Abstract
Snail family transcription factors are best known for regulating epithelial-mesenchymal transition (EMT). The Drosophila Snail family member Worniu is specifically transcribed in neural progenitors (neuroblasts) throughout their lifespan, and worniu mutants show defects in neuroblast delamination (a form of EMT). However, the role of Worniu in neuroblasts beyond their formation is unknown. We performed RNA-seq on worniu mutant larval neuroblasts and observed reduced cell cycle transcripts and increased neural differentiation transcripts. Consistent with these genomic data, worniu mutant neuroblasts showed a striking delay in prophase/metaphase transition by live imaging, and increased levels of the conserved neuronal differentiation splicing factor Elav. Reducing Elav levels significantly suppressed the worniu mutant phenotype. We conclude that Worniu is continuously required in neuroblasts to maintain self-renewal by promoting cell cycle progression and inhibiting premature differentiation.
Introduction
Stem cells must remain proliferative without becoming tumorigenic, and must remain competent to differentiate without actually differentiating. How stem cells maintain stemness – cell survival, cell cycle progression, and the capacity to differentiate – is a widely relevant question with clinical significance. Drosophila neural progenitors (neuroblasts) have become a good model system to study how neural stem cells self-renew and maintain stem cell identity. Larval neuroblasts undergo repeated rounds of asymmetric cell division, each time generating a smaller differentiating daughter cell and a larger self-renewing neuroblast (Doe, 2008). During neuroblast division, many proteins are asymmetrically partitioned into the neuroblast or daughter cell, where they often contribute to neuroblast self-renewal or daughter cell differentiation (Knoblich, 2008), however much less is known about the transcriptional program that maintains neuroblast self-renewal.
Worniu is a zinc finger transcription factor in the “Slug/Snail” family, and is transcribed in neuroblasts from the time of their birth Over 50 Snail family members have been characterized in metazoans; they can directly bind DNA, RNA or protein and regulate a wide range of cellular functions (Nieto, 2002; Thiery et al., 2009). Snail family members are best known for inducing epithelial-mesenchymal transition (EMT) during mesoderm development and neural-crest cell formation (Barrallo-Gimeno and Nieto, 2005; Cano et al., 2000). In Drosophila, 4 Snail family genes are known: wor, escargot, snail, and scratch (Ashraf and Ip, 2001; Roark et al., 1995). The genes wor, escargot and snail are expressed in neuroectoderm during embryogenesis to trigger EMT in neuroepithelial cells and transform them into newly-delaminated neuroblasts (Ashraf et al., 1999). Wor, Escargot, and Snail also act redundantly to promote expression of the apical polarity gene inscuteable (insc) and the cell cycle regulator string in newly formed embryonic neuroblasts (Ashraf and Ip, 2001; Cai et al., 2001).
The only Snail family member known to be expressed continuously in neuroblasts is Wor, but its function beyond neuroblast formation has not been investigated. Here we show that Wor maintains neuroblast self-renewal via dual functions: it promotes cell cycle progression (specifically the prophase-to-metaphase transition) and it inhibits premature differentiation (by suppressing Elav protein levels). These functions occur in neuroblasts well after their formation, highlighting the potential role of Snail family members in stem cell self-renewal.
Results
To analyze the wor mutant phenotype we used a deficiency that removes wor and several flanking genes, Df(2L)Exel8034, and a specific mutation within the wor gene, wor1 (Ashraf et al., 2004). We sequenced wor1 and found two mis-sense mutations (Figure S1), one of which alters the amino acid Pro443 to Ser in the conserved zinc finger domain and probably changes the conformation for DNA/RNA/protein binding. Because wor1/wor1 had a slightly weaker phenotype compared to wor1/Df(2L)Exel8034 due to lesser amount of Wor protein in the latter genotype, we conclude that wor1 is a strong hypomorph. We use wor1/Df(2L)Exel8034 for all experiments below (called “wor mutants”).
TU-tagging/RNA-seq shows that worniu mutant neuroblasts downregulate neuroblast genes and upregulate neuronal differentiation genes
Wor protein is nuclear and is predicted to be a transcription factor, so we compared the transcriptional profile of wild type (wt) and wor mutant neuroblasts to identify biological processes that were regulated by Wor. We used the TU-tagging method (Miller et al., 2009) to identify mRNAs that are actively transcribed in wt or wor mutant neuroblasts. TU-tagging is an spatial/temporal intersectional method to purify nascent RNA from designated tissues during a specific developmental stage (summarized in Figure 1A). We expressed UPRT in larval neuroblasts using wor-gal4 (Cabernard and Doe, 2009; Lee et al., 2006; Miller et al., 2009), which produced a high level of UPRT in wt and wor mutant larval neuroblasts (Figure 1B-C, dotted cyan circles) with some persistence into their new-born progeny (Figure 1B-C, dotted white circles, quantified in D).
Figure 1. TU-tagging/RNA-seq shows that wor mutants downregulate neuroblast genes and upregulate neuronal differentiation genes.
