Table 3.
Synthesis of N-acyl indole(cyclo)alkanoic acids
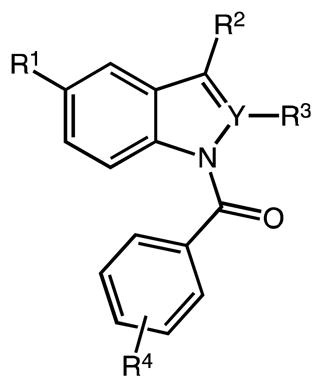
| |||||||
|---|---|---|---|---|---|---|---|
| # | Y | R1 | R2 | R3 | R4 | method | yield (%)a |
| 2 | C | OMe | CH2C(O)OH | H | p-Cl | D | 81 |
| 22a | C | OMe | CH2C(O)OH | Me | p-F | D | 90 |
| 22b | C | OMe | CH2C(O)OH | Me | m-CF3 | D | 97 |
| 22c | C | OMe | CH2C(O)OH | Me | p-CF3 | D | 90 |
| 22d | C | OMe | CH2C(O)OH | Me | p-Me | D | 82 |
| 22e | C | OMe | CH2C(O)OH | Me | p-CH2Cl | D | 98 |
| 22f | C | OMe | CH2C(O)OH | Me | p-OMe | D | 93* |
| 22g | C | OMe | CH2C(O)OH | H | p-F | D | 76 |
| 22h | C | OMe | CH2C(O)OH | H | m-CF3 | D | 96 |
| 22i | C | OMe | CH2C(O)OH | H | p-CF3 | D | 99 |
| 22j | C | OMe | CH2C(O)OH | H |
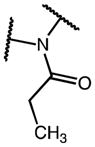
|
D | 94 |
| 22k | C | F | CH2C(O)OH | Me | p-Cl | D | 87 |
| 22l | C | F | CH2C(O)OH | Me | m-CF3 | D | 95 |
| 22m | C | F | CH2CH2C(O)OH | Me | p-Cl | D | 95 |
| 22n | C | F | CH2C(O)OH | H | p-Cl | D | 78 |
| 23 | C | OMe |
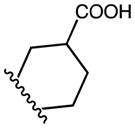
|
m-CF3 | D | 98* | |
| 24 | N | OMe | CH2C(O)OH | --- | Cl | D | No product (decomposition) |
Reagents and Conditions: [method D]: trimethyltin hydroxide, 1,2-DCE, microwave, 130 °C, 30 min.
isolated yield;
crude educts used; impurity of the starting material taken into account for the calculation of the percent yield (see Supporting Information)!
