase was greater in the intervention arm: ILI +21.3% vs. DSE +14.2%, relative difference 7.1%, p=0.046. We examined correlations between change in NT-proBNP in both arms and change in weight, glucose, blood pressure and lipid parameters (Table 3). Of note, there was an inverse relationship between change in weight variables and change in NT-proBNP among ILI participants, as well as between change in glucose in both groups. Adjusting for demographics, prior CVD and baseline HbA1c level did not appreciably change the estimated intervention effect (+7.7%, p=0.03) (Table 4, model 1). There was no evidence for an interaction between intervention arm and prior CVD, HbA1c, gender, race-ethnicity, or age (all p >0.1). The increase in NT-proBNP associated with ILI was similar among those with (+7.3%) or without (+7.8%) prior CVD (interaction p=0.8).
Figure 2.
Geometric mean NT-proBNP (pg/ml) at among participants randomized to intensive lifestyle intervention (ILI) (white bars) and diabetes support and education (DSE) (grey bars) at baseline and one year in the Look AHEAD trial. P values noted on graphic are for difference between arms at each time point.
Table 3.
Pair-wise correlation between change in log transformed NT-proBNP between year one and baseline, and change in selected parameters, by arm.
| Change (year 1-year 0) in log NT- proBNP |
||
|---|---|---|
| Change (year 1-year 0) | ILI | DSE |
| Systolic Blood Pressure | 0.090* | 0.080* |
| Diastolic Blood Pressure | 0.018 | 0.038 |
| Pulse Pressure | 0.111* | 0.077* |
| Weight | −0.123* | −0.043 |
| Body Mass Index | −0.120* | −0.044 |
| Hemoglobin A1c | −0.144* | −0.153* |
| Glucose | −0.199* | −0.124* |
| Estimated GFR | 0.062* | 0.100* |
| Triglycerides | −0.148* | −0.097* |
| LDL-Cholesterol | −0.084* | −0.069* |
| HDL-Cholesterol | 0.073* | 0.009 |
Statistical significance indicated by p<0.05.
Table 4.
Relative differences in NT-proBNP level at one-year follow-up associated with an intensive lifestyle intervention compared to a control condition.
| Relative Diff | P-value | ||
|---|---|---|---|
| No adjustment | ILI arm | 7.1% (1.1, 14.1%) | 0.046 |
| Model 1 | ILI arm | 7.7% (0.7,14.7%) | 0.030 |
| Model 2 | ILI arm BMI increase (SD) |
−1.6% (−9.9, 6.6%) −8.8% (−13, −5%) |
0.70 <0.001 |
| Model 3 | ILI arm SBP increase (SD) DBP increase (SD) |
9.4% (2.4, 16.4%) 10.7% (+6, 16%) −4.8% (−10, 0%) |
0.01 <0.001 0.05 |
| Model 4 | ILI arm HbA1c increase (SD) |
1.2% (−6.1, +8.4%) −12.5% (−16, −8%) |
0.75 <0.001 |
| Model 5 | ILI arm eGFR increase (SD) |
7.7% (0.7,14.7%) 7.1% (3.5, +10.6%) |
0.030 <0.001 |
| Model 6 | ILI Arm Triglycerides increase (SD) HDL increase (SD) |
5.5% (−1.7, 12.7%) −9.2% (−12,9, −5.5%) 0.9% (−2.8, 4.6%) |
0.13 <0.001 0.64 |
| Model 7 | ILI arm BMI increase (SD) SBP increase (SD) DBP increase (SD) HbA1c increase (SD) eGFR increase (SD) Triglycerides increase (SD) HDL increase (SD) |
−3.2% (−11.5, 5.2%) −6.4% (−10.7, −2.0%) 11.2% (6.2, 16.1%) −4.7% (−9.6, −0.2%) −9.6% (−13.9, −5.3%) 7.2% (3.5, 10.9%) −7.7% (−11.5,−4.0%) −0.8 (−4.5, 2.8%) |
0.46 <0.01 <0.001 0.06 <0.001 <0.001 <0.001 0.65 |
ILI- Intensive lifestyle arm (compared to the diabetes support and education arm). Model 1 adjusts for baseline age, gender, ethnicity, prior cardiovascular disease, and baseline HbA1c. Models 2, 3, 4 and 5 are additionally adjusted for the change in the variables listed. BMI= Body max index SBP =systolic blood pressure, DBP=diastolic blood pressure. Beta coefficients are per standard deviation change in the parameter indicated.
