Structures of ERK2 in complexes with ATP and with ADP are presented and are compared with the apo form of the protein in the same monoclinic crystal form and with the closest structural homolog cyclin-dependent kinase 2, for which an ATP-complex structure is available.
Keywords: mitogen-activated protein kinases, ERK2
Abstract
Extracellular signal-regulated kinases 1 and 2 (ERK1 and ERK2) are members of the mitogen-activated protein (MAP) kinase family. Constitutive activation of the ERK proteins contributes to the development and progression of numerous human tumors. Thus, ERK1 and ERK2 are promising targets for the design and the development of anticancer drugs. The detailed structural analysis of ERK complexed with ATP can provide valuable information for the design of new ligands that can bind in the ATP-binding pocket and inhibit ERK activity. In this study, the structures of apo-form ERK2 and of its complexes with the substrate ATP and the product ADP were determined. Comparison with the structural homolog cyclin-dependent kinase 2 reveals differences in the way that the ATP binding to the protein is mediated by magnesium. Only minor conformational changes are identified that occur upon substrate binding, and these are limited to the active-site residues.
1. Introduction
Protein kinases control and modulate a wide variety of biological processes by catalyzing the transfer of a phosphate group from adenosine triphosphate (ATP) to other proteins. Abnormal kinase activity has been associated with a number of diseases, including cancer (Cohen, 2002 ▶; Dibb et al., 2004 ▶; Sawyers, 2004 ▶). An essential catalytic step for every kinase involves the binding of an ATP molecule in its active site. Here, we report the crystal structures of ATP-bound and ADP-bound forms of the MAP kinase ERK2 and compare them with the apo form of the protein as well as with the structure of cyclin-dependent kinase 2 (CDK2), the closest homolog for which an ATP-bound crystal structure is available (we exclude the catalytically inactive K52R mutant of ERK2 because of its reportedly distorted ligand-binding mode; Robinson et al., 1996 ▶).
To date, 518 putative kinases have been identified in the human genome. By comparing the sequences of their catalytic domains, aided by knowledge of sequence similarity and domain structure outside the catalytic domains, known biological functions and a similar classification of the yeast, worm and fly kinomes, kinases can be classified into different groups, and MAPK and CDK belong to the same CMGC group (Manning et al., 2002 ▶). We therefore verify the prediction of the mode of ATP binding by ERK2. We also briefly discuss the only other previously reported crystal structure of ERK2 in complex with ATP, which used a catalytically inactive K52R mutant (Robinson et al., 1996 ▶), and the implications for enzyme kinetics.
2. Methods and materials
2.1. Protein purification
His6-tagged rat ERK2 was expressed as described previously (Burkhard et al., 2011 ▶). Briefly, cells were grown in LB medium with 100 µg ml−1 ampicillin at 310 K with shaking (250 rev min−1) to an OD600 of 0.8. ERK2 expression was induced with 0.5 mM isopropyl β-d-1-thiogalactopyranoside at 310 K for 4 h. Cells were harvested by centrifugation, resuspended in 300 mM NaCl, 50 mM sodium phosphate pH 7.8, 10% glycerol and lysed by sonication. The crude lysate was then centrifuged at 39 000g for 30 min at 277 K. The cleared lysate was applied onto Co2+-charged TALON Metal Affinity Resins (Clontech) and the column was washed with 20 column volumes of 300 mM NaCl, 50 mM sodium phosphate pH 7.8, 10% glycerol, 10 mM imidazole. ERK2 was then eluted from the column using a gradient of increasing imidazole concentration (50–400 mM). The protein was buffer-exchanged into buffer A [125 mM NaCl, 20 mM Tris–HCl pH 7.6, 10% glycerol, 5 mM β-mercaptoethanol (βME)] using a desalting column and was then concentrated using an Amicon Ultra centrifugal filter with a molecular-mass cutoff of 10 kDa. The concentrated protein was then loaded onto a Mono Q 10/100 GL column that had been equilibrated with buffer A and was eluted with a linear gradient from 125 to 600 mM NaCl. Two ERK2 peaks were eluted from the Mono Q column; protein from the peak which came out of the column first was exchanged into 200 mM NaCl, 50 mM Tris–HCl pH 7.6, 5 mM βME. The eluted protein was concentrated to a concentration of 6 mg ml−1 using an Amicon Ultra centrifugal filter.
