Abstract
Quantifying pain through assay of a human’s or animal’s response to a known stimulus as a function of time of day is a critical means of advancing chronotherapeutic pain management. Current methods for quantifying pain, even in the context of etiologies involving deep tissue, generally involve stimulation by quantifiable means of either cutaneous (heat-lamp tests, electrical stimuli) or both cutaneous and subcutaneous tissue (von Frey hairs, tourniquets, etc.) or study of proxies for pain (such as stress, via assay of cortisol levels). In this study, we evaluate the usefulness of intense Focused Ultrasound (iFU), already shown to generate sensations and other biological effects deep to the skin, as a means of quantifying deep diurnal pain using a standard animal model of inflammation. Beginning five days after injection of Complete Freund’s Adjuvant into the plantar surface of the rat’s right hind paw to induce inflammation, the rats were divided into two groups, the light-phase test group (09:00h–18:00h) and the dark-phase test group (23:00h–06:00h), both of which underwent iFU application deep to the skin. We used two classes of iFU protocol, motivated by the extant literature. One consisted of a single pulse (SP) lasting 0.375 seconds. The other, a multiple pulse (MP) protocol, consisted of multiple iFU pulses each of length 0.075s spaced 0.075s apart. We found the night group’s threshold for reliable paw withdrawal to be significantly higher than that of the day group as assayed by each iFU protocol. These results are consistent with the observation that the response to mechanical stimuli by humans and rodents display diurnal variations, as well as the ability of iFU to generate sensations via mechanical stimulation. Since iFU can provide a consistent method to quantify pain from deep, inflamed tissue, it may represent a useful adjunct to those studying diurnal pain associated with deep tissue as well as chronotherapeutics targeting that pain.
Keywords: inflammatory pain, diurnal, intense focused ultrasound, rat
INTRODUCTION
Many symptoms of inflammation-based pain diseases such as rheumatoid arthritis and fibromyalgia exhibit circadian rhythms (Spies et al., 2010; McLean et al., 2005; Bellamy et al., 2004). As such, the diurnal variation of pain has received much scrutiny, through direct study of humans as well as via animal studies (human studies: Bachmann et al., 2011; Bruguerolle and Labrecque, 2007; Bellamy et al., 2004; Strian, 1989; animal studies: Millecamps et al., 2005; Perissin et al., 2004; Nagakura et al., 2003, Perissin et al., 2003, Crockett et al 1977; Frederickson et al 1977; Konecka and Sroczynska 1998, Christina et al 2004). For example, numerous studies of the hypothalamic-pituitary-adrenal (HPA) axis have shown that many painful diseases exhibit circadian rhythms due to contributions from chronic stress, leading to a particularly active area of research in this field (McBeth et al., 2005; Bomholt et al., 2004; Sarlis, 1992). With an appreciation of the existence of diurnal pain rhythms comes the motivation for chronotherapeutic approaches to the treatment of pain, with particular attention paid to maximizing drug effects by administration at optimal times of day (Junker and Wirz, 2010; Boom et al., 2010; Karakucuk et al., 2006; Levi et al., 1985; Kowanko et al., 1981).
Current methods for quantifying pain in research, even in the context of etiologies involving deep tissue, generally involve application of a quantifiable source of stimulation of to cutaneous (heat-lamp tests) or to both cutaneous and subcutaneous tissue (von Frey hairs, tourniquets, etc.) or, by monitoring proxies for pain such as stress (Bruguerolle and Labrecque, 2007, Mellor et al., 2000). However, these methods are often inaccurate or incomplete tests for pain originating in deep tissue because they either do not specifically quantify the pain source of interest, or because they are only surrogate measures of pain.
Quantifying pain is also of clinical importance, and new techniques for this have recently emerged. These include biochemical sampling at trigger points to examine —near real-time concentrations of inflammatory markers and pH as compared with normal muscle tissue, as well as making use of magnetic resonance elastography and sonography to quantify variations in tissue stiffness as it relates to deep pain (reviewed in Basford and An, 2009).
Previous studies assessing diurnal pain variation in rats and mice through physical tests have utilized hot-plate or light tests (Crockett et al 1977; Frederickson et al 1977; Kubynin and Ignatov 1995, Konecka and Sroczynska 1998, Christina et al 2004) as well as mechanical compression and electrical stimulation of the base of a rat’s tail (for example, Kubynin and Ignatov, 1995). These approaches provide a useful metric for general pain measurement, but are non-specific and have little relevance for clinical usage. Moreover, there is little consensus in the literature about the specific trend observed in rodents regarding timing of highest and lowest sensitivity to stimulation. For example, some studies whose focus is heat stimulation have found that shortest latency times occur during the light-phase (Frederickson et al 1977; Crockett et al. 1977) and others during the dark-phase (Christina et al 2004).
With these challenges of experimental design and clinical practice in mind, we sought here to take the first steps towards testing the potential usefulness of intense focused ultrasound (iFU) as a quantifiable source of stimulation, capable of reliably interacting with deep, painful tissue without stimulating adjacent tissues.
iFU has already been shown to stimulate deep tissue, focusing its ultrasonic energy in a manner consistent with mechanically-based stimulation of tissue within a spot approximately the size and aspect of a grain of rice, with its focus at a prescribed depth below the surface of the skin (Dalecki, 2004; Wright et al., 2002; Davies et al., 1996; Gavrilov et al, 1996; Gavrilov et al., 1977). (Used with greater power, high intensity focused ultrasound – HIFU – can destroy deep tissue such as tumors without affecting intervening tissue — through a combination of heat (Ward, 2011; Orsi et al., 2010; Kennedy et al, 2003) and cavitation, by Hynynen, 1991, who also established the threshold for HIFU-induction of cavitation in vivo). Because the output of the transducer can be characterized through standard means one can quantify the amount of iFU delivered to the tissue of interest (Sutton et al., 2006; Hill et al., 1994). In this way, iFU may be used to quantify thresholds for stimulation of deep tissue in anatomically specific way, giving iFU potential applicability for both researchers and clinicians.