Table 4 demonstrates the impact of additionally adjusting for the change in blood pressure, body mass index, estimated GFR, HbA1c and lipids (table 4, Models 2-6) and for change in all these parameters (model 7) on the estimate of the intervention effect.. Of note, after accounting for the blood pressure changes, there was a larger effect of the intervention on NT-proBNP. In contrast, adjusting for change in BMI, HbA1c, triglycerides, and HDL resulted in no suggestion of an independent ILI effect. These suggest that the greater increase in NT-proBNP seen in the ILI arm was associated with the greater weight loss and improvement in metabolic parameters (glucose control and triglycerides).
An analysis of the subset with longitudinal measures of body composition revealed that at 1 year, participants in ILI lost more fat mass (−5.3 vs −0.1kg, p<0.001) as well as more lean body mass (−2.6 vs −0.3 kg, p<0.001) than DSE. Among this sample, log NT-proBNP increased 25.5% among those in ILI, vs. 13.6% in DSE (difference +11.9%, p=0.07). Longitudinal change in NT-proBNP was strongly associated with change in body composition. A one standard deviation lower fat mass was associated with 11.1% increased NT-proBNP (p=0.001), while a one SD lower lean mass was associated with a 7.7% increased NT-proBNP (p=0.02). When assessing for a difference in NT-proBNP by study arm and adjusting for baseline HbA1c, prior CVD status and change in fat mass, the beta coefficient for arm was +1.3%, p=0.9, while decreased fat mass remained significant (+11.1% per SD, p=0.004). When adjusting for baseline HbA1c, prior CVD status and change in lean mass, the beta coefficient for arm was +6.4%, p=0.4, while a decreased fat mass remained associated with increased NT-proBNP (+7.1% per SD, p=0.06).
Discussion
Among overweight and obese adults with diabetes who participated in a clinical trial the cross-sectional determinants of increased NT-proBNP level include older age, female gender, prior CVD, higher SBP, lower DBP, poorer kidney function, longer diabetes duration, and lower hemoglobin A1c. The inverse relationship between A1c and NT-proBNP levels was limited to those without prior CVD. After adjusting for demographic factors, we observed a significant positive association between degree of obesity (as measured by BMI) and baseline NT-proBNP; after full adjustment we found no association. In longitudinal analyses, an intensive lifestyle intervention that reduced weight and improved diabetes control and CVD risk factor measures at one-year, NT-proBNP was increased in both arms, and to a greater degree among ILI participants. The increased NT-proBNP associated with ILI was correlated most closely with changes in A1c, and BMI. In the sub-analysis including body composition measures, the change in fat mass was the strongest correlate of the change in NT-proBNP.
There are several strengths to this study. We are not aware of a larger sample of persons with diabetes who had NT-proBNP level assessed in the context of a randomized clinical trial which had beneficial effects on several CVD risk factors. The parent study (Look AHEAD) provided a diverse sample of overweight and obese adults with diabetes, with careful characterization of demographic, anthropometric, clinical parameters, and for a subset, body composition measures. Accordingly, we were able to consider and adjust for potential confounders in the cross-sectional associations between risk factors and NT-proBNP. There are also limitations to our study. By design, Look AHEAD did not enroll persons who were not overweight (limiting the range available to examine the relationship between BMI and NT-proBNP). Also, participants in clinical trials may not be representative of the overall population, which may limit the generalizability of our cross-sectional results. On the other hand, a randomized trial is the ideal setting for assessing relationships between interventions and outcomes. We performed assays of NT-proBNP 5-8 years after the blood was obtained and frozen. It is possible that the duration of storage could alter BNP level; however, we did not find evidence for a relationship between date of study entry and NT-proBNP. Finally, the Look AHEAD trial is ongoing and the cardiovascular outcomes which have occurred are not available; thus we cannot investigate the relationship between increased NT-proBNP and CVD events in this report.
