Crystal structures of the hexanucleotide d(CACGCG)·d(CGCGTG) were determined in the presence of Mn2+ ions in two crystal lattices and provide insights into ion interactions.
Keywords: Z-DNA, crystal packing, DNA–ion interactions, manganese ions
Abstract
Crystal structures of the hexanucleotide d(CACGCG)·d(CGCGTG) were determined in two crystal lattices when different concentrations of the counterion Mn2+ were used in crystallization. The availability of Mn2+ during the crystallization process appears to play an important role in inducing different crystal packings that lead to crystals belonging to the two space groups P21 and P65. Analysis of the molecular interactions of Mn2+ with the Z-form duplexes shows direct coordination to the purine residues G and A.
1. Introduction
Metal ions play a key role in the biological activity and structural stabilization of DNA. Transition-metal ions are known to simultaneously bind to phosphate sites and to bases (Sigel, 1993 ▶; Saenger, 1984 ▶; Martin, 1985 ▶). Mn2+ has different binding characteristics towards DNA in binding by almost exclusively coordinating to the N7 position of purine bases, especially guanine (Saenger, 1984 ▶). Mn2+ ions have been reported (Eichhorn & Shin, 1968 ▶) to decrease the melting temperature of several DNA sequences and to show greater destabilizing effects as the G+C content of the DNA increases. Mn2+ is known to alter the interaction between DNA and enzymes such as RNA polymerase and DNase (Anderson et al., 1971 ▶).
Our previous studies to explore the precise influence of sequence on the microstructure of Z-DNA, in particular the effect of the introduction of A·T base pairs into sequences that otherwise consist of C·G base pairs, have indicated that the position of the A·T base pair plays a role in determining the helical structure (Sadasivan & Gautham, 1995 ▶; Thiyagarajan et al., 2004 ▶). The design of the sequence used in this study was primarily based upon the canonical hexamer d(CGCGCG)2 (Wang et al., 1979 ▶). In other studies, we have reported a tautomeric shift in the A·T base pair in the sequence d(CGCGCA)·d(TGCGCG) that was induced by the binding of cobalt hexammine (Thiyagarajan et al., 2004 ▶, 2005 ▶) and by interaction with ruthenium hexammine (Bharanidharan et al., 2007 ▶). During crystallization attempts using hexameric sequences with a single A·T base pair in the presence of different metal ions (particularly cobalt hexamine), we observed the formation of unusual ring-shaped crystals (Kumar & Gautham, 1999 ▶; Mandal, Chandrasekaran et al., 2012 ▶). The present investigation was undertaken as part of studies in our laboratory to understand the mode of interaction of metal ions in general, and Mn2+ in particular, with DNA. The sequence was crystallized using 10 mM and 1 mM Mn2+ to obtain two crystal forms, which are named Mn_P21 (monoclinic) and Mn_P65 (hexagonal), respectively.
2. Materials and methods
2.1. Crystallization, X-ray diffraction data collection and data processing
The PAGE-purified DNA oligonucleotides d(CACGCG) and d(CGCGTG) were purchased from M/s Microsynth, Switzerland. The two cross-complementary sequences were annealed to form the duplex and used in crystallization experiments. Crystals were grown by the hanging-drop vapour-diffusion technique at room temperature. The crystallization conditions are described in Table 1 ▶. Crystals of Mn_P21 and Mn_P65 were obtained in the shape of trapezoidal rods and hexagonal prisms, respectively. For data collection at 100 K, the crystals were flash-cooled in liquid nitrogen; the mother liquor was sufficient for cryoprotection. X-ray diffraction data were collected in-house on a MAR Research image-plate system using Cu Kα radiation (λ = 1.5418 Å) generated by a rotating-anode X-ray generator (Bruker AXS) operated at 45 kV and 60 mA at the G. N. Ramachandran X-ray Diffraction Facility, University of Madras, Chennai, India. Data processing was performed using automar (Bartels & Klein, 2003 ▶). The diffraction data from the Mn_P21 crystal were processed in the monoclinic (P21) system. The diffraction data from the Mn_P65 crystal were indexed in hexagonal (P6), orthorhombic (C222) and monoclinic (P2) systems. After merging and scaling, we chose space group P65 (and the enantiomeric space group P61) for molecular-replacement trials based on the lowest R merge values, high symmetry and systematic absences. Data-collection statistics are shown in Table 1 ▶.
Table 1. Crystallization and X-ray diffraction data-collection statistics.
