Recombinant mevalonate kinase from M. mazei has been crystallized. Diffraction data were collected to 2.08 Å resolution.
Keywords: mevalonate kinase, Methanosarcina mazei, multiple-wavelength anomalous dispersion
Abstract
Mevalonate kinase (MVK), which plays an important role in catalysing the biosynthesis of isoprenoid compounds derived from the mevalonate pathway, transforms mevalonate to 5-phosphomevalonate using ATP as a cofactor. Mevalonate kinase from Methanosarcina mazei (MmMVK) was expressed in Escherichia coli, purified and crystallized for structural analysis. Diffraction-quality crystals of MmMVK were obtained by the vapour-diffusion method using 0.32 M MgCl2, 0.08 M bis-tris pH 5.5, 16%(w/v) PEG 3350. The crystals belonged to space group P21212, with unit-cell parameters a = 97.11, b = 135.92, c = 46.03 Å. Diffraction data were collected to 2.08 Å resolution.
1. Introduction
Isoprenoid compounds are ubiquitous in nature; they include hormones, insect pheromones, pigments, cell membranes, electron carriers and defensive agents and participate in important biological processes (Davis & Croteau, 2000 ▶; Sacchettini & Poulter, 1997 ▶; Liang et al., 2002 ▶). The isoprenoids, which include phytol, retinol and ubiquinone, are derived from a common structural motif: isoprene (2-methyl-1,3-butadiene). In the chemical industry, about 95% of the isoprene produced is used to make cis-1,4-polyisoprene, a synthetic version of natural rubber that is used in a wide variety of applications (Williams, 1859–1860 ▶). Today, isoprene produced by the thermal cracking of naphtha or crude oil is readily available. For environmental and energy-saving reasons, attempts are now being made to produce isoprene using biological processes. The precursors of isoprene units in biological systems are the five-carbon building blocks isopentenyl diphosphate (IPP) and its isomer dimethylallyl diphosphate (DMAPP) (Ogura & Koyama, 1998 ▶). IPP and DMAPP are synthesized in two different ways: the mevalonate (MVA) pathway and the methylerythritol phosphate (MEP) pathway (also called the non-mevalonate pathway) (Kuzuyama, 2002 ▶). The MVA pathway has evolved to provide a pool of the precursors in eukaryotes, archaea, eubacteria, plant cytoplasm and parasites of the genus Trypanosoma and Leishmania (Sgraja et al., 2007 ▶). In contrast, the MEP pathway is mainly utilized in plant chloroplasts and eubacteria (Rohdich et al., 2004 ▶).
Mevalonate kinase (MVK) catalyses the fourth step in the MVA pathway and is involved in the transfer of a phosphoryl group from ATP to mevalonate. Mevalonate kinase belongs to the distinct GHMP superfamily of ATP-dependent enzymes, which includes galactokinase (G), homoserine kinase (H), mevalonate kinase (M) and phosphomevalonate kinase (P) (Bork et al., 1993 ▶; Andreassi et al., 2009 ▶). These enzymes are involved in the biosynthesis of isoprenoids and amino acids as well as in carbohydrate metabolism and share several structural features. The ATP-binding site contains a conserved glycine-rich motif (PX 3GLGSSAA) in the N-terminal section that forms the phosphate-binding loop (Daugherty et al., 2001 ▶; Krell et al., 1998 ▶). As mevalonate kinase is an important regulatory point in the MVA pathway, intricate regulation is essential to ensure the production of isoprenoids in the proper amounts. Bacteria and eukaryotes regulate MVKs by feedback inhibition, which can be divided into two classes (Dorsey & Porter, 1968 ▶). The first class are inhibited by metabolites downstream of the diphosphomevalonate decarboxylase reaction [IPP, DMAPP, geranyl diphosphate (GPP), farnesyl diphosphate (FPP) and longer-chain isoprenoids; Gray & Kekwick, 1972 ▶; Hinson et al., 1997 ▶; Voynova et al., 2004 ▶]. The MVK from the thermostable archaeon Methanococcus jannaschii was also inhibited by IPP, GPP and FPP metabolites (Huang et al., 1999 ▶). The second class of MVKs are inhibited by diphosphomevalonate but not by metabolites downstream of the diphosphomevalonate decarboxylase (Andreassi et al., 2004 ▶). The recently characterized MVK from the archaeon Methanosarcina mazei displays a feedback-resistant regulation profile that differs from those of previously characterized MVKs from both bacteria and eukaryotes (Primak et al., 2011 ▶). Although it has been reported that M. mazei MVK has a much lower specific activity than M. jannaschii MVK, its different regulation profile without metabolite inhibition may be advantageous in optimizing the isoprenoid-biosynthesis pathway.