Many of the cross sectional associations found between NT-proBNP and demographic characteristics (age, gender), prior CVD, blood pressure, and renal function in this sample are consistent with prior findings in both the general population and those with diabetes (26-29). While several studies have demonstrated that BMI is negatively associated with BNP and/or NT-proBNP levels (11-15), there are a number of studies limited to persons with diabetes that did not show significant associations (28,30). In a cross-sectional study of 185 patients attending an obesity clinic, a history of gastric bypass was associated with an increased NT-proBNP level (31). While we observed an interaction between A1c level and CVD status, those without CVD had substantially lower NT-proBNP levels at a given level of A1c compared to those with CVD. The clinical significance of these findings remain unclear.
We are not aware of larger samples of patients with diabetes who were randomized to weight loss or a control condition and had NTpro-BNP measurements. These data demonstrate that one-year change in NT-proBNP is negatively associated with change in BMI, HbA1c, and triglycerides, and positively associated with change in systolic BP and estimated GFR. The increased NT-proBNP observed among ILI participants relative to DSE appeared to be more strongly related to changes in BMI and HbA1c. While these relationships remained after adjustment for other factors, these results should be interpreted cautiously because, in fact, changes in weight and risk factors were all interrelated. In a non-randomized prospective cohort of high coronary heart disease risk participants in a comprehensive lifestyle change program (N=125, 43% with diabetes) BNP (rather than NT-proBNP) was measured at baseline and three months; despite an average 7% weight loss there was a significant increase in BNP (32). Among 132 obese patients undergoing bariatric surgery experiencing an average weight loss of 27% at 6 months, NT-proBNP increased an average of 5 fold over pre-operative levels (33). There are few reports of change in BNP in relationship to diabetes therapy. A non-randomized study tested BNP at baseline and 1 year in 24 patients with diabetes involved in a multi-factorial risk reduction intervention; while HbA1c decreased from a mean of 7.8% to 7.1%, participants gained weight. They found no difference in BNP level between baseline and follow-up. (34) In 160 participants of the Steno-2 trial (patients with diabetes and microalbuminuria), high baseline NT-proBNP level was associated with longer diabetes duration, age, higher systolic BP, and impaired kidney function (but not BMI). Of note, at 2 years, NT-proBNP increased to a similar degree in patients randomly assigned to be intensely managed (lower glucose and BP goals) compared to conventional therapy (20). It has also been reported that thiazolidinedione initiation increases BNP levels (35,36); in the current study TZD use was cross-sectionally associated with a higher NT-proBNP level.
There are several potential explanations for our results. There is a well-described “obesity paradox” with respect to heart failure in the elderly, that is, that the overweight and obese have lower heart failure mortality, which would be consistent with the lower BNP levels typically seen with obesity. (37) In one study among heart failure patients which demonstrated that BMI was negatively associated with NT-proBNP, BMI was more strongly associated with lean-body mass than fat mass, and increased lean body mass was significantly associated with a lower NT-proBNP. (18) The implication is that BMI leads to misclassification of excess adiposity. In the analyses which included DXA measures, we found evidence that the increased NT-proBNP associated with ILI appeared to be largely due to the decreased fat mass observed, and may also be mediated by a decrease in lean mass. This novel finding suggest that rather than causing a misclassification by BMMI, there may be a fundamental, biological relationship between adipose tissue and BNP. There is also evidence that sex hormones (particularly androgens) affect natriuretic peptide levels, with increased testosterone leading to a decreased NT-proBNP (38, 39). Obesity and diabetes are associated with complex changes in sex hormones; with weight loss androgens may increase in men and decrease in women (40, 41). We do not have data regarding sex hormone levels, however. The known physiologic actions of BNP include arterial vasodilation, natriuresis, diuresis, and a reduction of the activity of the renal-angiotensin-aldosterone system (which leads to decreased hemodynamic stress) (4). Some have argued that lower natriuretic peptide levels in the obese reflect a BNP deficiency, thus weight loss produces a more physiologically appropriate level (33).