2.2. Protein crystallization, data collection and structure determination
Crystallization experiments were conducted using the sitting-drop vapor-diffusion method at 295 K. Initial conditions for ERK2 crystallization were identified using sparse-matrix screens from NeXtal and consisted of 100 mM MES pH 6.5, 5% PEG 400, 2 M ammonium sulfate. The protein crystallized readily and the crystals reached their maximum dimensions of 200 × 100 × 100 µm within five days.
ATP or ADP was soaked into the ERK2 crystals by replacing the mother liquor stepwise with cryoprotectant (100 mM HEPES pH 6.0, 27.5% PEG 3350, 17.5% glycerol) containing 100 mM ATP or ADP, 100 mM MgCl, 2 mM DTT. Data sets were collected at the Stanford Synchrotron Radiation Lightsource and were processed using XDS (Kabsch, 2010a ▶,b ▶) and SCALA (Evans, 2011 ▶) with the automated data-processing script developed by Ana Gonzalez and Yingssu Tsai (autoxds; http://smb.slac.stanford.edu/facilities/software/xds/). Notably, the microbeam was used (SSRL beamline 12-2) for the liganded-form crystals, which produced data sets of similar resolution to that obtained with the beam that matched the size of the crystal and an identical data-collection strategy, yet the microbeam data sets behaved significantly better in refinement, producing lower R free values and a smaller R/R free gap. Structures were solved by molecular replacement in Phaser using apo-form ERK2 as the initial model (PDB entry 1erk; Zhang et al., 1994 ▶). Models were generally refined using iterative cycles of refinement in REFMAC (Murshudov et al., 2011 ▶) and manual model building in Coot (Emsley et al., 2010 ▶). Clearly interpretable electron density was identified in (mF o − DF c) omit maps in the active sites of both the ATP-bound and the ADP-bound forms. These are shown in Fig. 1 ▶; electron density was also found in the apo ERK2 structure, which we interpreted as a polyethylene glycol fragment (see below). At the end of the refinement, likely TLS groups were identified with the help of the TLSMD server (Painter & Merritt, 2006a ▶,b ▶). Up to 20 different TLS groups were subjected to refinement in REFMAC and the optimal number was chosen that resulted in the lowest R free value. Data-collection and refinement statistics are given in Table 1 ▶.
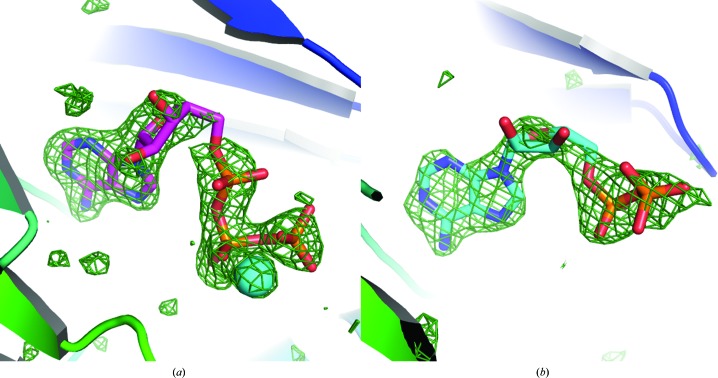
Figure 1.
Omit (mF o −DF c) density maps showing the presence of ATP (a) and ADP (b) in the corresponding ERK2–ligand complex structures.
Table 1. Data-collection and refinement statistics.