In this study, the MVK from M. mazei (MmMVK) was expressed in a soluble form in Escherichia coli, purified and crystallized for structural analysis. Structure determination of MmMVK may provide clues for elucidation of the specific catalytic activity related to its regulation mechanism. It also offers an opportunity for the design of novel enzymes of the GHMP superfamily for activity enhancement.
2. Experimental
2.1. Protein expression and purification
MmMVK (residues 1–301) was amplified from M. mazei genomic DNA by PCR with forward primer 5′-GCG CAT ATG GTT TCA TGT TCT GCG CCC-3′ and reverse primer 5′-GCG GGA TCC TCA ATC GAC CTT CAA CCC-3′ containing NdeI and BamHI restriction sites (bold), respectively. The resulting purified 900 bp PCR product was ligated into the bacterial expression vector pET-28a (Novagen, Madison, Wisconsin, USA) to produce recombinant MmMVK protein with a hexahistidine tag and thrombin cleavage site at the N-terminus (MGSSHHHHHHSSGLVPRGSH). The MmMVK cloned in the vector was confirmed by DNA sequencing. The calculated molecular mass of the tagged monomer was about 33.6 kDa and the theoretical isoelectric point was 6.6.
E. coli strain BL21 (DE3) transformed with the vector carrying MmMVK was used for protein expression. A 10 ml aliquot of an overnight culture in Luria–Bertani (LB) medium was seeded into 1000 ml fresh M9 minimal medium containing 30 µg ml−1 kanamycin and was grown to an OD600 of 0.6 at 310 K. 40 µg ml−1 selenomethionine (Acros Organics, New Jersey, USA) was added together with 17 other solid l-amino acids (BioShop Canada Inc., Burlington, Canada). The protein was expressed at 303 K for 1 d with 0.4 mM isopropyl β-d-1-thiogalactopyranoside (IPTG). Cells were harvested by centrifugation (6000 rev min−1, 6 min, 277 K), suspended in binding buffer (50 mM sodium phosphate pH 8.0, 300 mM NaCl, 5 mM β-mercaptoethanol, 1 mM MgCl2) and disrupted by sonication. After centrifugation (12 000 rev min−1, 1 h) at 277 K, the clear supernatant was filtered (qualitative filter paper, Advantec, Japan) and loaded onto an open column of nickel–NTA beads (Qiagen, Hilden, Germany) pre-equilibrated with binding buffer. The column was first washed with ten column volumes of binding buffer and then with 20 column volumes of washing buffer (50 mM sodium phosphate pH 8.0, 300 mM NaCl, 30 mM imidazole, 5 mM β-mercaptoethanol, 1 mM MgCl2). The recombinant MmMVK was eluted with elution buffer (50 mM Tris–HCl pH 8.0, 100 mM NaCl, 300 mM imidazole, 5 mM β-mercaptoethanol, 1 mM MgCl2). The fractions were examined by SDS–PAGE. Fractions containing MmMVK were pooled, concentrated and exchanged into 50 mM Tris–HCl pH 8.5, 1 mM DTT, 1 mM MgCl2 by ultrafiltration with a Corning Spin-X UF concentrator with 30K molecular-weight cutoff (Corning Inc., New York, USA). The MmMVK was further purified by anion-exchange chromatography on a Resource 15Q column (GE Healthcare, Piscataway, New Jersey, USA) with a linear gradient of 0–300 mM NaCl in 50 mM Tris–HCl pH 8.5, 1 mM DTT, 1 mM MgCl2. The eluted fractions were examined by SDS–PAGE and the fractions containing MmMVK were pooled and concentrated by ultrafiltration using a Corning Spin-X UF concentrator with 30K molecular-weight cutoff (Corning Inc.). The MmMVK was finally purified by size-exclusion chromatography using a Superdex 200 column (GE Healthcare) in 20 mM Tris–HCl pH 8.0, 150 mM NaCl, 1 mM DTT, 1 mM MgCl2. The MmMVK proteins separated by gel-filtration chromatography were concentrated to 10 mg ml−1 in 20 mM Tris pH 8.0, 1 mM DTT, 1 mM MgCl2 for crystallization by ultrafiltration with the Corning Spin-X UF concentrator (Corning Inc.). Protein purity was examined by SDS–PAGE and native PAGE. All purification steps were carried out at 293 K with ice-cooled buffers, which we believe help to stabilize the sample. The protein concentration was determined by Bradford assay (Bradford, 1976 ▶). The N-terminal His tag was not removed for crystallization.