These results are novel due to the rigorous design, large sample, and inclusion of body composition data, and confirm prior observation, Nonetheless, our findings remain somewhat puzzling, in the face of evidence that elevated NT-proBNP levels are predictive of adverse CVD events and mortality in the general population (8,9) as well as specifically those with diabetes (20). It is possible that the small changes in NT-proBNP seen in this study, among a population with few individuals having an abnormal NT-proBNP level, do not reflect an increased risk of events. Recently, McKie and colleagues suggest that NT-proBNP level within the normal range is not predictive of events in healthy adults (42). However, healthy was defined as the absence of major HF risk factors including diabetes, hypertension, or prior CVD as well as having a normal echocardiogram. In the non-healthy comparison group (11% of whom had diabetes) increasing NT-proBNP (even in the normal range) was strongly and independently associated with future cardiovascular events and mortality (42). One study suggests longitudinal increases in NT-proBNP levels are not benign. NT-proBNP levels were assessed at baseline and 2-3 years later in the Cardiovascular Health Study cohort (community dwelling elderly aged 65 and older; 18% with diabetes). Incident heart failure and CV death were associated with baseline levels of NT-proBNP ≥190pg/ml; additionally those with a 25% increase (including those with an initial NT-proBNP<190) had an approximately 2-fold increased risk of HF or CV death (10). Ultimately, it remains unclear if the changes observed reflect normal physiology, or portend unforeseen adverse consequences of a lifestyle intervention in this population.
In conclusion, among overweight and obese persons with diabetes, an intensive lifestyle intervention that reduced weight and improved glucose control was associated with an increase in NT-proBNP. The main implication is that NT-proBNP levels may be difficult to interpret among those who are obese and have diabetes, and therefore of limited utility for use as a screening test (for subclinical heart disease) or for prediction of future heart failure or CVD events. Further research, in particular, longitudinal data including CVD events, among those with diabetes and/or obesity would contribute to the understanding of the prognostic significance (if any) of natriuretic peptide levels in overweight and obese adults with diabetes who do not have clinically recognized heart failure.
Table 2.
Relationship of demographic and clinical characteristics to relative percent change in baseline NT-proBNP levels among 2128 participants of the Look AHEAD clinical trial, 2001-2004.
| Characteristic | Relative Percent Greater NT-proBNP (95%CI) |
Relative Percent Greater NT-proBNP (95%CI) |
|||
|---|---|---|---|---|---|
| Model 1a | p value | Model 2 b | p- value |
||
| Age | 1 year older | +4.8% (4.2, 5.4%) | <0.001 | +3.7% (3.0, 4.4%) | <0.001 |
| Gender | Female | +32% (23, 40%) | <0.001 | +25% (16, 34%) | <0.001 |
| Race/Ethnicity (vs. white) |
AA Hispanic other |
−41% (−40, −25%) −8.6% (−21, 4.1%) −14% (−34, 7%) |
<0.001 | −39%, (−51, −28%) −4.0%, (−17, 9%) −12% (−32, 8%) |
<0.001 |
| Prior CVD | yes vs. no | +58% (49, 70%) | <0.001 | +53% (44, 63%) | <0.001 |
| Systolic BP | per SD | +10.2% (6, 14%) | <.0001 | +16% (11, 21%) | <0.001 |
| Diastolic BP | per SD | −2.6% (−7, 2%) | 0.23 | −11% (−16, −6%) | <0.001 |
| Pulse Pressure | per SD | +15% (11, 19%) | <.0001 | --- | |
| Diabetes Duration |
1 year longer | +1.4% (0.8, 2%) | <.0001 | +1.0% (0.3, 1.6%) | 0.004 |
| Insulin Use | yes vs. no | +8% (−3, 20%) | 0.10 | −4.9% (−16, 7%) | 0.55 |
| TZD Use | yes vs. no | +26% (17, 35%) | <.0001 | +20% (11, 29%) | <0.001 |
| Hemoglobin HbA1c |
per SD | −2.9% (−7, 1%) | 0.16 | −5.0% (−9, −0.8%) | 0.02 |
| eGFR | per SD | −7.6% (−12, −3%) | 0.001 | −6.4% (−11, −2%) | 0.004 |
| Urine Albumin | per SD | +8.9% (5, 13%) | <.0001 | +7.2% ( 3, 11%) | <0.001 |
| HTN Drugs | yes vs. no | +21% (12, 31%) | <0.001 | +13% (2.7, 23%) | 0.013 |
| Lipid Lowering Drugs |
yes vs. no | +6% (−3, 14%) | 0.19 | +0.2% (−8, 9%) | 0.96 |
| BMI | per SD | +4.9% (1, 9%) | 0.017 | +1.6% (−3, 6%) | 0.45 |
| METS | per SD | 0% (−4, 4%) | 1.0 | --- | |
| Smoking (vs never) |
Former current |
+1.3% (−7, 10%) +2.2% (−18, 22%) |
1.0 | --- | |
BMI=body mass index, METS= Maximal MET value (at 80% HR), HTN=hypertension, eGFR=estimated glomerular filtration rate in ml/min/1.73m2, BP= blood pressure
Model 1 is age, gender, ethnicity and CVD adjusted, subsequent variables entered singly into the model.