Values in parentheses are for the outer shell.
| Mn_P21 | Mn_P65 | |
|---|---|---|
| Crystallization conditions | ||
| DNA† (mM) | 1.0 | 1.0 |
| Buffer‡ (mM) | 50.0 | 50.0 |
| Manganese dichloride (mM) | 10.0 | 1.0 |
| Spermine (mM) | 1.0 | 1.0 |
| Methyl pentanediol (%) | 50 | 50 |
| Diffraction data | ||
| Space group | P21 | P65 |
| Unit-cell parameters | ||
| a (Å) | 24.48 | 35.21 |
| b (Å) | 31.13 | 35.21 |
| c (Å) | 31.67 | 44.52 |
| β (°) | 103.34 | 90 |
| No. of unique reflections | 6048 | 3133 |
| Wilson B factor (Å2) | 22.24 | 29.92 |
| Resolution range (Å) | 30.82–1.61 (1.66–1.61) | 30.50–1.76 (1.82–1.76) |
| R merge (%) | 4.86 (21.81) | 11.16 (34.38) |
| Completeness (%) | 97.6 (81.7) | 97.4 (100) |
| 〈I/σ(I)〉 | 8.4 (2.2) | 4.7 (1.1) |
| Multiplicity | 4.43 (4.35) | 3.85 (3.98) |
d(CACGCG)·d(CGCGTG).
Sodium cacodylate trihydrate pH 7.0.
2.2. Structure determination and refinement
For both structures, molecular-replacement trials were carried out with the program AMoRe (Navaza, 1994 ▶) from the CCP4 suite (Winn et al., 2011 ▶) using fibre models of A-type, B-type and Z-type DNA (the fibre models were generated using Insight II release 98.0; Biosym/MSI, San Diego, USA) for the sequence d(CACGCG)·d(CGCGTG). The molecular-replacement searches were performed using data between 15 and 3 Å resolution. Map calculations were performed using Coot (Emsley & Cowtan, 2004 ▶).
In the case of Mn_P21, the best molecular-replacement solution was obtained with a correlation coefficient of 85% and an R factor of 29% when two Z-type DNA duplexes were present in the asymmetric unit. In the case of Mn_P65 we observed the presence of crystal twinning (0.44), and this was taken into account during refinement. The unit-cell parameters are similar to those obtained for previous crystal structure reports from our laboratory for a different sequence of the same length (Thiyagarajan et al., 2004 ▶, 2005 ▶; Bharanidharan et al., 2007 ▶). We carried out molecular-replacement trials using fibre models comprising a hexamer Z-type duplex and a dinucleotide (sitting over the 65 or 61 screw axis) for both the enantiomeric space groups P61 and P65. The best solution was obtained with a correlation coefficient of 80% and an R factor of 34% in space group P65. The helix axis of the dinucleotide was positioned along the 65 screw axis, thus generating an infinite disordered helix. Because of the disorder, the dinucleotide step could represent both CpG/CpG as well as CpA/TpG. The presence of the A·T base pair in the sequence destroys the twofold symmetry perpendicular to the helix axis that is present in sequences such as CGCGCG. The disordered helix could therefore be oriented either parallel to the c axis or antiparallel to it, or both. Therefore, the dinucleotide was constructed as a TpG/TpG step in which the C5 methyl group of thymine was assigned an occupancy of 1/6 and the N2 of guanine was assigned an occupancy of 5/6.
The numbering schemes of the hexameric helices of Mn_P21 and Mn_P65 are as follows: chain A, 5′-C1pA2pC3pG4pC5pG6-3′; chain B, 3′-G12pT11pG10pC9pG8pC7-5′. The numbering schemes of the dinucleotide steps in the Mn_P65 structure are as follows: chain C, 5′-pT13pG14-3′; chain D, 3′-pG16pT15-5′.