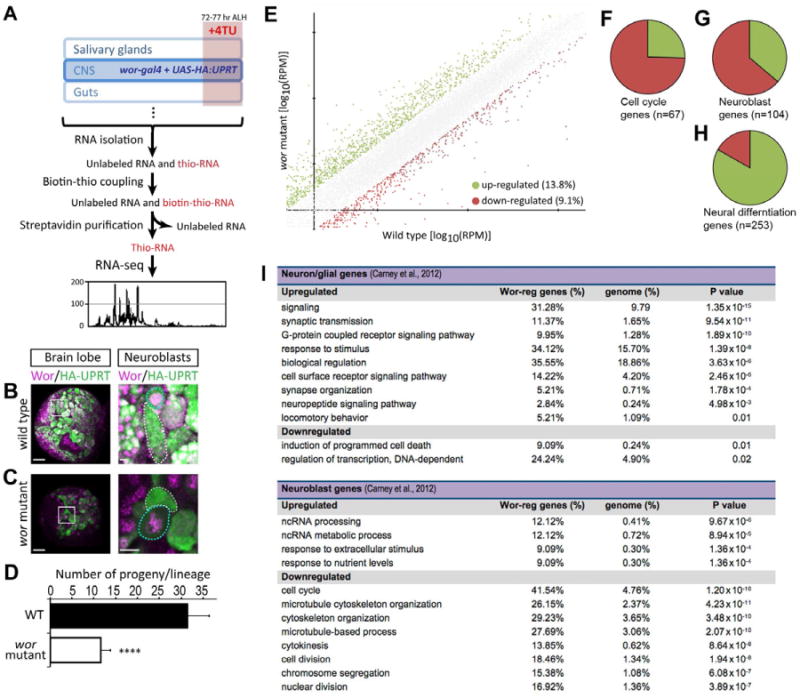
(A) Scheme of TU-tagging processes for transcriptome analysis. 4TU: 4-thioruacil.
(B-D) Wild type and wor mutant NBs both express HA-tagged UPRT (HA:UPRT) in third instar larvae. In both B and C, the left panel is a low magnification view of a brain lobe (scale bar, 20 μm) with the boxed region shown at high magnification (scale bar, 5 μm) in the right panels. HA:UPRT is expressed in NB (cyan dotted circles in the right panels) and persists in the new born neuroblast progeny (dotted white circles in right panels); quantified in D. ****, p-value < 0.0001. Small Wor+ cells adjacent to neuroblasts are newborn GMCs.
(E)Log to the base 10 of fold of gene activities in wt and wor mutants. Green dots are genes upregulated more than 2 fold; red dots are genes downregulated more than 2 fold.
(F)Pie chart represents the 67 of the 586 Drosophila “cell cycle” annotated genes(GO:0007049) that are differentially regulated in wor mutants (> or < 2-fold change); the majority are down-regulated (75%; red) and a minority are upregulated (25%; green).
(G-H) Pie charts represents the “neuroblast” genes or the “neuron differentiation” genes from Carney et al. (2012) that are differentially regulated in wor mutants (> or < 2-fold change). The majority of the 253 “neuron differentiation” genes are up-regulated (85%; green); whereas the majority of the 104 “neuroblast” genes are down-regulated (66%; red).
(I) GO terms that whose frequency is over-represented in wor upregulated (> 2-fold) or wor downregulated (< 2-fold) genes compared to their frequency in the genome. Only genes within the “neuron differentiated” (top) or “neuroblast” (bottom) gene lists from Carney et al. (2012) are analyzed.
See also Figure S1, Table S1, S2.
We fed early third instar larvae 4TU for 5 hr beginning at 72 hr after larval hatching (ALH) and then purified thio-labeled RNA, performed RNA-sequencing, and designed a custom computational pipeline to analyze the results. We performed two replicates from wor mutants and two from wt. We mapped an average of 5.49 million reads from wt and 5.35 million reads from wor mutants. A comparison of the averaged wt versus averaged wor data showed that wor mutants had 13.8% of genes upregulated at least 2-fold and 9.1% of genes downregulated at least 2-fold (Figure 1E; Table S1).
We first analyzed the upregulated genes. We found that genes upregulated in wor mutants were enriched for gene ontology (GO) terms linked to neuronal differentiation such as G-protein coupled receptor signaling, sensory perception, serotonin receptor signaling, and synaptic transmission (Table S2). In addition, we recently defined a group of “neuronal differentiation genes” in a transcriptomic analysis of larval brains enriched for neuroblasts or neurons (Carney et al., 2012). We found that 253 of the ∼1100 “neuronal differentiation” genes were differentially regulated in wor mutants (> or < 2-fold), with a strong bias towards being upregulated (Figure 1H). GO analysis of the upregulated genes shows significant over-representation of the terms signaling, synaptic transmission, synapse organization, and neuropeptide signaling pathway categories (all p <0.005; Figure 1I). We conclude that wor mutant neuroblasts aberrantly upregulate neuronal differentiation genes.
We next analyzed the downregulated genes. We asked whether previously defined “neuroblast genes” or “cell cycle genes” are downregulated in wor mutant neuroblasts -- the converse of the observed upregulation of neuronal differentiation genes. We found that 104 of the ∼970 “neuroblast” genes from Carney et al. (2012) were differentially expressed in wor mutants (> or < 2-fold), with a strong bias towards being downregulated (Figure 1G). The downregulated genes had a highly significant over-representation of the GO terms cell cycle, microtubule cytoskeleton organization, cytokinesis, cell division, and chromosome segregation (all p <0.000001; Figure 1I). Similarly, we found a downregulation of Drosophila genes annotated as “cell cycle” -- of the 586 cell cycle annotated genes (GO:0007049), 67 were differentially regulated in wor mutants versus wt, and most (74.6%) were downregulated (Figure 1F). We conclude that wor mutant neuroblasts fail to properly express “neuroblast” genes including those regulating the cell cycle.
Worniu maintains neuroblast stemness by promoting cell cycle, cortical polarity, and survival
Based on our transcriptomic analysis, we predicted that wor mutant neuroblasts would show defects in neuroblast attributes (cell cycle progression, cell polarity, and survival) and precocious neural differentiation. All of these phenotypes could lead to the smaller brain size and reduced neuroblast numbers observed in wor mutants (Ashraf et al., 2004; Figure 2A,B).
Figure 2. Worniu is required for brain development and neuroblast cell cycle progression.

(A) Merged confocal images of wt and wor mutant third instar larval brains stained with NB specific marker Dpn. Scale bars, 50μm.