Model 2 includes Model 1 variables plus systolic and diastolic blood pressure , diabetes duration, insulin use, thiazolidinediones use, hemoglobin A1c, glomerular filtration rate, urine albumin, antihypertensive drug use, lipid lowering drug use, and body mass index.
Acknowledgement
This project was supported by a grant from the National Institutes of Health/ NHLBI: 1R21HL089686-01A2. Additional information for the Look AHEAD Study including investigators, funding and support available in Diabetes Care 30:1374-1383, 2007
Footnotes
Trials Registration: ClinicalTrials.gov NCT00017953
Disclosure The authors have no conflicts of interest to report.
Reference List
- 1.Nichols GA, Gullion CM, Koro CE, Ephross SA, Brown JB. The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care. 2004;27:1879–1884. doi: 10.2337/diacare.27.8.1879. [DOI] [PubMed] [Google Scholar]
- 2.Bertoni AG, Hundley WG, Massing MW, Bonds DE, Burke GL, Goff DC., Jr. Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care. 2004;27:699–703. doi: 10.2337/diacare.27.3.699. [DOI] [PubMed] [Google Scholar]
- 3.Bell DS. Heart Failure: The frequent, forgotten, and often fatal complication of diabetes. Diabetes Care. 2003;26:2433–2441. doi: 10.2337/diacare.26.8.2433. [DOI] [PubMed] [Google Scholar]
- 4.Braunwald E. Biomarkers in Heart Failure. New England Journal of Medicine. 2009;358:2148–2159. doi: 10.1056/NEJMra0800239. [DOI] [PubMed] [Google Scholar]
- 5.Groenning BA, Nilsson JC, Sondergaard L, et al. Detection of left ventricular enlargement and impaired systolic function with plasma N-terminal pro brain natriuretic peptide concentrations. American Heart Journal. 2002;143:923–929. doi: 10.1067/mhj.2002.122168. [DOI] [PubMed] [Google Scholar]
- 6.McDonagh TA, Holmer S, Raymond I, Luchner A, Hildebrant P, Dargie HJ. NT-proBNP and the diagnosis of heart failure: a pooled analysis of three European epidemiological studies. European Journal of Heart Failure. 2004;6:269–273. doi: 10.1016/j.ejheart.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Groenning BA, Raymond I, Hildebrandt PR, Nilsson JC, Baumann M, Pedersen F. Diagnostic and prognostic evaluation of left ventricular systolic heart failure by plasma N-terminal pro-brain natriuretic peptide concentrations in a large sample of the general population. Heart (British Cardiac Society) 2004;90:297–303. doi: 10.1136/hrt.2003.026021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang TJ, Larson MG, Levy D, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–663. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 9.McKie PM, Rodeheffer RJ, Cataliotti A, et al. Amino-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide: biomarkers for mortality in a large community-based cohort free of heart failure. Hypertension. 2006;47:874–880. doi: 10.1161/01.HYP.0000216794.24161.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.deFilippi CR, Christenson RH, Gottdiener JS, et al. Dynamic Cardiovascular Risk Assessment in Elderly People: The Role of Repeated N-Terminal Pro-B-Type Natriuretic Peptide Testing. Journal of the American College of Cardiology. 2010;55:441–450. doi: 10.1016/j.jacc.2009.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehra MR, Uber PA, Park MH, et al. Obesity and suppressed B-type natriuretic peptide levels in heart failure. J Am Coll Cardiol. 2004;43:1590–1595. doi: 10.1016/j.jacc.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 12.Wang TJ, Larson MG, Levy D, et al. Impact of obesity on plasma natriuretic peptide levels. Circulation. 2004;109:594–600. doi: 10.1161/01.CIR.0000112582.16683.EA. [DOI] [PubMed] [Google Scholar]