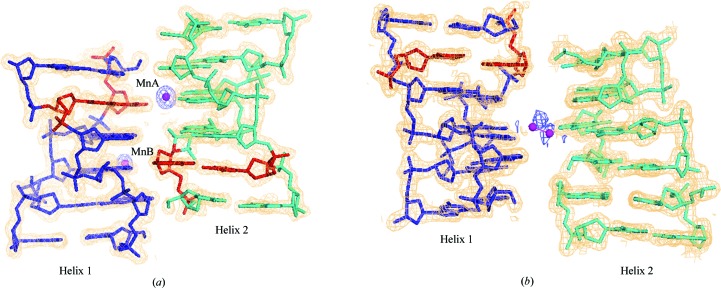
All refinements were carried out using REFMAC5 (Murshudov et al., 2011 ▶) with a maximum-likelihood target. Restraints were taken from the REFMAC5 dictionary (Vagin et al., 2004 ▶). 5% of the data were used for cross-validation and calculation of R free. No σ(F o) cutoff was applied. The f′ value for Mn2+ of 2.832e− is detectable with a laboratory X-ray source (Cu Kα), and the atom positions in the two structures were verified by calculating an anomalous difference map using the CCP4 suite. The map was calculated with Bijvoet differences (F + obs − F − obs) as the Fourier amplitudes along with the phases calculated from the refined model without the Mn atoms. The anomalous difference maps (Fig. 1 ▶) clearly indicated the positions of Mn2+ in the two crystal structures. Water molecules were added at the positions indicated in the F o − F c maps when viewed at the 3σ level in the cycles of refinement and map fitting. During the refinement of the Mn2+ positions, no distance restraints were applied to the coordination bonds. The occupancies of Mn2+ were manually adjusted, seeking to reach the best agreement with the refined B factors of the interacting atoms of the bases and phosphates. The atomic displacement parameters of all of the DNA atoms, water molecules and Mn2+ ions were found to be within acceptable ranges. Owing to the high cylindrical symmetry in the DNA helix, the uncertainties in the final models were verified using other models and OMIT-map calculations. The final refinement parameters are given in Table 2 ▶.
Figure 1.
(a) Helix 1 (blue), helix 2 (cyan) and two Mn2+ ions in Mn_P21. (b) Helix 1 (blue), an arbitrary helix 2 (cyan) comprising dinucleotide steps (x, y, z), (y, −x + y, z + 1/6) and (−x + y, −x, z + 1/3) and a disordered Mn2+ ion in Mn_P65. The anomalous difference Fourier map (blue) and 2F o − F c map (orange; for the helices only) are contoured at 3σ and 1σ levels, respectively. The A2·T11 base pairs are coloured red. The Mn2+ ions are shown as spheres. The water molecules are omitted for clarity.
Table 2. Structure-refinement statistics.
Values in parentheses are for the outer shell.
| Mn_P21 | Mn_P65 | |
|---|---|---|
| Resolution range (Å) | 30.82–1.61 | 30.50–1.76 |
| R factor (%) | 19.85 (30.03) | 23.03 (20.85) |
| R free (%) | 26.41 (34.03) | 25.86 (22.24) |
| Asymmetric unit components | 2 hexamer duplexes [helix 1 and helix 2] | 1 hexamer duplex [helix 1] and a dinucleotide step |
| No. of DNA atoms | 488 | 324 |
| No. of Mn2+ ions | 2 | 1 |
| No. of solvent molecules | 56 | 34 |
| Average B factor (Å2) | 18.33 | 18.85 |
| R.m.s.d. bond lengths (Å) | 0.02 | 0.01 |
| R.m.s.d. bond angles (°) | 2.88 | 2.34 |
| PDB code | 4dwy | 4dy8 |
Conformational and helical parameter calculations were performed using the program X3DNA (Lu & Olson, 2003 ▶). Graphical representations of molecular structures were prepared with PyMOL (DeLano, 2002 ▶). The coordinates and structure factors have been deposited in the PDB (Berman et al., 2000 ▶) as PDB entries 4dwy and 4dy8.
3. Results and discussion
Overall, the crystal structures of the hexanucleotide sequence d(CACGCG)·(CGCGTG) crystallized with Mn2+ fall within the Z-DNA family (Wang et al., 1979 ▶). The two strands of the duplexes form antiparallel left-handed helices with Watson–Crick base pairing. The phosphate groups follow a zigzag left-handed spiral. The helices possess a dinucleotide repeat with deep minor grooves and flat major grooves.
3.1. Overall structure and helicoidal parameters
Fig. 1 ▶ shows the helices and ions in the crystal structures Mn_P21 and Mn_P65. In Mn_P21 the asymmetric unit consists of two helices (helix 1 and helix 2). The r.m.s.d. value obtained by least-squares superposition of all atoms of the two helices helix 1 and helix 2 onto each other is 0.63 Å.
In Mn_P65 the asymmetric unit consists of one left-handed hexamer duplex (helix 1) and a dinucleotide step sitting on a 65 screw axis. The symmetry equivalents of this dinucleotide generate a full pseudo-continuous helix (Fig. 1 ▶ b) with its helical axis coinciding with the crystallographic c axis of the unit cell. We call this helix 2. This structure was found to be similar in conformation and crystal packing to that of the hexamer duplex d(CGCGCA)·d(TGCGCG) (which also crystallized in the hexagonal system) grown with 0.3 mM cobalt hexammine (Thiyagarajan et al., 2005 ▶; referred to as the hexamer duplex HexHA6). The r.m.s.d. in atomic positions between the hexamer duplexes helix 1 of Mn_P65 and HexHA6 after least-squares superposition of the phosphodiester backbone is 0.83 Å.