(B)Number of Dpn+ larval neuroblasts in wt (green), wor mutants (red), and wor mutant plus dronc RNAi (wor1/ Deficiency; wor-gal4 UAS-dronc RNAi) per brain lobe at the indicated developmental stages. We did not count optic lobe neuroblasts or the much smaller Dpn+ intermediate neural progenitors (Bayraktar et al., 2010; Bello et al., 2008; Boone and Doe, 2008; Bowman et al., 2008; Izergina et al., 2009). Asterisks indicate p values in (B-D): *, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001. p value over wor mutant plus dronc RNAi bar refers to both wt and wor mutant comparison.
(C)Percentage of Dpn+ larval neuroblasts that pass through S phase within the 2 hr pulse of EdU (EdU+) in wt (green) or wor mutants (red) at the indicated developmental stages.
(D)Percentage of Dpn+ larval neuroblasts that are mitotic (PH3+) in wt (green) or wor mutants (red) at the indicated developmental stages. The insets show representative images of the mitotic marker phosphohistoneH3 (PH3; green) and Dpn (red) staining in a wt (left panel) or a wor mutant brain lobe (right panel); central brain neuroblasts outlined with dashed line.
(E-G) Time-lapse imaging of larval wt (C) or wor mutants (D,E) brains with His2A:RFP and Zeus:GFP during mitosis. Imaging time is indicated at the upper-left corner of each frame. Cell cycle phase is labeled below. Scale bars, 5 μm.
(H) Cell cycle phase lengths in wt or wor neuroblasts from live imaging experiments; each bar represents a single larval neuroblast. Note the lengthened prophase (red) and prometaphase (orange) in wor mutants.
See also Figure S3.
Worniu promotes neuroblast cell cycle progression
To determine whether wor mutant neuroblasts have a normal cell cycle, we performed EdU incorporation and and counted the number of EdU+ neuroblasts immediately after the pulse. In this and subsequent experiments, we identify larval neuroblasts as large (>8 μm) Dpn+ cells within the central brain; optic lobe neuroblasts were not characterized. Most wt neuroblasts were EdU+ (Figure 2C, green bar), consistent with their reported cell cycle time of ∼2 hr (Cabernard and Doe, 2009). In contrast, very few wor mutant neuroblasts were EdU+ (Figure 2C, red bar), indicating a cell cycle delay between G2-M-G1. To determine if the wor mutants were delayed in mitosis, we measured the mitotic index of wt and wor mutant brains by staining for the M-phase marker phosphohistone H3 (PH3). By late third instar (96-120 hr ALH) there was a striking increase in the PH3+ neuroblasts in wor mutant compared to wt (Figure 2D). We conclude that third instar wor mutant neuroblasts have a delay in completing mitosis.
To determine more precisely the nature of the M-phase delay in wor mutants, we performed live imaging of neuroblast mitosis within the intact brain (Cabernard and Doe, 2009; Cabernard et al., 2010; Siller et al., 2006; Siller et al., 2005). We imaged third instar larval neuroblasts expressing both His2A:RFP to monitor chromosomes (Schuh et al., 2007) and Zeus:GFP to image spindle microtubules (Cabernard and Doe, 2009). Wild type neuroblasts showed the expected mitosis length of ∼20 min (Cabernard and Doe, 2009; Siller and Doe, 2008; Siller et al., 2005) (Figure 2E, quantified in 2H). In contrast, wor mutant neuroblasts showed a dramatically extended prophase and/or pro-metaphase (Figure 2F,G; quantified in H). We also observed failure in centrosomal separation and bent mitotic spindles. In two cases we observed neuroblasts that “escaped” prophase arrest, and these had a relatively normal length of anaphase (Figure 2H, top two neuroblasts). We conclude that wor mutant neuroblasts show an arrest or delay in the prophase/metaphase transition, a stage of the cell cycle where microtubules are dramatically reorganized (see Discussion).
Worniu promotes neuroblast cortical polarity
We have previously observed cell cycle delays in neuroblasts lacking aPKC or Dap160 apical cortical polarity proteins (Chabu and Doe, 2008), and wor-escargot-snail triple null mutants lack apical localization of Insc in embryonic neuroblasts (Ashraf and Ip, 2001). We stained for apical and basal polarity proteins, and observed a failure of all proteins to be properly localized during prophase; yet localization was normal by metaphase (Figure S2), most likely by a microtubule-dependent mechanism (Andersen et al., 2012; Siegrist and Doe, 2005). We conclude that Wor is required to establish neuroblast polarity at prophase.
Worniu promotes neuroblast survival
wor mutants have fewer neuroblasts compared to the wt brains (Figure 2B), which could be caused by neuroblast apoptosis or differentiation. To determine if this reduction was due to neuroblast apoptosis, we first used a genetic sensor for caspase-mediated apoptosis, in which caspase activity induces nuclear localization of GFP by cleaving a membrane tether (Bardet et al., 2008), and found that wor mutant second instar brains have multiple large GFP+ cells at cell death (Figure S3). We next used a more general cell death marker, TUNEL staining, and the Dpn antibody to unambiguously identify neuroblasts. We found 0 ± 0 (n=4 brain lobes) TUNEL+ Dpn+ neuroblasts in the wt brains; in contrast, 6.1 ± 1.7 (n=7; p < 0.0001) TUNEL+ Dpn+ neuroblasts were observed in wor mutants (Figure S3). RNAi depletion of the Dronc caspase gave a significant but partial rescue of the neuroblast numbers (wor1/ Deficiency; wor-gal4 UAS-dronc RNAi) (Figure 2B, Figure S3); partial rescue is probably because wor-gal4 is only expressed in a subset of neuroblasts (84.8 ± 2.2 neuroblasts per brain lobe) or the incomplete knock-down by RNAi. We conclude that the loss of neuroblasts seen in wor mutants is largely due to apoptosis.