2.2. Crystallization
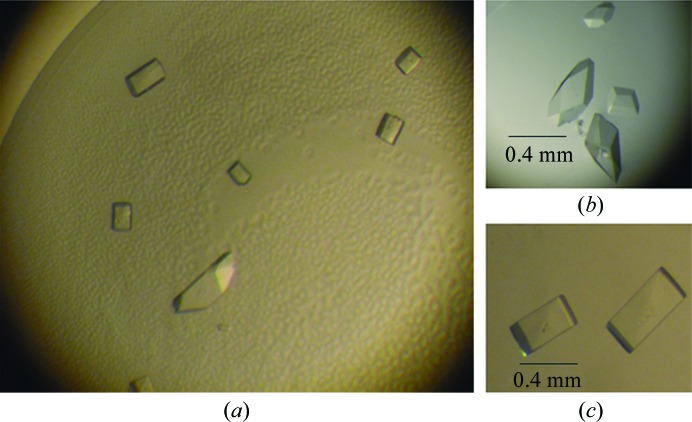
Crystallization of MmMVK was initially carried out with Crystal Screen, Crystal Screen 2, Index (Hampton Research, California, USA), Wizard Screens I and II, Cryo Screens I and II (Emerald BioStructures, Bainbridge Island, Washington, USA) and laboratory-made solutions using a microbatch crystallization method at 291 K. Drops consisting of equal volumes (1 µl) of protein solution (10 mg ml−1 in 20 mM Tris–HCl pH 8.0, 1 mM DTT, 1 mM MgCl2; buffer A) and screening solution were equilibrated under Al’s oil in a 72-well microbatch plate (D’Arcy et al., 2003 ▶). Crystals were initially produced in Index solution No. 82. Further screening to find optimal crystallization conditions was performed by hanging-drop vapour-diffusion trials, varying the salt and precipitant concentrations and the volume of the drop. The best crystals were grown at 291 K using 10 mg ml−1 MmMVK protein in buffer A and reservoir solution consisting of 0.32 M MgCl2, 0.08 M bis-tris pH 5.5, 16%(w/v) PEG 3350. The drop consisted of 3 µl protein solution and 3 µl reservoir solution. Orthogonal-shaped and rhombohedral-shaped crystals appeared after two weeks and grew to maximum dimensions of 0.4 × 0.3 × 0.1 and 0.4 × 0.3 × 0.2 mm, respectively (Fig. 1 ▶).
Figure 1.
Crystals of MmMVK obtained by the hanging-drop vapour-diffusion method. (a) Both orthogonal-shaped crystals and rhombohedral-shaped crystals formed in the same condition. (b) The rhombohedral-shaped crystals were finally used for data collection. (c) The orthogonal-shaped crystals in cryosolution were easily damaged during freezing and could not be used for data collection.
2.3. X-ray data collection and processing
Before data collection, crystals were quickly transferred into a cryosolution consisting of 0.32 M MgCl2, 0.08 M bis-tris pH 5.5, 17%(w/v) PEG 3350, 10% glycerol for less than 10 s. Only the rhombohedral-shaped crystals were stable during the quick soaking; the orthogonal-shaped crystals were always damaged in cryosolutions supplemented with different cryoprotectants such as glycerol, ethylene glycol, PEG 400, glucose and MPD. The rhombohedral-shaped crystals were flash-cooled in liquid nitrogen. X-ray diffraction data were collected on beamline 5C at the Pohang Accelerator Laboratory, Republic of Korea using an ADSC Quantum 210 CCD detector. For multiple-wavelength anomalous dispersion (MAD), three data sets were collected at wavelengths of 0.97947 Å (peak), 0.97954 Å (edge) and 0.97179 Å (remote) at 100 K. 180 images of data were collected with an oscillation angle of 1° and an exposure time of 1 s at each wavelength. All diffraction images were indexed, integrated and scaled using the HKL-2000 suite (Otwinowski & Minor, 1997 ▶). Data statistics are shown in Table 1 ▶. The intensities were converted to structure-factor amplitudes using TRUNCATE from the CCP4 suite (French & Wilson, 1978 ▶; Winn et al., 2011 ▶).