| Crystal | Apo ERK2 | ERK2–ATP | ERK2–ADP |
|---|---|---|---|
| PDB code | 4gsb | 4gt3 | 4gva |
| Data collection | |||
| Wavelength | 1.000 | 0.980 | 0.980 |
| Space group | P21 | P21 | P21 |
| Unit-cell parameters (Å, °) | a = 48.8, b = 70.0, c = 59.9, β = 109.0 | a = 48.6, b = 69.3, c = 59.8, β = 109.0 | a = 48.6, b = 69.1, c = 60.0, β = 108.5 |
| Resolution range (Å) | 46.1–1.80 (1.90–1.80) | 38.3–1.68 (1.77–1.68) | 38.4–1.83 (1.93–1.83) |
| Multiplicity | 3.6 (3.6) | 3.5 (3.5) | 3.4 (3.2) |
| Unique reflections | 35077 | 41032 | 32458 |
| R p.i.m. (%) | 2.7 (10.7) | 2.4 (50.5) | 3.4 (58.2) |
| Completeness (%) | 99.3 (99.4) | 96.2 (94.4) | 97.4 (93.3) |
| 〈I/σ(I)〉 | 16.9 (5.9) | 15.3 (1.3) | 12.1 (1.3) |
| Refinement | |||
| R work/R free (%) | 15.4/19.9 | 16.6/20.0 | 18.9/24.1 |
| No. of protein atoms | 2976 | 2930 | 2848 |
| No. of waters | 275 | 202 | 172 |
| No. of heteroatoms | 58 | 58 | 33 |
| R.m.s.d. bonds (Å) | 0.022 | 0.022 | 0.018 |
| R.m.s.d. angles (°) | 2.1 | 2.3 | 2.0 |
| Cruickshank DPI (Å) | 0.12 | 0.10 | 0.14 |
| Average B factors (Å2) | |||
| Protein | 28.5 | 37.9 | 39.3 |
| Waters | 35.5 | 44.8 | 42.9 |
| Ligand | — | 60.6 | 66.4 |
3. Results and discussion
3.1. Comparison with CDK2
According to the structural classification of the human kinases, ERK2 is predicted to belong to the CMGC group that includes cyclin-dependent kinases. To validate that ERK2 binds ATP in a manner consistent with this prediction, we compared the ATP-bound form of the protein with that of CDK2 (Schulze-Gahmen et al., 1996 ▶).
As expected, ATP is bound in the cleft between the N-terminal and C-terminal domains of ERK2. However, there are detectable differences in the way that the two proteins coordinate the ATP cofactor molecule. Specifically, in the ERK2–ATP structure the magnesium ion that is required for binding is coordinated by six O atoms. Two of these ligands are provided by the triphosphate moiety of the ATP and two more by the side chains of Asn152 and Asp165; the last two are water molecules. In the structure of the CDK2–ATP complex the side-chain ligands (Asn132/Asp145) and one water molecule are preserved; however, the three remaining ligands are all supplied by the triphosphate. Interestingly, this replaces both water molecules observed in the ERK2–ATP structure, and the single water ligand in the CDK2–ATP complex occupies the position equivalent to the Oβ of the ATP bound to ERK2 (Fig. 2 ▶).
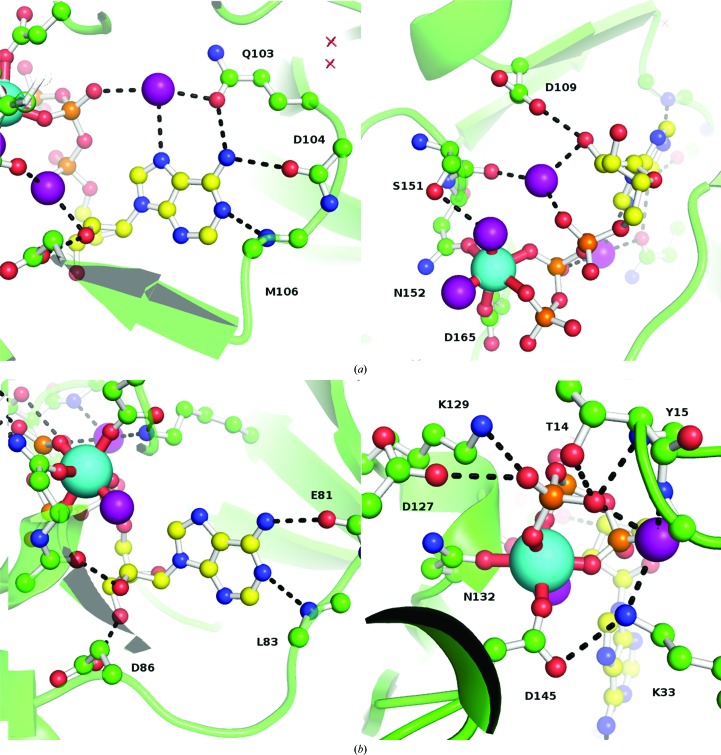
Figure 2.