In the Mn_P21 structure the average twist at the pyrimidine–purine base step and the purine–pyrimidine base step in both helices is −8.7° and −49.3°, respectively. In the Mn_P65 structure the average helical twist per dinucleotide is −50.6° in helix 1 and −51° in helix 2. At the pyrimidine–purine step the average twist is −5.5° in helix 1 and −8.7° in helix 2. At the purine–pyrimidine step the average twist is −55.5° in helix 1 and −50.6° in helix 2. The base-geometry parameters slide, rise, roll, twist and X-displacement in both structures do not show significant differences from the fibre-model Z-DNA duplex.
3.2. Crystal packing
The majority of structures of Z-type DNA hexamers deposited in the NDB (Berman et al., 1992 ▶) are in space group P212121; the packing mode is ‘mode 1’ (Gessner et al., 1989 ▶). The second packing mode is ‘mode 2’, in which hexamers of Z-type DNA normally crystallize in a hexagonal space group (Egli et al., 1991 ▶). In the Mn_P21 structure the columns of helices are positioned such that the twofold screw axis is perpendicular to the helix axis, i.e. the crystallographic screw axis does not overlap with the molecular screw axis. This is different from the packing in crystals of the Z-DNA hexamer d(CGCACG)·d(CGTGCG) (Sadasivan & Gautham, 1995 ▶), also in space group P21, in which the crystallographic 21 axis coincides with the helix axis. In the present structure, the O3′ atom of G6 of helix 1 interacts with the phosphate atoms of T11 of the symmetry-related helix 2 (x − 1, y, z − 1); that of G12 interacts with G4 of the symmetry-related helix 2 (−x, y − ½, −z). In helix 2 the O3′ atom of G6 interacts with the phosphate of C9 of the symmetry-related helix 2 (−x + 1, y + ½, −z + 1). These interactions indicate that helix 1 and helix 2 deviate only slightly from mode 1 packing in terms of helical orientation, i.e. by a small rotation around the helical axis (Gessner et al., 1989 ▶). However, the presence of a disordered phosphate group for residues C5 in helix 1 and C3 and C5 in helix 2 indicates mode 2 packing (Egli et al., 1991 ▶). Thus, in the Mn_P21 structure the packing mode is of a mixed mode 1/mode 2 form. In the Mn_P65 structure the hexamer helices follow mode 1 packing: the O3′ atom of G6 of helix 1 interacts with the O2P atom of G10 of the symmetry-related helix 1.
The symmetry-related hexamers stack one over the other to form infinite helical columns. Even though the gross packing motif of the helices remains the same as that observed in canonical Z-DNA crystal structures, the presence of the single A·T base pair breaks the twofold sequence symmetry of the hexamer (Fig. 2 ▶) and gives the stacked columns a direction (Sadasivan et al., 1994 ▶; Thiyagarajan et al., 2005 ▶; Mandal et al., 2008 ▶). Fig. 2 ▶(a) shows the packing of helical columns in the Mn_P21 structure when viewed down the helical axis. The two helices (helix 1 and helix 2) point in opposite directions in an antiparallel arrangement (Fig. 2 ▶ b). The interhelical interactions, together with ion-mediated interactions, may be responsible for the stability of the helical arrangement in the monoclinic lattices. The presence of the A·T base pair and the Mn2+ ion may serve to ‘lock’ the packing of the helices into the observed pattern.
Figure 2.
Packing of the helical columns in Mn_P21 (a, b) and Mn_P65 (c, d). (a) and (c) are views down the helical axis, while (b) and (d) are views perpendicular to the helical axis. Helix 1 and helix 2 [except in (a)] are coloured blue and cyan, respectively. The A·T base pairs in the ordered hexamer helices are coloured red. Spheres represent Mn2+ bound to helices. The column with the 65 screw symbol indicates the disordered column built from the dinucleotide step. No direction for these columns could be assigned since the position of the A·T base pair is disordered. The unit cell is shown as thin lines.