worniu mutant neuroblasts undergo premature differentiation due to an increase in Elav protein levels
Based on our transcriptomic analysis, we predicted that wor mutant neuroblasts would show precocious neural differentiation. To determine if wor mutant neuroblasts initiate premature differentiation, we stained for well-characterized evolutionarily-conserved neural differentiation marker Elav (Embryonic lethal abnormal visual system; Hu family in mammals); the Elav protein is normally only detected in mature post-mitotic neurons where it promotes neuron-specific alternate splicing (Carney et al., 2012; Koushika et al., 1996; Lee et al., 2006; Lisbin et al., 2001; Robinow and White, 1988). Wild type larval neuroblasts transcribe elav (data not shown) but have low or no Elav protein (Figure 3A; quantified in C), whereas many wor mutant neuroblasts showed detectable Elav protein (Figure 3B; quantified in C). We conclude that wor mutant neuroblasts have an abnormally high level of the Elav neuronal differentiation marker, consistent with premature differentiation.
Figure 3. Worniu represses Elav to permit neuroblast cell polarity and cell cycle progression.
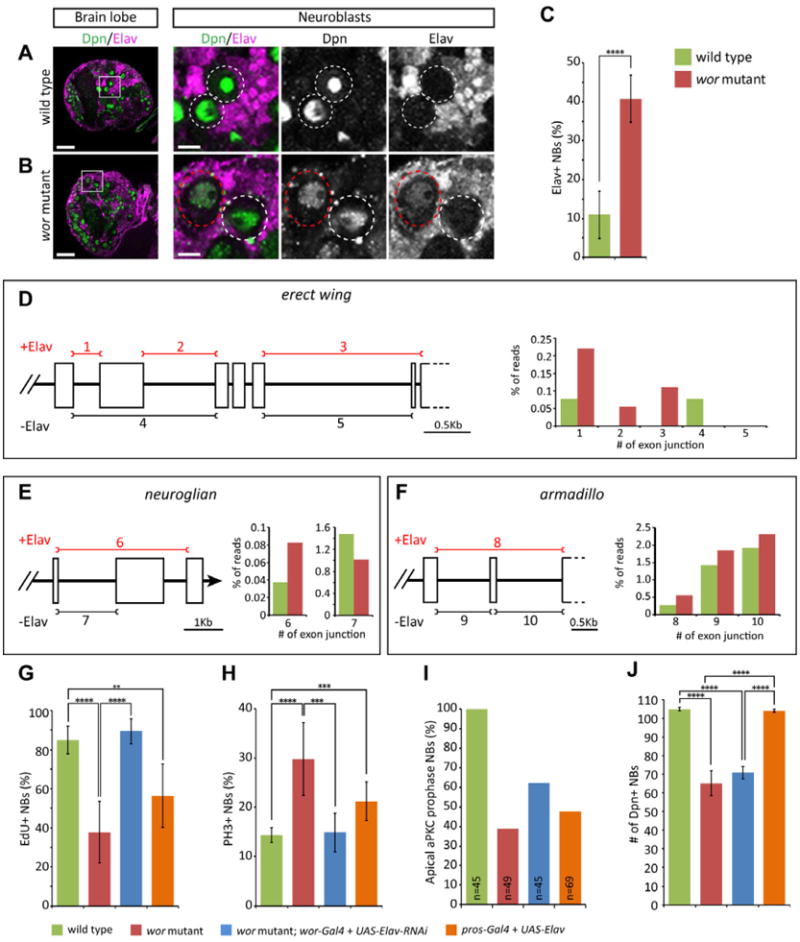
(A-B) Worniu represses Elav in neuroblasts. (A) Wild type third instar larvae (96 hrs ALH). (B) wor mutant third instar larvae (96 hrs ALH).- Note the detectable Elav protein in Dpn+ neuroblasts (white circle). Scale bars, 20 μm in left panel, 5μm in right panels.
(C) Quantification of Elav+ neuroblasts in wt and wor mutant larval brains. ****, p-value < 0.0001.
(D-I) Elav-dependent and –independent alternative splicing junctions of nrg, ewg and arm and the percentage of reads spanning splicing junctions in wt and wor mutant RNA-seq data. Left panels show partial gene structure in the genomic region of ewg (X:163000-167500) for the transcripts of ewg-RC, ewg-RE, ewg-RF and ewg-RG (D), nrg (X:8442400.8446200) for the transcripts of nrg-RA and nrg-RB (E), and arm (X:1786000.1788850) for the transcripts of arm-RB and arm-RC (F). The bars represent the exon junctions used in Elav-dependent (red) or – independent manner and each junction is numbered on top or bottom of the bar. The right panels show percentage (%) of reads spanning Elav-dependent (Elav+) exon splicing junctions over total reads of each respective gene in wt (green) or in wor mutant (red).
(G-J) Reduction of Elav rescues wor mutant cell cycle and cell polarity phenotypes, whereas misexpression of Elav in wt mimics these phenotypes. (G) Neuroblasts progressing through S phase (EdU+ following a 2 hr pulse). (H) Neuroblasts in mitosis (phosphohistone H3+). (I) Neuroblasts with normal cell polarity (prophase apical aPKC crescents). (J) Number of neuroblasts. The brains were dissected from the larvae 96 hours after larval hatching. p values: **, p<0.01; ***, p<0.001; ****, p<0.0001.
See also Figure S2, Table S3.