While already shown to stimulate deep, healthy tissue, iFU has not been used to differentially stimulate inflamed tissue, nor has that stimulation been shown to vary in a diurnal way. We demonstrate both, here. Specifically, we have tested the hypothesis that stimulation generated by each of a single acoustic pulse as well as a series of iFU pulses preferentially stimulates inflamed tissue relative to contralateral tissue in an animal model of inflamatory pain, and that the amount of iFU necessary to stimulate inflamed tissue exhibits a diurnal pattern. As such, our results suggest that researchers could use iFU as a way to quantify diurnal pain patterns from deep tissue by providing a consistent method by which researchers can accurately and objectively stimulate deep inflamed tissue.
MATERIALS AND METHODS
Animals
All animal procedures were approved by the Institutional Animal Care and Use Committees (IACUC) of both the University of Washington and the Veterans Administration of Puget Sound as well as conformed to relevant national guidelines.
Adult male Fischer rats (Charles River) weighing approximately 180g were housed 3 per cage under housing conditions of 12h light:12h dark (light on at 06:00h and light off at 18:00h) and temperature of 20–22 °C. Animals were kept at this standardized light/dark regimen for at least one week to establish synchronization. The animals had free access to food and water.
Animal Model of Peripheral Inflammatory Pain
Thirty-two adult male Fischer rats (approximately 180 g, Charles River) were used for the study. The rats were deeply anesthetized with a 5% isoflurane (Pitman-Moore, Mundelein, IL) and oxygen mixture via nose cone for induction and 2% isoflurane for maintenance of the anesthetic plane. Inflammation was induced using methods adapted from Nagakura et al. 0.2 ml of Complete Freund’s Adjuvant (CFA, Sigma Aldrich) was injected subcutaneously over 45 seconds into the plantar surface of the right hind paw at the base of the toes using a 25 g 5/8″ needle. This produced significant inflammation throughout the right hind paw — from skin to periostium — relative to the left (Nagakura et al., 2003).
Ultrasound Devices and Acoustic Protocols
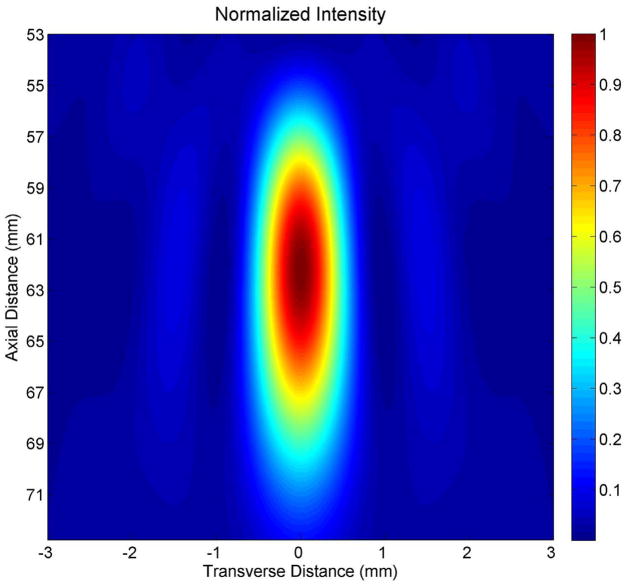
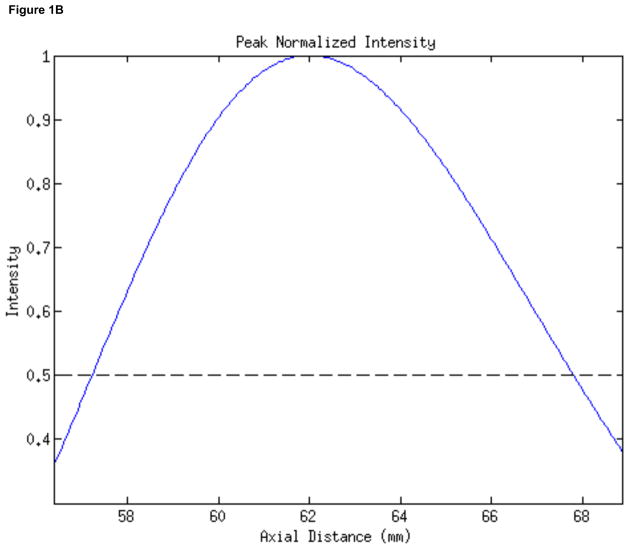
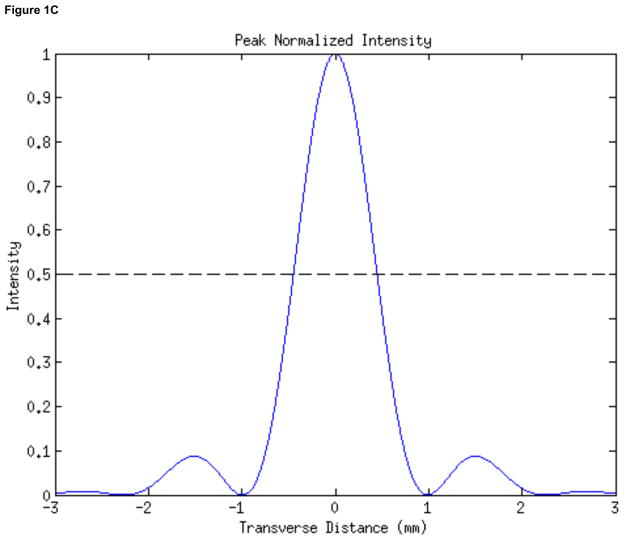
In order to apply ultrasound for stimulation we used the inner element (22.6 mm inner diameter, 48.5 mm outer diameter) of a two-element, 2 MHz annular array transducer (H-106 S/N-01, Sonic Concepts, Inc., Woodinville, WA), placed within a brass housing that facilitated hand-held deployment of the device. The radius of curvature of the device measured 62.6 mm. We quantified the focus of the device (Figure 1) using numerical simulations (Kossoff, 1979; O’Neil, 1949, using MATLAB, The Mathworks, Inc, Natick, MA), appropriate at the relatively high intensities given off by our transducer (Sutton et al., 2006; Hill et al., 1994). The transducer had its focus at 7.0mm beyond the proximal surface of the device, with less than twenty percent of the intensity of the ultrasound at the focus found at that surface.