Details of the protein–ligand interactions in the ERK2–ATP (a) and CDK2–ATP (b) complexes. The cyan spheres show the position of the magnesium ion; purple spheres are water molecules.
These changes in the geometry of the magnesium-binding site result in an overall shift of the ATP molecule in ERK2 away from the magnesium ion by ∼2.2 Å. As a consequence of this shift, the sugar moiety no longer makes a hydrogen bond to the backbone O atom of Ser151 (corresponding to Gln131 in CDK2), while the hydrogen bond to Asp109 is preserved but uses the O2′ oxygen (O3′ in CDK2). An additional water molecule is also observed that forms hydrogen bonds to the O2′ and Oα atoms and the backbone O atom of Ser151 (the latter is also involved in stabilizing one of the waters that coordinate magnesium via a side-chain hydrogen bond).
The adenine moiety forms a pair of hydrogen bonds to the backbone of Asp104/Met106 (Glu81/Leu83 in CDK2). A new interaction is also observed that involves N6 of ATP and O∊ of Gln103, which is further stabilized by a water molecule that links Gln103 and the N7 and Oβ atoms of the ATP molecule. Importantly, the corresponding residue in CDK2 is Phe80, which cannot form the same hydrogen-bonding pattern, thus creating a different environment.
The two proteins are only 36% identical and it is therefore difficult to point out specific mutations as providing a major contribution to the observed differences in ATP coordination. One notable difference is Ser151 (Gln131 in CDK2), which stabilizes one of the magnesium-coordinating waters. Another important difference appears to be Ala33 (Thr14 in CDK2), which may prevent proper coordination of the triphosphate moiety. However, this residue is located in a loop that connects two β-strands in the N-terminal domain and this whole structural element is shifted in ERK2.
Another possibility is that the primary reason for the differences in ATP-binding mode is the shift of the residues that coordinate the adenine moiety. CDK2 is about 6 kDa smaller than ERK2, mostly owing to a much shorter C-terminal sequence. The additional residues in ERK2 form a long loop that connects back to the N-terminal domain and ends with an α-helix. The much shorter C-terminal fragment in CDK2 instead loops towards the ATP-binding site, which may result in a different positioning of the Glu81/Leu83 element, as well as a significant rearrangement of the whole N-terminal domain.
It also has to be emphasized that the ERK2–ATP structure presented here was obtained by soaking and thus the possibility remains that the ATP-binding mode is influenced by the constraints of the crystal lattice. Future cocrystallization studies are needed to determine whether further conformational change may occur upon ATP binding.
3.2. Conformational changes upon ATP binding
Curiously, some electron density was identified in the ATP-binding site of the apo ERK2 crystal structure that overlaps with the position of the adenine moiety. This electron density was interpreted as a polyethylene glycol fragment, possibly coordinated to the protein via hydrogen bonds to the backbone O atoms of Asp104 and Met106. It must be acknowledged that this interpretation is based simply on the presence of polyethylene glycol in the cryoprotecting solution and cannot be unequivocally verified given the available experimental data.
The percentile-based spread (Pozharski, 2010 ▶) between the ERK2 and ERK2–ATP structures is 0.21 and 0.19 Å for all atoms and for backbone atoms, respectively. This is compatible with the expectation based on the Cruickshank DPI as an estimate of the coordinate error (0.12 and 0.10 Å, respectively; hence, a difference in atomic positions of ∼0.16 Å between the two models is expected). Local conformational changes upon ATP binding are minor (including the rotation of some side chains) and are easily explained by the new pattern of hydrogen bonds formed by the ATP and as a result of magnesium coordination.