Elav is a RNA-binding protein known to promote neuronal-specific splicing of at least three direct target genes: neuroglian(nrg), erect wing(ewg), and armadillo(arm) (Koushika et al., 2000; Lisbin et al., 2001). We counted RNA-seq reads spanning the junctions of alternatively-spliced exons of all three genes, and found that the neural-specific, Elav-dependent splice isoforms for all three transcripts were increased in wor mutants compared to wt (Figure 3D-F). Thus, the increased level of Elav in wor mutant neuroblasts appears sufficient to bias splicing towards the neuronal-specific isoforms for all three of its known target genes.
To determine the effect of increased Elav levels on the self-renewal of wor mutant neuroblasts, we tested whether wor mutant phenotypes could be rescued by reducing Elav levels. We used wor-gal4 to drive UAS-elav-RNAi in larval neuroblasts, and observed a complete rescue of the wor mutant cell cycle phenotype (Figure 3G,H) and a substantial rescue of the wor mutant cell polarity phenotype (Figure 3I). Thus, the increased level of Elav protein in wor mutant neuroblasts results in most of the cell cycle and cell polarity defects. Reducing Elav levels was not able to restore normal neuroblast numbers (Figure 3J), suggesting that it is an Elav-independent pathway. To provide an independent test for the role of Elav in neuroblast cell cycle and cell polarity, we increased Elav levels in otherwise wt neuroblasts, and observed cell cycle and cell polarity phenotypes similar to wor mutants, without altering neuroblast number (Figure 3G-J). We conclude that Wor keeps Elav protein levels low in neuroblasts, which is necessary for establishing neuroblast cell polarity and cell cycle progression -- both key stem cell features.
Worniu overexpression induces nuclear Prospero and cell cycle arrest in neuroblasts
Having established that Wor is necessary to maintain neuroblast properties (proliferation, polarity, survival), we wanted to see if ectopic Wor was sufficient to induce neuroblast attributes in GMCs or prevent neuronal differentiation. We used prospero-gal4 to overexpress Wor in larval neuroblasts and their progeny (abbreviated as WorOXN hereafter). Unexpectedly, the WorOXN larval brains were smaller than wt brains (Figure 4A,B), their larval neuroblasts were smaller in diameter (Figure 4F), and the neuroblasts exhibited a severe cell cycle delay (Figure 4E). We observed no change in the number of Dpn+ central brain neuroblasts (Figure 4G). To determine the cause of the WorOXN phenotype, we tested for ectopic Prospero (Pros) protein in neuroblasts, because Pros is known to inhibit cell cycle progression in larval neuroblasts (Bayraktar et al., 2010). Whereas both wt neuroblasts and wor mutant neuroblasts lack nuclear Pros (Figure 4C and data not shown), WorOXN neuroblasts had clearly detectable nuclear Pros (Figure 4D). Furthermore, when we reduced Pros levels in WorOXN larvae (WorOXN; pros17/+) we found partial but significant rescue of the cell cycle and cell size phenotypes, and a slight increase in neuroblast numbers (Figure 4E,G). This latter result suggests that Wor overexpression has the ability to transform GMCs/neurons into neuroblasts, but that this is usually masked by Pros-mediated cell cycle arrest. We conclude that overexpression of Wor does not lead to a transformation of GMC/neurons into neuroblasts, and that wt neuroblasts must precisely regulate Wor levels – too little Wor leads to Elav-induced premature differentiation, while too much Wor leads to Pros-induced cell cycle arrest.
Figure 4. Worniu misexpression in neuroblasts induces nuclear Prospero and cell cycle arrest.

(A-D) Worniu misexpression in neuroblasts induces nuclear Pros protein. (A,C) Wild type third instar larvae at 96 hrs ALH. (B,D) Misexpression of Wor in third instar larval Dpn+ neuroblasts (pros-gal4 UAS-wor; WorOXN). Both A and B panels are a low magnification view of a brain lobe (scale bar, 20 μm) with the boxed region shown at high magnification of neuroblasts (dotted circles; scale bar, 5 μm) in the C and D, respectively.
(E-G) Misexpression of Wor in third instar larval Dpn+ neuroblasts (pros-gal4 UAS-wor; WorOXN) induces cell cycle arrest and neuroblasts size reduction without decreasing neuroblast numbers, and the phenotypes can be partially rescued by reducing Pros levels. (E) EdU incorporation following a 2hr pulse. The number of EdU+Dpn+ neuroblasts: wt, 87.8±5.6; WorOXN, 16.2±5.4; WorOXNpros17/+, 56.3±6.8. (F) The diameter of neuroblasts: wt, 9.5±1.2 μm; WorOXN, 14.1±1.4 μm; WorOXN pros17/+, 10.8±1.2 μm. (G) The neuroblast number: wt, 106.0±0.8; WorOXN, 104.5±1.3; WorOXN pros17/+, 113.6±3.5. p values: *, p<0.05; ***, p<0.001; ****, p<0.0001. The brains were dissected from the larvae 96 hours after larval hatching.
Discussion
Wor and Dpn mark all neuroblasts throughout their entire lineage, yet Wor function in maintaining neuroblast biology has never been explored. Because wor mRNA and protein are specifically detected in neuroblasts, not in neurons or glia (Figure 1 and data not shown), the brain phenotypes described here are most likely to be due to cell autonomous function of Wor within neuroblasts. Here we show that Wor prevents premature differentiation of neuroblasts, a conclusion based in part on the upregulation of neuronal differentiation transcripts in wor mutant neuroblast lineages. The observed increase in neuronal differentiation transcripts is likely to be an underestimation, because wor mutant neuroblast lineages have 3× fewer UPRT+ neurons than wt neuroblast lineages (due to the neuroblast cell cycle delay in wor mutant neuroblasts; Figure 1). The reduced number of neurons in the wor mutant clones makes it all the more striking that we find neuronal differentiation transcripts upregulated in wor mutant neuroblast lineages.