Figure 1. Characterization of the stimulating ultrasound iFU field.
(A) Two-dimensional mathematical simulation of the intensity field of our iFU transducer as a function of the transverse distance across the transducer face and the axial distance from the transducer, through the proximal surface of the cone (at 55 mm) to the focus of the transducer (at 62 mm) and beyond. (B) Plot of intensity versus axial distance from the proximal face of the ultrasound delivery system, calculated along the center of the ultrasound field. (C) Plot of calculated intensity distribution at the focus as a function of the transverse distance.
The transducer was driven by two function generators (33120A, Hewlett Packard/Agilent, Palo Alto, CA) and an amplifier (A150 RF Power Amplifier, ENI, Chesnut Ridge, NY). The first generator gated the pulse to a specific duration. The second generator, in series with the first, modified the acoustic output and ensured that the pulse was emitted at a specific frequency. The amplifier increased the signal from the function generators and sent it to the solid cone device. An oscilloscope (Wave Runner LT 322, LeCroy, Chesnut Ridge, NY) measured the duration of the pulse, its carrier frequency and the voltage delivered to the iFU device by the amplifier during each experiment. This voltage was correlated to acoustic intensity emitted by the iFU device via a force balance’ technique (Sutton et al., 2006; Hill et al., 1994). In particular, the displacement of a scale produced by ultrasound energy emitted by the device, along with mathematical calculations of the spatial distribution of ultrasound energy (the half-maximum-pressure contour – Figure 1), is translated mathematically into a measure of intensity (ISATA). Specifically, ISATA is the spatially and temporally averaged intensity over the area enclosed by the half-pressure-maximum contour in the focal plane, a standard measure of ultrasound intensity. We have also measured the peak positive and peak negative pressures associated with representative intensity values (Table 1) using a calibrated hydrophone (Onda, Sunnyvale CA), using linear extrapolation at large values of pressure and intensity.
Table 1.
Relationship between intensity, peak positive pressure and peak negative pressure.
| Intensity (W/cm2) | Peak positive pressure (MPa) | Peak negative pressure (MPa) |
|---|---|---|
| 250 | 0.96 | −0.85 |
| 500 | 1.39 | −1.18 |
| 750 | 1.76 | −1.45 |
| 1000 | 2.06 | −1.66 |
| 1250 | 2.31 | −1.80 |
| 1500* | 2.61 | −1.97 |
| 2000* | 3.27 | −2.32 |
| 2500* | 3.87 | −2.67 |
| 3000* | 4.47 | −3.02 |
extrapolated values
The paw withdrawal data were collected for two acoustic protocols. One protocol consisted of multiple pulses (MP) made up of five 0.075-second pulses spaced by 0.075 seconds, similar to those previously used by other researchers (Dalecki, 2004; Wright et al., 2002). The other acoustic protocol consisted of a single pulse (SP) with length of 0.375 seconds, motivated by protocols explored by Gavrilov, Wright, Dalecki and colleagues (Wright et al., 2002; Dalecki et al., 1995; Davies et al., 1996; Gavrilov et al., 1996; Wright et al., 1993; Wright and Davies, 1989).
iFU Application to Rats
Beginning five days after CFA injection, the rats were divided into two groups, the light-phase group and the dark-phase group. The testing was done in the time between 10:00h and 16:00h for the light-phase group and between 23:00h and 04:00h for the dark-phase group.