Table 1. Data statistics for the SeMet derivative.
Values in parentheses are for the highest resolution shell.
| Peak | Edge | Remote | |
|---|---|---|---|
| Space group | P21212 | ||
| Unit-cell parameters (Å) | a = 97.11, b = 135.92, c = 46.03 | ||
| No. of chains in the asymmetric unit | 2 | ||
| V M (Å3 Da−1) | 2.24 | ||
| Solvent content (%) | 45.19 | ||
| Wavelength (Å) | 0.97947 | 0.97954 | 0.97179 |
| Resolution (Å) | 50–2.08 (2.12–2.08) | 50–2.12 (2.15–2.12) | 50–2.23 (2.28–2.23) |
| Unique reflections | 37136 (1420) | 36224 (1843) | 30700 (1889) |
| Completeness (%) | 98.5 (76.8) | 99.9 (100) | 99.7 (100) |
| R merge † (%) | 8.5 (63.3) | 7.7 (82.1) | 7.4 (76.4) |
| Multiplicity | 7.1 (6.2) | 7.2 (6.9) | 7.2 (7.3) |
| 〈I/σ(I)〉 | 13.7 | 12.4 | 10.8 |
R
merge = 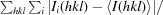
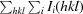 , where 〈I(hkl)〉 is the average intensity of the ith observations.
, where 〈I(hkl)〉 is the average intensity of the ith observations.
3. Results
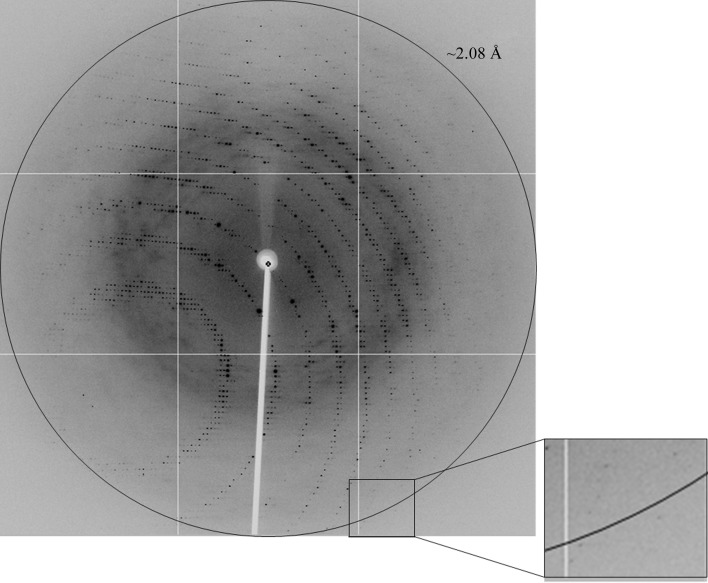
MmMVK (residues 1–301) was expressed in E. coli and sufficiently purified for crystallization. The apparent mass of the protein was estimated to be about 33 kDa from SDS–PAGE, which is similar to the theoretical molecular mass of 33.6 kDa including the histidine tag. On gel-filtration chromatography the protein separated with an effective mass of 64.6 kDa, suggesting that the protein exists as a dimer in solution, which is consistent with previously reported results (Primak et al., 2011 ▶). We collected a 2.08 Å resolution MAD data set (Fig. 2 ▶) at three wavelengths, which was processed in the primitive orthorhombic space group P21212, with unit-cell parameters a = 97.11, b = 135.92, c = 46.03 Å (Table 1 ▶). The asymmetric unit contained two MmMVK molecules with a Matthews coefficient V M of 2.24 Å3 Da−1 and a solvent content of 45.19% (Matthews, 1968 ▶). A detailed discussion of the structure determination will be published elsewhere.