In case of the Mn_P65 structure the symmetry-related hexamers stack one over the other to form infinite helical columns, all of which are oriented in the same direction (Figs. 2 ▶ c and 2 ▶ d). The other duplex is placed on the crystallographic sixfold screw axis. The sixfold screw symmetry of the Z-type helix coincides with the crystallographic sixfold. Thus, the asymmetric unit includes a dinucleotide step (which repeats infinitely to generate the helical column) along with a regular hexamer duplex. Owing to partially occupied phosphate groups at all base steps as well as the presence of an A·T base pair in the sequence, this column of helices is disordered and we cannot distinguish its direction. The disordered and ordered columns alternate along both the a and the b axes (Fig. 2 ▶ d).
3.3. Ion interactions
In general, Mn2+ ions interact wth DNA by coordinating to the N7 position of purines, especially guanine (Saenger, 1984 ▶). Previous reports have demonstrated that AT-rich sequences localize monovalent and divalent cations in the minor groove in a sequence-specific manner (Hud & Feigon, 1997 ▶; Hud et al., 1999 ▶). An analysis of divalent cations in a number of high-resolution DNA crystal structures reveals that divalent cations can be bound at the top of a minor groove in DNA-sequence elements with a narrow minor groove (e.g. AATT; Minasov et al., 1999 ▶; Tereshko & Subirana, 1999 ▶; Sines et al., 2000 ▶). In B-type duplexes, for example the oligonucleotide d(CGTTAATTAACG)2 crystallized in the presence of Mn2+ (10 mM) and a low spermine concentration (0.25 mM) (Millonig et al., 2009 ▶), the Mn2+ ions form direct bridges between neighbouring duplexes in the crystal. Recently, we have reported the crystal structure of the tetradecanucleotide sequence d(CCCCGGTACCGGGG)2 as an A-type duplex in which one Mn2+ ion was identified with direct coordination to the N7 position of G13 and a water molecule at the major-groove side of the C2·G13 base pair (Mandal, Venkadesh et al., 2012 ▶). There are no previous reports on the nature of Mn2+ interactions with Z-type DNA helices.
Table 3 ▶ gives details of the ion interactions in both Mn_P21 and Mn_P65. In the Mn_P21 structure two Mn2+ ions, MnA and MnB, are present in the interstitial space (Fig. 1 ▶). The MnA site is fully occupied, while the MnB site has an occupancy of 0.7. The coordination geometry around MnA is octahedral, comprising three direct interactions with N7 of A2 (helix 1), N7 of G4 (helix 2) and O1P of T11 of a symmetry-related helix 2 and interactions with three water molecules (Fig. 3 ▶ a). The MnA ion shows three water-mediated interaction with O1P and O2P of C3 (helix 1) and O5′ of C1 (of helix 2). This ion bridges the N7 atoms of two planar purines facing each other. The coordination geometry around MnB is distorted trigonal, comprising three direct interactions with the N7 and O6 atoms of residue G4 of helix 1 and the N7 atom of the terminal residue G12 of the symmetry-related helix 2 (Fig. 3 ▶ b). The three direct interactions cause disruption of the nearby base pairing, leading to bifurcation of the G4·C9 and G12·C1 base-pair hydrogen bonds (Fig. 3 ▶ b). The ion pushes the guanine away from the helix axis in the crystal lattice without destabilizing the crystal.
Table 3. Details of Mn2+–Z-DNA interactions in Mn_P21 and Mn_P65 .
Details of the bifurcation of hydrogen bonds in base pairs are also included.