We habituated sets of three rats to their free-ranging presence within individual cages containing three separate enclosures, each with a mesh bottom whose individual holes were large enough to allow the researcher to pass through the proximal tip of the ultrasound device to the bottom of the rat’s feet (Figure 2). We also habituated the rats to the touch, through the mesh, of the iFU transducer and acoustic gel (Aquasonic 100 Ultrasound Transmission gel, Parker Laboratories Inc.) to the plantar aspect of the rat’s paw (again Figure 2). After habituation we again placed the proximal surface of the device up through the holes within the bottom of the mesh cage until that proximal surface touched the bottom of one of the rat’s hind paws, using ultrasound gel to ensure adequate coupling. (Note that if during the iFU test procedure a rat began to withdraw its paw in response to contact with the device plus gel but without iFU application, it was re-habituated to the touch of the device plus gel before re-starting iFU threshold testing.) This time we applied one of our MP or SP acoustic protocols to the plantar surface of the paw. During and immediately after iFU application we looked for an immediate and rapid withdrawal of the stimulated hind paw, before returning to the other hind paw in each of the three rats after a minimum of 30 seconds. In the absence of a hind paw withdrawal response the intensity of ultrasound was increased in increments beginning at 30% and tapering to 10% increase as intensity increased, starting with an initial acoustic intensity of approximately 50 W/cm2. Rats showing only one out of two withdrawal responses to a given level of iFU stimulation at a given power by a given paw were considered negative tests and the intensity was increased as described until the minimum iFU threshold intensity at a given duration was achieved that induced two consecutive withdrawal responses from a given paw. If rats withdrew both hind paws to a given iFU intensity the acoustic intensity was decreased and iFU was re-applied until only one paw withdrawal response was observed twice after each of two consecutive applications of iFU to that paw or we determined that we could not identify a single sensitive paw. In our experience this entire experimental procedure required one to two hours of effort.
Figure 2. Photograph of our experimental design.
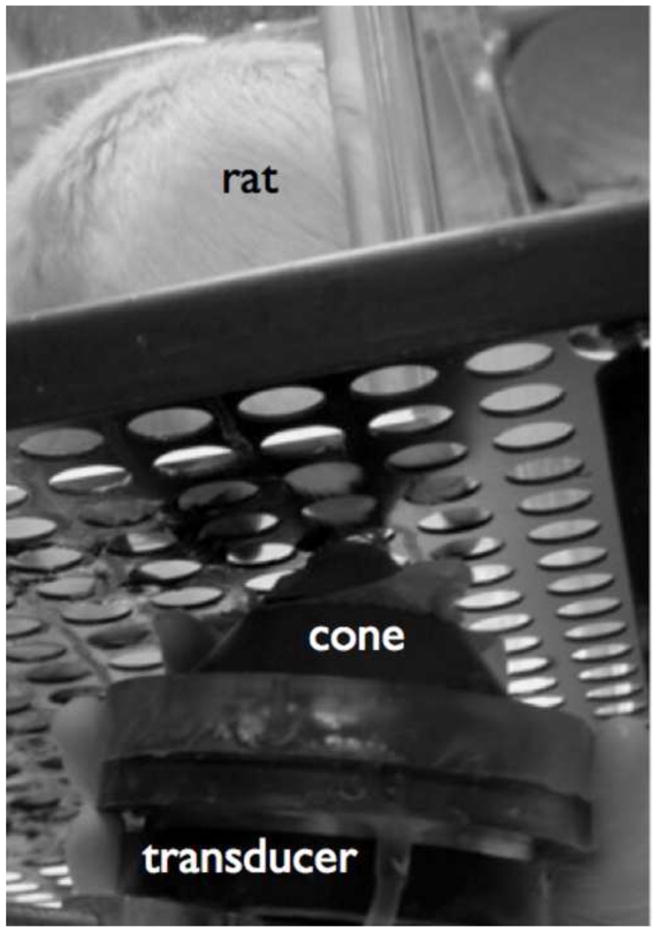
Here a rat resides within in a Plexiglas box on top of a perforated aluminum plate. The holes in the plate allowed us to deliver ultrasound to the plantar aspect of the rat’s paws in a serial fashion, using our iFU device. That device consists of a transducer – characterized in Figure 1, covered by a water-filled cone to facilitate propagation of the ultrasound from the transducer into the rat’s paw, as well as to aid in the aiming of the iFU into the paw.
The intensity and dose of iFU that caused two consecutive withdrawals on the same paw for a given rat was defined as the iFU threshold value for that rat on that day. iFU was then applied six more times at that same intensity to facilitate calculations of sensitivity and specificity. We applied the MP and SP protocols to each rat on separate testing days. No rat received an entire iFU test more than once per day, with at least 1 day between each test. After testing, rats were returned to their cages and then returned to the animal housing facility.
Data Analysis
Data was entered into an Excel (Microsoft, Redmond, WA) spreadsheet where the intensity and acoustic dose at each acoustic protocol were calculated, reported as aggregates in terms of means ± standard deviation. The acoustic dose was defined as the time duration of iFU application multiplied by the intensity. Differences in intensity and acoustic dose between groups were evaluated by analyses of variance with the Student’s t-test used for appropriate post-hoc comparisons (GB Stat; Dynamic Microsystems; Silver Springs, Maryland). Differences between two groups of data are reported as statistically significant if the p-value is smaller than the significance level of 0.05.
The sensitivity and specificity of iFU application to the paws were calculated using data from the first two consecutive withdrawals that identified a given iFU threshold value as well as the subsequent six iFU applications of that same iFU threshold value to each of the rat’s rear paws. Sensitivity was defined as the number of withdrawal responses on the injured paw to the threshold intensity divided by the total applications to that paw at that intensity. Similarly, the specificity was defined as the number of applications of iFU at the threshold intensity to the uninjured paw that elicited no response divided by the total number of applications to that paw.