3.3. Comparison with ADP binding
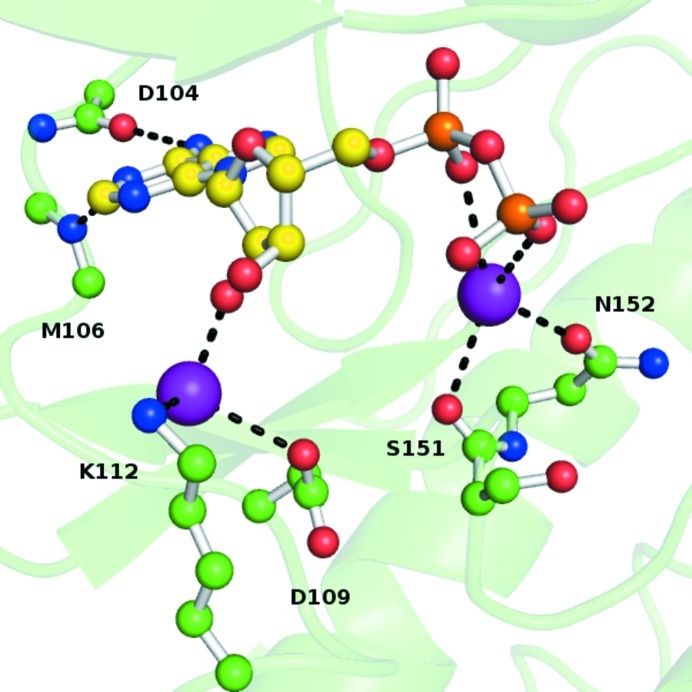
Compared with the ERK2–ATP complex, direct protein–ligand interactions are significantly reduced when ADP binds to ERK2 (Fig. 3 ▶). Magnesium is not present in the crystal structure (despite being present in the solution used for ligand soaking) and the diphosphate moiety of the ADP ligand is coordinated to Ser151/Asn152 indirectly via a water molecule. Similarly, the sugar moiety no longer forms a direct hydrogen bond to Asp109, although it is coordinated by a water molecule that forms hydrogen bonds to it and to Lys112. The adenine ring still forms backbone interactions with Asp104/Met106, but the interactions with Gln103 and the additional water molecule are no longer observed. All of these observations indicate that ADP binds more weakly to the ERK2 kinase, thereby facilitating its dissociation from the protein upon completion of the catalytic cycle.
Figure 3.
Protein–ligand interactions in the ERK2–ADP complex. Purple spheres are water molecules.
3.4. Comparison with the structure of the ATP complex with the K52R ERK2 mutant
The only structure of the ERK2–ATP complex reported previously is that of K52R mutant, which has been shown to have significantly reduced catalytic activity (Robinson et al., 1996 ▶). It has been suggested that this mutation results in significant distortion of the adenine ring of the ATP, affecting the rate of catalysis. The structure was solved at 2.8 Å resolution, which is generally considered to be too low to allow the detection of significant deviations from ideal geometry. As expected, we observed no such distortion with the wild-type protein. Curiously, the conformation of the triphosphate moiety in the published K52R–ATP complex structure resembles what was observed in this work for the ERK2–ADP complex. This suggests that the mechanism by which the K52R mutation affects the rate of catalysis may be related to the conformation of the triphosphate and not the adenine. The percentile-based spread between the two models is 0.48 and 0.42 Å for all atoms and for backbone atoms, respectively. Structural differences between the two models are restricted to the disordered 326–336 loop (which is not involved in ATP binding) and the N-terminal part of the protein.
3.5. β-Mercaptoethanol-mediated crystal contact
The crystal form used in this work can only be obtained in the presence of β-mercaptoethanol. Initial analysis of the apo-form structure revealed that several solvent-exposed cysteines formed disulfide bonds to a β-mercaptoethanol molecule. In order to minimize the potential influence of such modifications on the ERK2 structure, crystals were soaked in mother liquor supplemented with DTT. All but one of the modified cysteines were then reduced. The only cysteine that remained modified upon this treatment is apparently involved in crystal formation and is part of a crystal contact with the protein molecule shifted by one unit cell in the a-axis direction, as shown in Fig. 4 ▶. Notably, this particular crystal form could not be obtained in our experiments in the absence of β-mercaptoethanol, revealing the crucial role played in crystallogenesis by the hydrogen bond formed between the hydroxyl group of the modified cysteine residue and the backbone N atom of Leu256.