| Atom (occupancy) | B factor (Å2) | Atom | Distance (Å) | Remarks |
|---|---|---|---|---|
| Mn_P21 | ||||
| MnA (1.00) | 12.88 | N7/A2/helix 1 | 2.36 | Octahedral geometry, three direct interactions |
| N7/G4/helix 2 | 2.28 | |||
| O1P/T11/helix 1† | 2.09 | |||
| O/2 HOH | 2.23 | |||
| O/3 HOH | 2.29 | |||
| O/7 HOH | 2.30 | |||
| O/7 HOH (1.00) | 16.13 | O/7 HOH–O1P/C3/helix 1 | 2.43 | Water-mediated interactions |
| O/2 HOH (1.00) | 12.27 | O/2 HOH–O2P/C3/helix 1 | 2.73 | |
| O/3 HOH (1.00) | 15.05 | O/3 HOH–O5′/C1/helix 2 | 3.13 | |
| MnB (0.70) | 20.17 | N7/G4/helix 1 | 2.45 | Distorted trigonal geometry, three direct interactions, bifurcation of hydrogen bond in G4·C9 and C1·G12 base pairs |
| O6/G4/helix 1 | 2.47 | |||
| N7/G12/helix 2‡ | 2.31 | |||
| O6/G4/helix 1 | N4/C9/helix 1 | 2.90 | ||
| O6/G4/helix 1 | N3/C9/helix 1 | 3.50 | ||
| N1/G4/helix 1 | N3/C9/helix 1 | 2.90 | ||
| N1/G4/helix 1 | O2/C9/helix 1 | 3.53 | ||
| N2/G4/helix 1 | O2/C9/helix 1 | 2.87 | ||
| O6/G12/helix 2‡ | N4/C1/helix 2‡ | 2.81 | ||
| O6/G12/helix 2‡ | N3/C1/helix 2‡ | 3.32 | ||
| N1/G12/helix 2‡ | N3/C1/helix 2‡ | 2.84 | ||
| N1/G12/helix 2‡ | O2/C1/helix 2‡ | 3.37 | ||
| N2/G12/helix 2‡ | O2/C1/helix 2‡ | 2.88 | ||
| Mn_P65 | ||||
| Mn2+ (0.65) | 24.81 | O6/G4/helix 1 | 2.74 | Two direct interactions, bifurcation of hydrogen bond in G4·C9 base pair |
| N7/G4/helix 1 | 2.94 | |||
| Mn2+ (0.35) | 26.77 | O6/G16/helix 2 | 2.57 | Two direct interactions |
| N7/G16/helix 2 | 3.04 | |||
| O6/G4/helix 1 | N4/C9/helix 1 | 3.30 | ||
| O6/G4/helix 1 | N3/C9/helix 1 | 3.43 | ||
| N1/G4/helix 1 | N3/C9/helix 1 | 3.01 | ||
| N1/G4/helix 1 | O2/C9/helix 1 | 3.28 | ||
| N2/G4/helix 1 | O2/C9/helix 1 | 2.68 | ||
Related to helix 1 in Mn_P21 by symmetry (−x, y + 1/2, −z).
Related to helix 2 in Mn_P21 by symmetry (−x − 1, y + 1/2, −z).
Figure 3.
Interactions of ions: (a) MnA and (b) MnB in Mn_P21 and (c) the disordered Mn2+ ion in Mn_P65 (viewed down the helical axis). The Mn2+ ions are shown as spheres, water molecules are shown as crosses, ion-mediated and water-mediated interactions are shown as red dashed lines and hydrogen bonds of base pairs are shown as black dashed lines. The ion induces distortion of the hydrogen-bond base-pair schemes in (b) and (c).
In the Mn_P65 structure the two ions represent two positions for a single disordered ion. One is located at the centre of the major groove near the G4 base of the hexamer helix 1. Another is found near the G16 base of helix 2. Thus, the ion binds to either G4 or to G16, but not to both at the same time. This disordered binding mode is also reflected in the anomalous difference Fourier map (Fig. 1 ▶), in which an elongated electron-density envelope can be fitted with two positions for the manganese ion (with occupancies of 0.65 and 0.35) separated by a distance of 1.92 Å. Fig. 3 ▶(c) illustrates the ion interactions in Mn_P65. The ion at the major position coordinates to the ligand atoms N7 and O6 of G4 in helix 1 (ordered column). This direct coordination causes disruption of the nearby base pairing, leading to bifurcation of the G4·C9 base-pair hydrogen bonds. The ion pushes G4 away from the helix axis in the crystal lattice without destabilizing the crystal. The distances between the ion in the major position and the N7 (4.18 Å) and O6 (4.29 Å) atoms of G16 in helix 2 are large and the ion in this position cannot be considered as making coordination bonds to the disordered column. However, the ion in the minor position (occupancy 0.35) coordinates to the N7 and O6 atoms of G16 in helix 2. Again, the ion in the minor position is at a large distance from the N7 (4.07 Å) and O6 (4.25 Å) atoms of G4 in the hexamer duplex helix 1. Thus, at any point in time the ion interacts with either the ordered or the disordered column, but not with both simultaneously.
3.4. Ion binding and helix packing in the two structures
While the molecular structure of the hexamers in the two crystals is the same, there are differences in the packing that lead in turn to differences in the space groups and crystal systems. It has previously been demonstrated (Sadasivan et al., 1994 ▶) that crystals of Z-DNA hexamers can be indexed in a variety of different space groups, despite the fact that the primary packing mode is the same. DNA molecules, and in particular Z-DNA duplexes, may be approximately modelled as cylinders. Differences in the details of the sequence, structure and ion interactions then lead to differences in the space groups in which the crystals are best indexed.