RESULTS
Diurnal variation of iFU thresholds
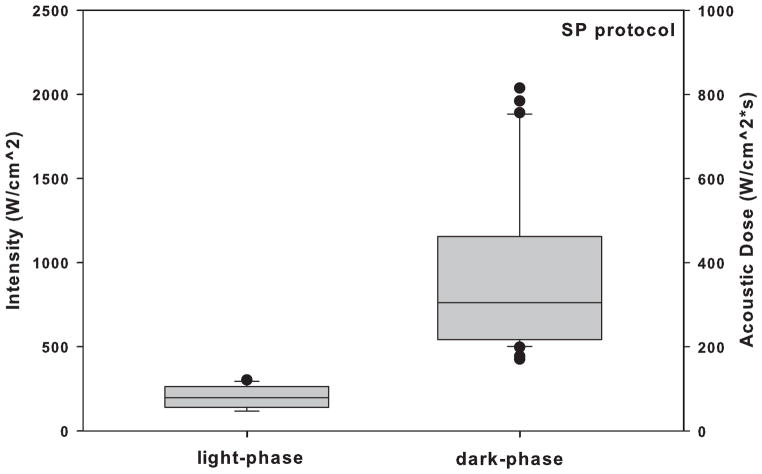
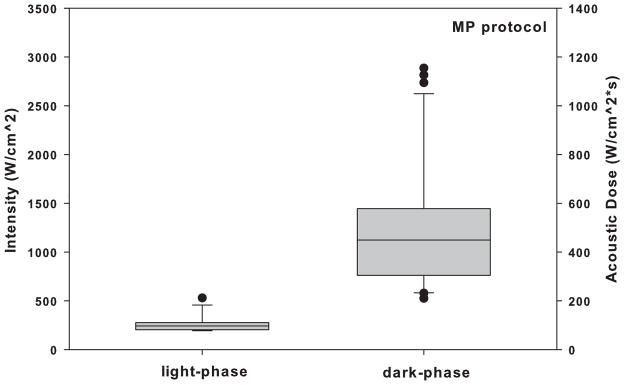
Twenty-three rats were tested during the dark-phase and nine rats were tested during the light-phase, in order to manage the observed larger variance in iFU thresholds we observed for the dark-phase group in a manner consistent with Fitts (2010). iFU thresholds values of intensity and acoustic dose for both SP and MP protocols vary significantly (P < 0.05) between day and night (Fig. 3 and Fig. 4). Specifically, the thresholds of intensity and acoustic dose during the dark-phase for both SP and MP protocols are significantly higher than those during the light-phase (Table 2 and 3, respectively).
Figure 3.
Comparison of the intensity and acoustic dose of iFU necessary to produce a reliable withdrawal response in animals using the single pulse (SP) protocol, for the dark-phase group of rats versus the light-phase group of rats.
Figure 4.
Comparison of the intensity and acoustic dose of iFU necessary to produce a reliable withdrawal response in animals using the multiple pulse (MP) protocol, for the dark-phase group of rats versus the light-phase group of rats.
Table 2.
MEAN VALUES (μ) WITH STANDARD DEVIATION (S.D) FOR THE INTENSITY AND ACOUSTIC DOSE iFU THRESHOLDS OF LIGHT-PHASE AND DARK-PHASE COHORTS: SP PROTOCOL. The number of data points (n) included and the P values for the student’s t test for differences between the light-phase data and the dark-phase data are also given.
| Light-phase (μ ± S.D.) | n | Dark-phase (μ ± S.D.) | n | P | Significance | |
|---|---|---|---|---|---|---|
| Intensity | 216.0 ± 75 | 12 | 1017 ± 520 | 31 | 2.5E-06 | Yes |
| Acoustic dose | 80.99 ± 28 | 12 | 381.4 ± 196 | 31 | 2.5E-06 | Yes |
Table 3.
MEAN VALUES (μ) WITH STANDARD DEVIATION (S.D) FOR THE INTENSITY AND ACOUSTIC DOSE iFU THRESHOLDS OF LIGHT-PHASE AND DARK-PHASE COHORTS: MP PROTOCOL. The number of data points (n) included and the P values for the student’s t test for differences between the light-phase data and the dark-phase data are also given.
| Light-phase (μ ± S.D.) | n | Dark-phase (μ ± S.D.) | n | P | Significance | |
|---|---|---|---|---|---|---|
| Intensity | 280.6 ± 96 | 12 | 1355 ± 720 | 31 | 3.6E-06 | Yes |
| Acoustic dose | 105.2 ± 36 | 12 | 508.1 ± 270 | 31 | 3.6E-06 | Yes |
All rats withdrew their inflamed paw first after sufficient application of iFU, thereby defining the iFU threshold value. Combining that initial behavioral data with the additional six iFU applications described above, the average sensitivity and specificity measured greater than 89% for the SP protocol and 95% for the MP protocol for the light-phase rats (Table 4 and 5). The average sensitivity and specificity measured greater than 88% for the SP protocol and 73% for the MP protocol for the dark-phase rats. Student’s t-test highlighted a significant difference between the sensitivity and specificity of the dark-phase versus the light-phase group for each of the MP protocol (Table 5) though not for the SP protocol (Table 4).
Table 4.
MEAN VALUES (μ) WITH STANDARD DEVIATION (S.D) FOR THE SENSITIVITY AND SPECIFICITY OF iFU STIMULATION MEASURED DURING LIGHT-PHASE AND DARK-PHASE: SP PROTOCOL. The number of data points (n) included and the P values for the student’s t test for differences between the light-phase data and the dark-phase data are also given.
| Light-phase (μ ± S.D.) | n | Dark-phase (μ ± S.D.) | n | P | Significance | |
|---|---|---|---|---|---|---|
| iFU sensitivity | 0.90 ± 0.10 | 12 | 0.88 ± 0.12 | 31 | 0.30 | No |
| iFU specificity | 0.89 ± 0.12 | 12 | 0.92 ± 0.09 | 31 | 0.20 | No |
Table 5.