A second reason we conclude that Wor prevents premature differentiation of neuroblasts is our finding that wor mutants have increased levels of the differentiation marker Elav within neuroblasts. How does Wor normally keep Elav protein levels low in neuroblasts? Wor may repress elav at the transcriptional or post-transcriptional levels. Although we see no change in elav transcript abundance between wt and wor mutant neuroblast lineages by RNA-seq (Table S1), wor mutants have 3× fewer UPRT+ neurons than wor mutants (see above). These extra cells should result in more elav transcripts in wt; the fact that we see equal levels suggests that wor mutant neuroblasts may have increased levels of wor transcription. On the other hand, Wor may repress Elav at a post-transcriptional level. Wild type embryonic and larval neuroblasts transcribe the elav gene but little of the mRNA is translated (Berger et al., 2007)(data not shown); it is likely that elav is also post-transcriptionally regulated in larval neuroblasts, and this step could be subject to direct or indirect regulation by Wor. Thus, Wor may regulate elav at the transcriptional and/or post-transcriptional level to keep Elav protein low in neuroblasts.
How does Elav promote premature differentiation of neuroblasts? Elav may act by inducing neuronal-specific splicing of its direct targets neuroglian, erect wing and armadillo (which we observe to be upregulated in wor mutants), or additional targets that have yet to be identified. In addition, other RNA splicing factors, many of which are up- or down-regulated at least 2 fold in wor mutants (Table S1), may co-regulate Elav targets and/or splicing of additional pre-mRNAs. Genomic analysis of alternative splicing junction usage in wor mutants showed a profound change of global splicing events: 15.0% of all potentially alternatively-spliced exons (14476 junctions from 3430 genes; see methods) showed >2-fold change in wor mutants compared to wt (Table S3). Because the function of different splice isoforms are so poorly understood, we can only speculate that some or all of the upregulated splice isoforms promote neural differentiation and inhibit cell cycle in wor mutants. Neuronal differentiation seen in wor mutant neuroblasts is not complete, because wor mutant neuroblasts maintain expression of neuroblast markers such as Dpn, Ase, and Miranda. Thus, wor mutant neuroblasts have a mixed fate, in which both neuroblast and neuronal genes are expressed.
Wor is required to promote cell polarity at prophase. The defect in apical protein localization seen in wor mutants is similar to that seen in the absence of an external polarizing cue in embryonic neuroblasts (Siegrist and Doe, 2006, 2007; Yoshiura et al., 2011), or in sgt1 mutant larval neuroblasts (Andersen et al., 2012). It is also coincident with the prophase cell cycle delay observed by live imaging, but the relationship between loss of polarity proteins and prophase delay is unknown. Wor is also required to prevent neuroblast apoptosis. In mammals, Snail family members are known to protect cells from apoptosis triggered by loss of survival signals (Vega et al., 2004). It remains unknown whether Wor acts in a similar manner; all we can say is that Wor acts via an Elav-independent pathway to maintain neuroblast survival.
Wor is required for cell cycle progression from prophase to metaphase. It is interesting that loss of wor causes cell cycle delays at the precise time when the microtubule cytoskeleton is dramatically reorganized into a bipolar spindle. In addition, our RNA-seq data shows that wor mutants are depleted for “microtubule cytoskeleton organization” annotated transcripts (Figure 1I). Mammalian Snail family proteins confer migratory properties to epithelial cells during EMT or metastasis, which also involves a dramatic reorganization of the cytoskeleton (Barrallo-Gimeno and Nieto, 2005; Cano et al., 2000; Nieto, 2011). Thus, Wor may have a conserved function in regulating the microtubule cytoskeleton. Because reducing Elav levels can rescue cell cycle progression, Wor appears to regulate the microtubule cytoskeleton indirectly, via keeping Elav protein levels low. High levels of Elav in neuroblasts may induce microtubule organization characteristic of mature neurons, such as using a single centrosome to nucleate unidirectional microtubule outgrowth into the axon. Thus, neuroblasts with high levels of Elav may be unable to efficiently duplicate their centrosomes or form a bipolar mitotic spindle, leading to the observed prophase arrest phenotype.
Materials and methods
Fly genetics
See supplemental methods.
TU-tagging, RNA-seq and RNA sequence analysis
TU-tagging is a method for labeling of newly transcribed RNAs in cells containing the T. gondii enzyme UPRT and exposed to 4-thiouracil (4TU) (Miller et al., 2009; Zeiner et al., 2008). Larvae were raised at 25°C. After 3 days, 50 larvae were transferred to agar plates with 500 μM 4- thiouracil (Sigma, St. Louis, MO) for 5 hours. We added 1 mM oxonic acid (Sigma, St. Louis, MO) to avoid a salvage pathway which can use 4-thiouracil without the presence of UPRT (M. Cleary, unpublished data). Standard methods were used to purify RNA (Miller et al., 2009; Zeiner et al., 2008). For each experiment, 5-10 ng of streptavidin purified (TU-tagged) RNA was converted to double stranded cDNA (ds-cDNA) using the Ovation RNA-seq System V2 (NuGEN), and 1 μg of ds-cDNA was used to generate an indexed Illumina library using NEBNext DNA Sample Prep Master Mix Set 1 (NE BioLabs). The resulting libraries were sequenced together in one lane of an HiSeq 2000 using version 3 sequencing reagents. This resulted in 13.7-14.8 million single-end 100 bp reads from each indexed library. The sequence reads were aligned against the D melanogaster release 5 genome sequence using Bowtie 2 (bowtie-bio.sourceforge.net/bowtie2/) in ‘sensitive-local’ mode, and the number of reads mapping to each gene region or each alternatively spliced exon junction of the release 5.25 genome annotation was determined using the SAMtools (Li et al., 2009) ‘view’ command. Reads per million (RPM) expression values were calculated by normalizing the number of reads that mapped to each gene region or each alternatively spliced exon junction to the total number of reads that mapped to all gene regions. RPM values from biological replicate experiments were averaged.