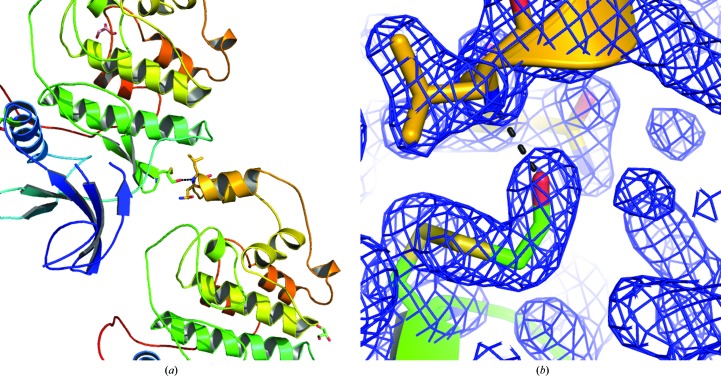
Figure 4.
Cysteine modifications stabilize the monoclinic crystals of ERK2. (a) shows two molecules from adjacent unit cells. (b) The (2F o − F c) electron-density map is shown for the modified Cys159 (green) and the hydrogen bond that it makes to the symmetry-related copy of Leu256 (yellow).
Supplementary Material
PDB reference: ERK2, apo, 4gsb
PDB reference: ATP complex, 4gt3
PDB reference: ADP complex, 4gva
Acknowledgments
This work was supported by NIH R01 CA120215. Portions of this research were carried out at the Stanford Synchrotron Radiation Lightsource, a Directorate of SLAC National Accelerator Laboratory and an Office of Science User Facility operated for the US Department of Energy Office of Science by Stanford University. The SSRL Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research and by the National Institutes of Health, National Institute of General Medical Sciences (including P41GM103393) and the National Center for Research Resources (P41RR001209). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of NIGMS, NCRR or NIH.
References
- Burkhard, K. A., Chen, F. & Shapiro, P. (2011). J. Biol. Chem. 286, 2477–2485. [DOI] [PMC free article] [PubMed]
- Cohen, P. (2002). Nature Rev. Drug Discov. 1, 309–315. [DOI] [PubMed]
- Dibb, N. J., Dilworth, S. M. & Mol, C. D. (2004). Nature Rev. Cancer, 4, 718–727. [DOI] [PubMed]
- Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. (2010). Acta Cryst. D66, 486–501. [DOI] [PMC free article] [PubMed]
- Evans, P. R. (2011). Acta Cryst. D67, 282–292. [DOI] [PMC free article] [PubMed]
- Kabsch, W. (2010a). Acta Cryst. D66, 125–132. [DOI] [PMC free article] [PubMed]
- Kabsch, W. (2010b). Acta Cryst. D66, 133–144. [DOI] [PMC free article] [PubMed]
- Manning, G., Whyte, D. B., Martinez, R., Hunter, T. & Sudarsanam, S. (2002). Science, 298, 1912–1934. [DOI] [PubMed]
- Murshudov, G. N., Skubák, P., Lebedev, A. A., Pannu, N. S., Steiner, R. A., Nicholls, R. A., Winn, M. D., Long, F. & Vagin, A. A. (2011). Acta Cryst. D67, 355–367. [DOI] [PMC free article] [PubMed]
- Painter, J. & Merritt, E. A. (2006a). Acta Cryst. D62, 439–450. [DOI] [PubMed]
- Painter, J. & Merritt, E. A. (2006b). J. Appl. Cryst. 39, 109–111.
- Pozharski, E. (2010). Acta Cryst. D66, 970–978. [DOI] [PubMed]
- Robinson, M. J., Harkins, P. C., Zhang, J., Baer, R., Haycock, J. W., Cobb, M. H. & Goldsmith, E. J. (1996). Biochemistry, 35, 5641–5646. [DOI] [PubMed]
- Sawyers, C. (2004). Nature (London), 432, 294–297. [DOI] [PubMed]
- Schulze-Gahmen, U., De Bondt, H. L. & Kim, S.-H. (1996). J. Med. Chem. 39, 4540–4546. [DOI] [PubMed]
- Zhang, F., Strand, A., Robbins, D., Cobb, M. H. & Goldsmith, E. J. (1994). Nature (London), 367, 704–711. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PDB reference: ERK2, apo, 4gsb
PDB reference: ATP complex, 4gt3
PDB reference: ADP complex, 4gva