MEAN VALUES (μ) WITH STANDARD DEVIATION (S.D) FOR THE SENSITIVITY AND SPECIFICITY OF iFU STIMULATION MEASURED DURING LIGHT-PHASE AND DARK-PHASE: MP PROTOCOL. The number of data points (n) included and the P values for the student’s t test for differences between the light-phase data and the dark-phase data are also given.
| Light-phase (μ ± S.D.) | n | Dark-phase (μ ± S.D.) | n | P | Significance | |
|---|---|---|---|---|---|---|
| iFU sensitivity | 0.95 ± 0.06 | 12 | 0.73 ± 0.13 | 31 | 9.6E-07 | Yes |
| iFU specificity | 0.96 ± 0.06 | 12 | 0.82 ± 0.16 | 31 | 0.0036 | Yes |
DISCUSSION
We have tested in vivo the hypothesis that stimulation by intense focused ultrasound (iFU) favors inflamed tissue relative to contralateral, uninflamed tissue, and that that stimulation exhibits diurnal variability. Specifically, the rats always withdrew their sensitized paws first to iFU stimulation; this withdrawal occurred at lower intensity and dose values of iFU during the light phase than the dark phase. The increased variance in the results and reduced sensitivity and specificity during the dark phase relative to the light phase is consistent with the nocturnal nature of the rodents we tested, where greater activity, hence distractibility, among other factors, offers a plausible explanation for their response to iFU stimulation (Moont et al, 2010).
As to how iFU generates a sensation with diurnal variability, we note that studies of the circadian variation in experimental pain show a variety of results for both humans and rodents as a function of stimulation source (Bruguerolle and Labrecque, 2007; Kubynin and Ignatov 1995; Strian, 1989). Interestingly, thermal stimuli (both hot and cold) generally fail to generate responses with a diurnal component for humans, with mixed results for rodents. Instead, mechanical stimulation has a well-documented diurnal rhythm in a manner consistent with our results. (See also Kubynin and Ignatov (1995), who observed a reduced sensitivity of rodents to mechanical stimulation during the dark phase relative to the light phase.) Studies of stimulation by iFU support the view that it is iFU’s ability to mechanically palpate tissue within its focal region via the acoustic radiation force that generates sensations (Dalecki, 2004; Wright et al., 2002; Davies et al., 1996; Gavrilov et al., 1996; Gavrilov et al., 1977). Also, cavitation is an unlikely mechanism of mechanical stimulation, given the work of Hynynen (1991). Specifically, he showed in vivo that at 1 MHz one would require a minimum dose of 800 W/cm2 applied for one second to generate cavitation. The dose values we required to generation sensations measured substantially less than this threshold value except for a few data points. Our work here is therefore consistent with the view that iFU represents a mechanical source of stimulation via tissue palpation induced by the acoustic radiation force.
With regard to the safety of this procedure, we note that a number of authors have applied comparable amounts of ultrasound to themselves and to test subjects without incident (Dickey et al, 2012; Dalecki, 2004; Wright et al., 2002; Davies et al., 1996; Gavrilov et al., 1996; Gavrilov et al., 1977). Moreover, Foley et al (2008) found that an intensity of 7,890 W/cm2 and duration 5 seconds [hence a dose of almost 40,000 (W*s)/(cm2)] was required to cause acute damage to peripheral nerves. This is well beyond the intensity and dose values we (or anyone else, to our knowledge) have used to generate sensations with iFU. Therefore, extant evidence suggests that eventual use of iFU in the clinic with amounts of ultrasound comparable to what we used here will likely be safe. Useful next steps would include experimental measurement of the margin of safety of iFU’, that is, determination of how much iFU is required to cause damage relative to that necessary to generate reliable sensations.
CONCLUSION
The response to intense focused ultrasound stimulation of deep tissue exhibits a diurnal variation. Given that ultrasound can be applied deep to the skin and can also be quantified, these results suggest that iFU may one day serve as a useful tool for animal and human research targeting the study of diurnal variations of deep pain as well as the study of therapeutic interventions sensitive to those diurnal variations.
Highlights.
Intense focused ultrasound (iFU) can generate diagnostic sensations deep to the skin.
We evaluate iFU as a way to quantify diurnal variation of inflammatory pain.
We applied iFU to the inflamed paws of rats during the day and the night.
Rats responded to lower iFU intensities during the day than the night.
Consistent iFU response variation makes it useful to study diurnal pain.