Dissection, antibody and TUNEL staining, EdU incorporation and time-lapse imaging
Antibody staining followed published protocols (Lee et al., 2006). Primary antibodies: rat anti-Pins; rat anti-Dpn; guinea pig anti-bazooka; guinea pig anti-Mira; rat anti-Mira; mouse anti-Pros; rabbit anti-PH3; rabbit anti-aPKC; mouse anti-α-tubulin; guinea pig anti-Numb; mouse anti-Insc; mouse anti-Elav (details in supplemental methods). TUNEL staining was done by manufacture's protocol (Roche, Indianapolis, IN). For EdU incorporation, larval brains explants were incubated in S2 medium (Sigma, St. Louis, MO) containing 100μg/mL EdU (Molecular Probes, Eugene, OR) at 25°C for 2 hours. After completing standard fixation and antibody staining procedures, EdU was detected by following manufacturer's protocols (Molecular Probes, Eugene, OR). Microscopy was done using a Zeiss LSM700, and images were processed with FIJI (http://fiji.sc). Time-lapse imaging was done by standard protocols using a Leica spinning disc microscope (Siller et al., 2005) and montaged with FIJI. All quantifications are presented as average±standard deviation.
Supplementary Material
Each point needs to be less than 85 characters (including spaces)
Worniu regulates multiple processes to maintain neuroblast “stemness”
worniu prevents the upregulation of neural differentiation genes, including Elav
Upregulated Elav in worniu mutants inhibits neuroblast cell cycle and polarity
Overexpressing Worniu elevates nuclear Prospero, which inhibits self-renewal
Acknowledgments
We thank L Gay, C Cabernard, R Andersen, and J Kroll for technical assistance; Tory Herman, Yan Yan, Omer Bayraktar, Travis Carney, Minoree Kohwi, Tony Ip and Alex Gould for comments on the manuscript; and Jim Skeath and Bill Chia for antibodies. The work was supported by HHMI, where C.Q.D. is an investigator.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen RO, Turnbull DW, Johnson EA, Doe CQ. Sgt1 acts via an LKB1/AMPK pathway to establish cortical polarity in larval neuroblasts. Dev Biol. 2012;363:258–265. doi: 10.1016/j.ydbio.2011.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf SI, Ganguly A, Roote J, Ip YT. Worniu, a Snail family zinc-finger protein, is required for brain development in Drosophila. Dev Dyn. 2004;231:379–386. doi: 10.1002/dvdy.20130. [DOI] [PubMed] [Google Scholar]
- Ashraf SI, Hu X, Roote J, Ip YT. The mesoderm determinant snail collaborates with related zinc-finger proteins to control Drosophila neurogenesis. Embo J. 1999;18:6426–6438. doi: 10.1093/emboj/18.22.6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf SI, Ip YT. The Snail protein family regulates neuroblast expression of inscuteable and string, genes involved in asymmetry and cell division in Drosophila. Develop. 2001;128:4757–4767. doi: 10.1242/dev.128.23.4757. [DOI] [PubMed] [Google Scholar]
- Bardet PL, Kolahgar G, Mynett A, Miguel-Aliaga I, Briscoe J, Meier P, Vincent JP. A fluorescent reporter of caspase activity for live imaging. PNAS U S A. 2008;105:13901–13905. doi: 10.1073/pnas.0806983105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrallo-Gimeno A, Nieto MA. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Develop. 2005;132:3151–3161. doi: 10.1242/dev.01907. [DOI] [PubMed] [Google Scholar]
- Bayraktar OA, Boone JQ, Drummond ML, Doe CQ. Drosophila type II neuroblast lineages keep Prospero levels low to generate large clones that contribute to the adult brain central complex. Neural Dev. 2010;5:26. doi: 10.1186/1749-8104-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello BC, Izergina N, Caussinus E, Reichert H. Amplification of neural stem cell proliferation by intermediate progenitor cells in Drosophila brain development. Neural Dev. 2008;3:5. doi: 10.1186/1749-8104-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger C, Renner S, Luer K, Technau GM. The commonly used marker ELAV is transiently expressed in neuroblasts and glial cells in the Drosophila embryonic CNS. Dev Dyn. 2007;236:3562–3568. doi: 10.1002/dvdy.21372. [DOI] [PubMed] [Google Scholar]
- Boone JQ, Doe CQ. Identification of Drosophila type II neuroblast lineages containing transit amplifying ganglion mother cells. Dev Neurobiol. 2008;68:1185–1195. doi: 10.1002/dneu.20648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman SK, Rolland V, Betschinger J, Kinsey KA, Emery G, Knoblich JA. The tumor suppressors Brat and Numb regulate transit-amplifying neuroblast lineages in Drosophila. Dev Cell. 2008;14:535–546. doi: 10.1016/j.devcel.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabernard C, Doe CQ. Apical/basal spindle orientation is required for neuroblast homeostasis and neuronal differentiation in Drosophila. Dev Cell. 2009;17:134–141. doi: 10.1016/j.devcel.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Cabernard C, Prehoda KE, Doe CQ. A spindle-independent cleavage furrow positioning pathway. Nature. 2010;467:91–94. doi: 10.1038/nature09334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Chia W, Yang X. A family of snail-related zinc finger proteins regulates two distinct and parallel mechanisms that mediate Drosophila neuroblast asymmetric divisions. EMBO J. 2001;20:1704–1714. doi: 10.1093/emboj/20.7.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nature Cell Bio. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- Carney TD, Miller MR, Robinson KJ, Bayraktar OA, Osterhout JA, Doe CQ. Functional genomics identifies neural stem cell sub-type expression profiles and genes regulating neuroblast homeostasis. Dev Biol. 2012;361:137–146. doi: 10.1016/j.ydbio.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabu C, Doe CQ. Dap160/intersectin binds and activates aPKC to regulate cell polarity and cell cycle progression. Develop. 2008;135:2739–2746. doi: 10.1242/dev.024059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doe CQ. Neural stem cells: balancing self-renewal with differentiation. Development. 2008;1587:1575–1587. doi: 10.1242/dev.014977. [DOI] [PubMed] [Google Scholar]