- 13.Rivera M, Cortes R, Salvador A, et al. Obese subjects with heart failure have lower N-terminal pro-brain natriuretic peptide plasma levels irrespective of aetiology. Eur J Heart Fail. 2005;7:1168–1170. doi: 10.1016/j.ejheart.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Daniels LB, Clopton P, Bhalla V, et al. How obesity affects the cut-points for B-type natriuretic peptide in the diagnosis of acute heart failure. Results from the Breathing Not Properly Multinational Study. Am Heart J. 2006;151:999–1005. doi: 10.1016/j.ahj.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 15.Richards M, Nicholls MG, Espiner EA, et al. Comparison of B-type natriuretic peptides for assessment of cardiac function and prognosis in stable ischemic heart disease. J Am Coll Cardiol. 2006;47:52–60. doi: 10.1016/j.jacc.2005.06.085. [DOI] [PubMed] [Google Scholar]
- 16.Tsuruda T, Kato J, Sumi T, et al. Combined use of brain natriuretic peptide and C-reactive protein for predicting cardiovascular risk in outpatients with type 2 diabetes mellitus. Vasc Health Risk Manag. 2007;3:417–423. [PMC free article] [PubMed] [Google Scholar]
- 17.Das SR, Drazner MH, Dries DL, et al. Impact of body mass and body composition on circulating levels of natriuretic peptides: results from the Dallas Heart Study. Circulation. 2005;112:2163–2168. doi: 10.1161/CIRCULATIONAHA.105.555573. [DOI] [PubMed] [Google Scholar]
- 18.Oreopoulos A, Ezekowitz JA, McAlister FA, et al. Association between direct measures of body composition and prognostic factors in chronic heart failure. Mayo Clin Proc. 2010;85:609–617. doi: 10.4065/mcp.2010.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magnusson M, Melander O, Israelsson B, Grubb A, Groop L, Jovinge S. Elevated plasma levels of Nt-proBNP in patients with type 2 diabetes without overt cardiovascular disease. Diabetes Care. 2004;27:1929–1935. doi: 10.2337/diacare.27.8.1929. [DOI] [PubMed] [Google Scholar]
- 20.Gaede P, Hildebrandt P, Hess G, Parving HH, Pedersen O. Plasma N-terminal pro-brain natriuretic peptide as a major risk marker for cardiovascular disease in patients with type 2 diabetes and microalbuminuria. Diabetologia. 2005;48:156–163. doi: 10.1007/s00125-004-1607-0. [DOI] [PubMed] [Google Scholar]
- 21.Deffur A, Ker JA, Rheeder P, Van Niekerk MM, Quinton SJ. NT-proBNP measurements in high-risk diabetic patients--a case series from Mamelodi (Gauteng) Cardiovasc J S Afr. 2006;17:56–59. [PubMed] [Google Scholar]
- 22.Ryan DH, Espeland MA, Foster GD, et al. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24:610–628. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 23.The Look AHEAD Research Group Reduction in Weight and Cardiovascular Disease Risk Factors in Individuals With Type 2 Diabetes: One-year results of the Look AHEAD trial. Diabetes Care. 2007;30:1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bray G, Gregg E, Haffner S, et al. Baseline characteristics of the randomised cohort from the Look AHEAD (Action for Health in Diabetes) study. Diab Vasc Dis Res. 2006;3:202–215. doi: 10.3132/dvdr.2006.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heshka S, Ruggiero A, Bray GA, et al. Altered body composition in type 2 diabetes mellitus. Int J Obes (Lond) 2008;32:780–787. doi: 10.1038/sj.ijo.0803802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olsen MH, Hansen TW, Christensen MK, et al. N-terminal pro brain natriuretic peptide is inversely related to metabolic cardiovascular risk factors and the metabolic syndrome. Hypertension. 2005;46:660–666. doi: 10.1161/01.HYP.0000179575.13739.72. [DOI] [PubMed] [Google Scholar]
- 27.Costello-Boerrigter LC, Boerrigter G, Redfield MM, et al. Amino-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide in the general community: determinants and detection of left ventricular dysfunction. J Am Coll Cardiol. 2006;47:345–353. doi: 10.1016/j.jacc.2005.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tildesley HD, Aydin CM, Ignaszewski A, Strelzow JA, Yu E, Bondy G. Sulfonylurea therapy is associated with increased NT-proBNP levels in the treatment of type 2 diabetes. Int J Cardiol. 2007;115:312–317. doi: 10.1016/j.ijcard.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 29.Cosson E, Nguyen MT, Pham I, Pontet M, Nitenberg A, Valensi P. N-terminal pro-B-type natriuretic peptide: an independent marker for coronary artery disease in asymptomatic diabetic patients. Diabet Med. 2009;26:872–879. doi: 10.1111/j.1464-5491.2009.02788.x. [DOI] [PubMed] [Google Scholar]