Acknowledgments
This work received financial support from the Life Sciences Discovery Fund of Washington State, NIH (UL1 RR025014, R41 NS 049719-01), and the Veterans Administration. The author’s contributions are as follows: experimental design (Garcia, Kliot, Loeser, McClintic, Mourad, Ward); data collection (Garcia, Illian, McClintic, Ward); data analysis (Garcia, Illian, McClintic, Mourad, Ward, Yao); write-up (Garcia, Gofeld, Kliot, Loeser, McClintic, Mourad, Yao). Dr. Mourad has a significant financial interest in the research described in this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bachmann C, Nitsche M, Pfingsten M, Gersdorff N, Harder C, Baier P, Antal A, Treede R, Paulus W, Happe S. Diurnal time course of heat pain perception in healthy humans. Neuroscience Letters. 2011;489:122–125. doi: 10.1016/j.neulet.2010.11.080. [DOI] [PubMed] [Google Scholar]
- 2.Basford J, An K. New Techniques for the Quantification of Fibromyalgia and Myofascial Pain. Current Pain and Headache Reports. 2009;13:376–378. doi: 10.1007/s11916-009-0061-6. [DOI] [PubMed] [Google Scholar]
- 3.Bellamy N, Sothern R, Campbell J. Aspects of Diurnal Rhythmicity in Pain, Stiffness, and Fatigue in Patients with Fibromyalgia. Journal of Rheumatology. 2004;31: 379–389. [PubMed] [Google Scholar]
- 4.Boom M, Grefkens J, can Dorp E, Ologsen E, Lourenssen G, Aarts L, Dahan A, Sarton E. Opioid chronopharmacology: influence of timing of infusion on fentanyl’s analgesic efficacy in healthy human volunteers. Journal of Pain Research. 2010;2: 183–190. doi: 10.2147/JPR.S13616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bomholt S, Harbuz M, Blackburn-Munro G, Blackburn-Munro R. Involvement and Role of the Hypothalamo-pituitary-adrenal (HPA) Stress Axis in Animal Models of Chronic Pain and Inflammation. Stress. 2004;7:1–14. doi: 10.1080/10253890310001650268. [DOI] [PubMed] [Google Scholar]
- 6.Bruguerolle B, Labrecque G. Rhythmic pattern in pain and their chronotherapy. Advanced Drug Delivery Reviews. 2007;59:883–895. doi: 10.1016/j.addr.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Christina AJM, Merlin NJ, Vijaya C, Jayaprakash S, Murugesh N. Daily rhythm of nociception in rats. Journal of Circadian Rhythms. 2004;2:2. doi: 10.1186/1740-3391-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crockett RS, Bornschein RL, Smith RP. Diurnal variation in response to thermal stimulation: mouse-hotplate test. Physiology & Behavior. 1977;18:193–196. doi: 10.1016/0031-9384(77)90120-2. [DOI] [PubMed] [Google Scholar]
- 9.Dalecki Diane. Mechanical Bioeffects of Ultrasound. Annual Review of Biomedical Engineering. 2004;6:229–248. doi: 10.1146/annurev.bioeng.6.040803.140126. [DOI] [PubMed] [Google Scholar]
- 10.Dalecki D, Child S, Raeman C, Carstensen E. Tactile perception of ultrasound. Journal of Acoustical Society of America. 1995;97:3165–70. doi: 10.1121/1.411877. [DOI] [PubMed] [Google Scholar]
- 11.Davies I, Gavrilov L, Tsirulnikov E. Application of focused ultrasound for research on pain. Pain. 1996;67:17–27. doi: 10.1016/0304-3959(96)03042-4. [DOI] [PubMed] [Google Scholar]
- 12.Dickey TC, Tyche RE, Kliot M, Loeser JD, Pederson K, Mourad PD. Intense focused ultrasound can reliably induce sensations in human test subjects in a manner correlated with the density of their mechanoreceptors. Ultrasound in Medicine and Biology. 2012;38(1):85–90. doi: 10.1016/j.ultrasmedbio.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fitts DA. The variable-criteria sequential stopping rule: generality to unequal sample sizes, unequal variances, or to large ANOVAs. Behav Res Methods. 2010;42(4):918–929. doi: 10.3758/BRM.42.4.918. [DOI] [PubMed] [Google Scholar]
- 14.Foley JL, Vaezy S, Little JW. Effects of High-Intensity Focused Ultrasound on Nerve Conduction. Muscle Nerve. 2008;37:241–250. doi: 10.1002/mus.20932. [DOI] [PubMed] [Google Scholar]
- 15.Frederickson RCA, Burgis V, Edwards JD. Hyperalgesia Induced by Naloxone Follows Diurnal Rhythm in Responsivity to Painful Stimuli. Science. 1977;198:756–758. doi: 10.1126/science.561998. [DOI] [PubMed] [Google Scholar]
- 16.Gavrilov L, Gersuni G, Ilyinski O, Tsirulnikov E, Shchekanov E. A study of reception with the use of focused ultrasound I. Effects on the skin and deep receptor structures in man. Brain Research. 1977;135:265–277. doi: 10.1016/0006-8993(77)91030-7. [DOI] [PubMed] [Google Scholar]
- 17.Gavrilov L, Gersuni G, Ilyinski O, Tsirulnikov E, Shchekanov E. A study of reception with the use of focused ultrasound II. Effects on the animal receptor structures. Brain Research. 1977;135:279–285. doi: 10.1016/0006-8993(77)91031-9. [DOI] [PubMed] [Google Scholar]
- 18.Gavrilov L, Tsirulnikov E, Davies I. Application of focused ultrasound for the stimulation of neural structures. Ultrasound in Medicine and Biology. 1996;22:179–192. doi: 10.1016/0301-5629(96)83782-3. [DOI] [PubMed] [Google Scholar]
- 19.Hill C, Rivens I, Vaughan M, Ter Harr G. Lesion development in focused ultrasound surgery: A general model. Ultrasound in Medicine and Biology. 1994;20:259–69. doi: 10.1016/0301-5629(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 20.Hynynen K. The threshold for thermally significant cavitation in dog’s thigh muscle in vivo. Ultrasound in Medicine and Biology. 1991;17(2):157–169. doi: 10.1016/0301-5629(91)90123-e. [DOI] [PubMed] [Google Scholar]