- Izergina N, Balmer J, Bello B, Reichert H. Postembryonic development of transit amplifying neuroblast lineages in the Drosophila brain. Neural Dev. 2009;4:44. doi: 10.1186/1749-8104-4-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblich JA. Mechanisms of asymmetric stem cell division. Cell. 2008;132:583–597. doi: 10.1016/j.cell.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Koushika SP, Lisbin MJ, White K. ELAV, a Drosophila neuron-specific protein, mediates the generation of an alternatively spliced neural protein isoform. Curr Biol. 1996;6:1634–1641. doi: 10.1016/s0960-9822(02)70787-2. [DOI] [PubMed] [Google Scholar]
- Koushika SP, Soller M, White K. The neuron-enriched splicing pattern of Drosophila erect wing is dependent on the presence of ELAV protein. Mol Cell Biol. 2000;20:1836–1845. doi: 10.1128/mcb.20.5.1836-1845.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CY, Robinson KJ, Doe CQ. Lgl, Pins and aPKC regulate neuroblast self-renewal versus differentiation. Nature. 2006;439:594–598. doi: 10.1038/nature04299. [DOI] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R. The Sequence Alignment/Map format and SAMtools. Bioinform. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisbin MJ, Qiu J, White K. The neuron-specific RNA-binding protein ELAV regulates neuroglian alternative splicing in neurons and binds directly to its pre-mRNA. Genes Dev. 2001;15:2546–2561. doi: 10.1101/gad.903101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MR, Robinson KJ, Cleary MD, Doe CQ. TU-tagging: cell type-specific RNA isolation from intact complex tissues. Nat Methods. 2009;6:439–441. doi: 10.1038/nmeth.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3:155–166. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- Nieto MA. The ins and outs of the epithelial to mesenchymal transition in health and disease. Annu Rev Cell Dev Biol. 2011;27:347–376. doi: 10.1146/annurev-cellbio-092910-154036. [DOI] [PubMed] [Google Scholar]
- Roark M, Sturtevant MA, Emery J, Vaessin H, Grell E, Bier E. scratch, a pan-neural gene encoding a zinc finger protein related to snail, promotes neuronal development. Genes Dev. 1995;9:2384–2398. doi: 10.1101/gad.9.19.2384. [DOI] [PubMed] [Google Scholar]
- Robinow S, White K. The locus elav of Drosophila melanogaster is expressed in neurons at all developmental stages. Dev Biol. 1988;126:294–303. doi: 10.1016/0012-1606(88)90139-x. [DOI] [PubMed] [Google Scholar]
- Schuh M, Lehner CF, Heidmann S. Incorporation of Drosophila CID/CENP-A and CENP-C into centromeres during early embryonic anaphase. Curr Biol. 2007;17:237–243. doi: 10.1016/j.cub.2006.11.051. [DOI] [PubMed] [Google Scholar]
- Siegrist SE, Doe CQ. Microtubule-induced Pins/Galphai cortical polarity in Drosophila neuroblasts. Cell. 2005;123:1323–1335. doi: 10.1016/j.cell.2005.09.043. [DOI] [PubMed] [Google Scholar]
- Siegrist SE, Doe CQ. Extrinsic cues orient the cell division axis in Drosophila embryonic neuroblasts. Develop. 2006;133:529–536. doi: 10.1242/dev.02211. [DOI] [PubMed] [Google Scholar]
- Siegrist SE, Doe CQ. Microtubule-induced cortical cell polarity. Genes Dev. 2007;21:483–496. doi: 10.1101/gad.1511207. [DOI] [PubMed] [Google Scholar]
- Siller KH, Cabernard C, Doe CQ. The NuMA-related Mud protein binds Pins and regulates spindle orientation in Drosophila neuroblasts. Nature Cell Bio. 2006;8:594–600. doi: 10.1038/ncb1412. [DOI] [PubMed] [Google Scholar]
- Siller KH, Doe CQ. Lis1/dynactin regulates metaphase spindle orientation in Drosophila neuroblasts. Dev Biol. 2008;319:1–9. doi: 10.1016/j.ydbio.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siller KH, Serr M, Steward R, Hays TS, Doe CQ. Live imaging of Drosophila brain neuroblasts reveals a role for Lis1/dynactin in spindle assembly and mitotic checkpoint control. Mol Biol Cell. 2005;16:5127–5140. doi: 10.1091/mbc.E05-04-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Vega S, Morales AV, Ocana OH, Valdes F, Fabregat I, Nieto MA. Snail blocks the cell cycle and confers resistance to cell death. Genes Dev. 2004;18:1131–1143. doi: 10.1101/gad.294104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiura S, Ohta N, Matsuzaki F. Tre1 GPCR Signaling Orients Stem Cell Divisions in the Drosophila Central Nervous System. Dev Cell. 2011 doi: 10.1016/j.devcel.2011.10.027. [DOI] [PubMed] [Google Scholar]
- Zeiner GM, Cleary MD, Fouts AE, Meiring CD, Mocarski ES, Boothroyd JC. RNA analysis by biosynthetic tagging using 4-thiouracil and uracil phosphoribosyltransferase. Methods Mol Biol. 2008;419:135–146. doi: 10.1007/978-1-59745-033-1_9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