- 30.Park SJ, Cho KI, Jung SJ, et al. N-terminal pro-B-type natriuretic Peptide in overweight and obese patients with and without diabetes: an analysis based on body mass index and left ventricular geometry. Korean Circ J. 2009;39:538–544. doi: 10.4070/kcj.2009.39.12.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.St Peter JV, Hartley GG, Murakami MM, Apple FS. B-type natriuretic peptide (BNP) and N-terminal pro-BNP in obese patients without heart failure: relationship to body mass index and gastric bypass surgery. Clin Chem. 2006;52:680–685. doi: 10.1373/clinchem.2005.062562. [DOI] [PubMed] [Google Scholar]
- 32.Chainani-Wu N, Weidner G, Purnell DM, et al. Relation of B-type natriuretic peptide levels to body mass index after comprehensive lifestyle changes. Am J Cardiol. 2010;105:1570–1576. doi: 10.1016/j.amjcard.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 33.Chen-Tournoux A, Khan AM, Baggish AL, et al. Effect of weight loss after weight loss surgery on plasma N-terminal pro-B-type natriuretic peptide levels. Am J Cardiol. 2010;106:1450–1455. doi: 10.1016/j.amjcard.2010.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim HS, Patel JV, Nadar S, Hughes EA, Lip GY. Comparison of brain natriuretic peptide and left ventricular diastolic function determined by tissue Doppler in patients with diabetes mellitus, patients with hypertension without diabetes, and in healthy subjects. Am J Cardiol. 2005;95:905–908. doi: 10.1016/j.amjcard.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 35.Sambanis C, Tziomalos K, Kountana E, et al. Effect of pioglitazone on heart function and N-terminal pro-brain natriuretic peptide levels of patients with type 2 diabetes. Acta Diabetol. 2008;45:23–30. doi: 10.1007/s00592-007-0014-7. [DOI] [PubMed] [Google Scholar]
- 36.Turkmen KY, Guvener DN, Yildirir A, Atar A, Dogruk UA, Biyiklioglu Z. Effects of rosiglitazone on plasma brain natriuretic peptide levels and myocardial performance index in patients with type 2 diabetes mellitus. Acta Diabetol. 2007;44:149–156. doi: 10.1007/s00592-007-0256-4. [DOI] [PubMed] [Google Scholar]
- 37.Lavie CJ, Milani RV, Ventura HO, Romero-Corral A. Body composition and heart failure prevalence and prognosis: getting to the fat of the matter in the “obesity paradox”. Mayo Clin Proc. 2010;85:605–608. doi: 10.4065/mcp.2010.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang AY, Abdullah SM, Jain T, et al. Associations among androgens, estrogens, and natriuretic peptides in young women: observations from the Dallas Heart Study. J Am Coll Cardiol. 2007;49:109–116. doi: 10.1016/j.jacc.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 39.Saenger AK, Dalenberg DA, Bryant SC, Grebe SK, Jaffe AS. Pediatric brain natriuretic peptide concentrations vary with age and sex and appear to be modulated by testosterone. Clin Chem. 2009;55:1869–1875. doi: 10.1373/clinchem.2009.123778. [DOI] [PubMed] [Google Scholar]
- 40.Grossmann M, Gianatti EJ, Zajac JD. Testosterone and type 2 diabetes. Curr Opin Endocrinol Diabetes Obes. 2010;17:247–256. doi: 10.1097/MED.0b013e32833919cf. [DOI] [PubMed] [Google Scholar]
- 41.Harvie MN, Pegington M, Mattson MP, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond) 2010 doi: 10.1038/ijo.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McKie PM, Cataliotti A, Lahr BD, et al. The prognostic value of N-terminal pro-B-type natriuretic peptide for death and cardiovascular events in healthy normal and stage A/B heart failure subjects. J Am Coll Cardiol. 2010;55:2140–2147. doi: 10.1016/j.jacc.2010.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]