Fig. 2 ▶ illustrates the packing of the helices in the present unit cell, with unit-cell parameters a = 24.48, b = 31.13, c = 31.67 Å, β = 103.3° with b as the unique axis. In Mn_P21 we were able to identify two Mn2+ ions bound to the asymmetric unit. Here, the position of the ion (MnA) is such that it forms a bridge between the A2·T11 base pair on one helix (helix 1) and the G4·C9 base pair (Fig. 3 ▶ a) on the other helix (helix 2). The presence of a second Mn2+ ion (MnB in Mn_P21) bound to the G4·C9 base pair (Fig. 3 ▶ b) of helix 1 and the G12·C1 base pair of the symmetry-related helix 2 did not significantly alter the helical orientation. Considered from a point of view perpendicular to the helix axis, this indicates that the terminal base pairs of the duplexes are not in the same plane. The A·T base pairs are also not in the same plane as observed in many other structures (Sadasivan & Gautham, 1995 ▶; Thiyagarajan et al., 2004 ▶, 2005 ▶; Bharanidharan et al., 2007 ▶) of sequences with a single A·T base pairs. Therefore, the hexamers are arranged along the helix axis so that successive duplexes alternate in direction (when we consider that the position of the A·T base pair defines a direction for each hexamer).
The consequence of the alternation in the column directions is that it does not allow the positioning of the 21 screw axis to coincide with the helical axis, as might have very easily happened in a crystal formed by hexamers with no sequence-defined direction or if the hexamers in a column all pointed in the same direction. Such a crystal could still have been indexed in monoclinic P21, although the unit-cell parameters and their relationship to the helical axis would have been different (Sadasivan et al., 1994 ▶). The present arrangement in which the MnA ion allows the close packing of two of the helical columns also allows a close approach of the partially negatively charged backbone phosphate O atom to the cation, with a weak ionic bond interaction. Thus, the packing of the three helical columns is tight and this is reflected in the volume of the unit cell occupied per base pair in the crystal, which is the lowest of the two crystal forms reported here (Table 4 ▶).
Table 4. Volume per base pair.
| Mn_P21 | Mn_P65 | |
|---|---|---|
| Unit-cell volume (Å3) | 23453 | 47723 |
| No. of base pairs in unit cell | 24 | 48 |
| No. of Mn2+ ions in unit cell | 2 | 2 |
| Unit-cell volume per base pair (Å3) | 977 | 994 |
In the hexagonal crystal the helices are arranged in the conventional manner (Fig. 2 ▶) as observed in other hexagonal structures of d(CGCGCA)·d(TGCGCG) (Thiyagarajan et al., 2004 ▶, 2005 ▶; Bharanidharan et al., 2007 ▶). In both the monoclinic and hexagonal crystals each unit cell contains two Mn2+ ions. However, in the hexagonal crystals, which were grown at a lower ion concentration, the ion positions are disordered and relatively sparsely occupied. Thus, adjacent helical columns are not as tightly packed as in the case of the monoclinic crystals. This is also apparent from the unit-cell volume per base pair for the two structures (Table 4 ▶). Clearly, the closest helical packing is observed for the monoclinic system.
To summarize, binding of Mn2+ to DNA has the following effects. When the concentration of Mn2+ is varied in the crystallization drop, it leads to slightly different packing modes, essentially by a process of ‘loosening’ the lattice at the lower ion concentration. The differences in the lattice may be traced to the different binding modes of Mn2+ and differences in the nature of the ion interactions and interhelical contacts. In addition, the binding of the ion induces a perturbation of the G·C base-pairing scheme, leading to bifurcated hydrogen bonds between the bases.
Supplementary Material
PDB reference: Mn_P21, 4dwy
PDB reference: Mn_P65, 4dy8
Acknowledgments
This work was financially supported by the following agencies of the Government of India: DBT under a research grant and DST under the FIST programme. PKM thanks CSIR for Senior Research Fellowships.
References
- Anderson, J. A., Kuntz, G. P., Evans, H. H. & Swift, T. J. (1971). Biochemistry, 10, 4368–4374. [DOI] [PubMed]
- Bartels, K. S. & Klein, C. (2003). The Automar Manual v.1.4. Norderstedt, Germany: MAR Research GmbH.
- Berman, H. M., Olson, W. K., Beveridge, D. L., Westbrook, J., Gelbin, A., Demeny, T., Hsieh, S. H., Srinivasan, A. R. & Schneider, B. (1992). Biophys. J. 63, 751–759. [DOI] [PMC free article] [PubMed]
- Berman, H. M., Westbrook, J., Feng, Z., Gilliland, G., Bhat, T. N., Weissig, H., Shindyalov, I. N. & Bourne, P. E. (2000). Nucleic Acids Res. 28, 235–242. [DOI] [PMC free article] [PubMed]
- Bharanidharan, D., Thiyagarajan, S. & Gautham, N. (2007). Acta Cryst. F63, 1008–1013. [DOI] [PMC free article] [PubMed]
- DeLano, W. L. (2002). PyMOL. http://www.pymol.org.