Figure 2.
A diffraction image of MmMVK. The circle corresponds to 2.08 Å resolution. The inset represents a magnified view of the area indicated by the square.
Acknowledgments
We thank the staff at beamline 5C of the Pohang Accelerator Laboratory, Republic of Korea, for their technical assistance and beamline support. This work was supported by Korea National Research Foundation/MEST grant Nos. NRF-2010-C1AAA001-0029084 (KHL) and 20120001112. NZ was supported by the BK21 program.
References
- Andreassi, J. L. II, Dabovic, K. & Leyh, T. S. (2004). Biochemistry, 43, 16461–16466. [DOI] [PubMed]
- Andreassi, J. L. II, Vetting, M. W., Bilder, P. W., Roderick, S. L. & Leyh, T. S. (2009). Biochemistry, 48, 6461–6468. [DOI] [PMC free article] [PubMed]
- Bork, P., Sander, C. & Valencia, A. (1993). Protein Sci. 2, 31–40. [DOI] [PMC free article] [PubMed]
- Bradford, M. M. (1976). Anal. Biochem. 72, 248–254. [DOI] [PubMed]
- D’Arcy, A., Mac Sweeney, A., Stihle, M. & Haber, A. (2003). Acta Cryst. D59, 396–399. [DOI] [PubMed]
- Daugherty, M., Vonstein, V., Overbeek, R. & Osterman, A. (2001). J. Bacteriol. 183, 292–300. [DOI] [PMC free article] [PubMed]
- Davis, E. M. & Croteau, R. (2000). Top. Curr. Chem. 209, 53–95.
- Dorsey, J. K. & Porter, J. W. (1968). J. Biol. Chem. 243, 4667–4670. [PubMed]
- French, S. & Wilson, K. (1978). Acta Cryst. A34, 517–525.
- Gray, J. C. & Kekwick, R. G. (1972). Biochim. Biophys. Acta, 279, 290–296. [DOI] [PubMed]
- Hinson, D. D., Chambliss, K. L., Toth, M. J., Tanaka, R. D. & Gibson, K. M. (1997). J. Lipid Res. 38, 2216–2223. [PubMed]
- Huang, K.-X., Scott, A. I. & Bennett, G. N. (1999). Protein Expr. Purif. 17, 33–40. [DOI] [PubMed]
- Krell, T., Coggins, J. R. & Lapthorn, A. J. (1998). J. Mol. Biol. 278, 983–997. [DOI] [PubMed]
- Kuzuyama, T. (2002). Biosci. Biotechnol. Biochem. 66, 1619–1627. [DOI] [PubMed]
- Liang, P.-H., Ko, T.-P. & Wang, A. H.-J. (2002). Eur. J. Biochem. 269, 3339–3354. [DOI] [PubMed]
- Matthews, B. W. (1968). J. Mol. Biol. 33, 491–497. [DOI] [PubMed]
- Ogura, K. & Koyama, T. (1998). Chem. Rev. 98, 1263–1276. [DOI] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol. 276, 307–326. [DOI] [PubMed]
- Primak, Y. A., Du, M., Miller, M. C., Wells, D. H., Nielsen, A. T., Weyler, W. & Beck, Z. Q. (2011). Appl. Environ. Microbiol. 77, 7772–7778. [DOI] [PMC free article] [PubMed]
- Rohdich, F., Bacher, A. & Eisenreich, W. (2004). Bioorg. Chem. 32, 292–308. [DOI] [PubMed]
- Sacchettini, J. C. & Poulter, C. D. (1997). Science, 277, 1788–1789. [DOI] [PubMed]
- Sgraja, T., Smith, T. K. & Hunter, W. N. (2007). BMC Struct. Biol. 7, 20. [DOI] [PMC free article] [PubMed]
- Voynova, N. E., Rios, S. E. & Miziorko, H. M. (2004). J. Bacteriol. 186, 61–67. [DOI] [PMC free article] [PubMed]
- Williams, C. G. (1859–1860). Proc. R. Soc. Lond. 10, 516–519.
- Winn, M. D. et al. (2011). Acta Cryst. D67, 235–242.