- Egli, M., Williams, L. D., Gao, Q. & Rich, A. (1991). Biochemistry, 30, 11388–11402. [DOI] [PubMed]
- Eichhorn, G. L. & Shin, Y. A. (1968). J. Am. Chem. Soc. 90, 7323–7328. [DOI] [PubMed]
- Emsley, P. & Cowtan, K. (2004). Acta Cryst. D60, 2126–2132. [DOI] [PubMed]
- Gessner, R. V., Frederick, C. A., Quigley, G. J., Rich, A. & Wang, A. H.-J. (1989). J. Biol. Chem. 264, 7921–7935. [DOI] [PubMed]
- Hud, N. V. & Feigon, J. (1997). J. Am. Chem. Soc. 119, 5756–5757.
- Hud, N. V., Sklenár, V. & Feigon, J. (1999). J. Mol. Biol. 286, 651–660. [DOI] [PubMed]
- Kumar, P. S. & Gautham, N. (1999). Curr. Sci. 77, 1076–1078.
- Lu, X.-J. & Olson, W. K. (2003). Nucleic Acids Res. 31, 5108–5121. [DOI] [PMC free article] [PubMed]
- Mandal, P. K., Chandrasekaran, A. R., Madhanagopal, B., Venkadesh, S. & Gautham, N. (2012). J. Cryst. Growth, 354, 20–26.
- Mandal, P. K., Venkadesh, S. & Gautham, N. (2008). J. Indian Inst. Sci. 88, 73–93.
- Mandal, P. K., Venkadesh, S. & Gautham, N. (2012). Acta Cryst. F68, 393–399. [DOI] [PMC free article] [PubMed]
- Martin, R. B. (1985). Acc. Chem. Res. 18, 32–38.
- Millonig, H., Pous, J., Gouyette, C., Subirana, J. A. & Campos, J. L. (2009). J. Inorg. Biochem. 103, 876–880. [DOI] [PubMed]
- Minasov, G., Tereshko, V. & Egli, M. (1999). J. Mol. Biol. 291, 83–99. [DOI] [PubMed]
- Murshudov, G. N., Skubák, P., Lebedev, A. A., Pannu, N. S., Steiner, R. A., Nicholls, R. A., Winn, M. D., Long, F. & Vagin, A. A. (2011). Acta Cryst. D67, 355–367. [DOI] [PMC free article] [PubMed]
- Navaza, J. (1994). Acta Cryst. A50, 157–163.
- Sadasivan, C. & Gautham, N. (1995). J. Mol. Biol. 248, 918–930. [DOI] [PubMed]
- Sadasivan, C., Karthe, P. & Gautham, N. (1994). Acta Cryst. D50, 192–196. [DOI] [PubMed]
- Saenger, W. (1984). Principles of Nucleic Acid Structure, pp. 201–219. New York: Springer-Verlag.
- Sigel, H. (1993). Chem. Soc. Rev. 22, 255–267.
- Sines, C. C., McFail-Isom, L., Howerton, S. B., VanDerveer, D. & Williams, L. D. (2000). J. Am. Chem. Soc. 122, 11048–11056.
- Tereshko, V. & Subirana, J. A. (1999). Acta Cryst. D55, 810–819. [DOI] [PubMed]
- Thiyagarajan, S., Rajan, S. S. & Gautham, N. (2004). Nucleic Acids Res. 32, 5945–5953. [DOI] [PMC free article] [PubMed]
- Thiyagarajan, S., Rajan, S. S. & Gautham, N. (2005). Acta Cryst. D61, 1125–1131. [DOI] [PubMed]
- Vagin, A. A., Steiner, R. A., Lebedev, A. A., Potterton, L., McNicholas, S., Long, F. & Murshudov, G. N. (2004). Acta Cryst. D60, 2184–2195. [DOI] [PubMed]
- Wang, A. H.-J., Quigley, G. J., Kolpak, F. J., Crawford, J. L., van Boom, J. H., van der Marel, G. & Rich, A. (1979). Nature (London), 282, 680–686. [DOI] [PubMed]
- Winn, M. D. et al. (2011). Acta Cryst. D67, 235–242.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PDB reference: Mn_P21, 4dwy
PDB reference: Mn_P65, 4dy8