- 21.Junker U, Wirz S. Review article: Chronobiology: influence of circadian rhythms on the therapy of severe pain. Journal of Oncology Pharmacy Practice. 2010;16: 81–87. doi: 10.1177/1078155209337665. [DOI] [PubMed] [Google Scholar]
- 22.Karakucuk E, Yamanoglu T, Demirel O, Bora N, Zengil H. Temporal variation in drug interaction between lithium and morphine induced analgesia. Chronobiology International. 2006;23: 675–682. doi: 10.1080/07420520600650745. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy J, Haar G, Cranstron D. High intensity focused ultrasound: surgery of the future? The British Journal of Radiology. 2003;76:590–599. doi: 10.1259/bjr/17150274. [DOI] [PubMed] [Google Scholar]
- 24.Konecka AM, Sroczynska I. Circadian rhythm of pain in male mice. General Pharamcology. 1998;5:809–810. doi: 10.1016/s0306-3623(98)00076-7. [DOI] [PubMed] [Google Scholar]
- 25.Kossoff G. Analysis Of Focusing Action Of Spherically Curved Transducers. Ultrasound In Medicine And Biology. 1979;5(4):359–365. doi: 10.1016/0301-5629(79)90006-1. [DOI] [PubMed] [Google Scholar]
- 26.Kowanko I, Pownall R, Knapp M, Swannell A, Mahoney P. Circadian Variations in the Signs and Symptoms of Rheumatoid Arthritis and in the Therapeutic Effectiveness of Flurbiprogen at Different Times of Day. British Journal of Clinical Pharmacology. 1981;11:477–484. doi: 10.1111/j.1365-2125.1981.tb01153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kubynin AN, Ignatov YD. Chronobiological Parameters of Pain Sensitivity of Rats and Mice. Bulletin of Experimental Biology and Medicine. 1995;119(5):520–523. [Google Scholar]
- 28.Levi F, Le Louarn C, Reinberg A. Timing optimizes sustained-release indomethacin treatment of osteoarthritis. Clinical Pharmacology and Therapeutics. 1985;37:77–84. doi: 10.1038/clpt.1985.15. [DOI] [PubMed] [Google Scholar]
- 29.McBeth J, Chui Y, Silman A, Ray D, Morriss R, Dickens C, Gupta A, Macfarlane G. Hypothalamic-pituitary-adrenal stress axis function and the relationship with chronic widespread pain and its antecedents. Arthritis Research and Therapy. 2005;7:992–1000. doi: 10.1186/ar1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McLean S, Williams D, Harris R, Kop W, Groner K, Ambrose K, Lyden A, Gracely R, Crofford L, Geisser M, Sen A, Biswas P, Clauw D. Momentary Relationship Between Cortisol Secretion and Symptoms in Patients with Fibromyalgia. Arthritis and Rheumatism. 2005;52:3660–3669. doi: 10.1002/art.21372. [DOI] [PubMed] [Google Scholar]
- 31.Mellor D, Cook C, Stafford K. Quantifying Some Responses to Pain as a Stressor. The Biology of Animal Stress. 2000:171–198. [Google Scholar]
- 32.Millecamps M, Jourdan D, Leger S, Etienne M, Eschalier A, Ardid D. Circadian pattern of spontaneous behavior in monarthritic rats: a novel global approach to evaluation of chronic pain and treatment effectiveness. Arthritis & Rheumatism. 2005;52: 3470–3478. doi: 10.1002/art.21403. [DOI] [PubMed] [Google Scholar]
- 33.Moont R, Pud D, Sprecher E, Sharvit G, Yarnitsky D. ‘Pain inhibits pain’ mechanisms: Is pain modulation simply due to distraction? Pain. 2010;150(1):113–20. doi: 10.1016/j.pain.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 34.Mourad PD. Biological effects of ultrasound. In: Webster JL, editor. —Encyclopedia of Electronics and Electrical Engineering. V2. John Wiley & Sons; Philadelphia: 1999. pp. 368–386. [Google Scholar]
- 35.Nagakura Y, Okada M, Kohara A, Kiso T, Toya T, Iwai A, Wanibuchi F, Yamaguchi T. Allodynia and Hyperalgesia in Adjuvant-Induced Arthritic Rats: Time Course of Progression and Efficacy of Analgesics. The Journal of Pharmacology and Experimental Therapeutics. 2003;306:490–497. doi: 10.1124/jpet.103.050781. [DOI] [PubMed] [Google Scholar]
- 36.O’Neil H. Theory Of Focusing Radiators. Journal Of The Acoustical Society Of America. 1949;21(5):516–526. [Google Scholar]
- 37.Orsi F, Arnone P, Chen W, Zhang L. High intensity focused ultrasound ablation: A new therapeutic option for solid tumors. Journal of Cancer Research and Therapeutics. 2010;6: 414–420. doi: 10.4103/0973-1482.77064. [DOI] [PubMed] [Google Scholar]
- 38.Perissin L, Boccalon S, Scaggiante B, Petrelli L, Ortonlani F, Porro C. Diurnal changes of tonic nociceptive responses in mice: evidence for a proalgesic role of melatonin. Pain. 2004;110: 250–258. doi: 10.1016/j.pain.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 39.Perissin L, Facchin P, Porro C. Tonic pain response in mice: effects of sex, season and time of day. Life Sciences. 2003;72: 897–907. doi: 10.1016/s0024-3205(02)02344-5. [DOI] [PubMed] [Google Scholar]
- 40.Sarlis N, Chowdrey H, Stephanou A, Lightman S. Chronic Activation of the Hypothalamo-Pituitary-Adrenal Axis and Loss of Circadian Rhythm during Adjuvant-Induced Arthritis in the Rat. Endocrinology. 1992;130: 1775–1779. doi: 10.1210/endo.130.4.1312424. [DOI] [PubMed] [Google Scholar]
- 41.Simon E. The enigma of deep-body thermosensory specificity. International Journal of Biometeorology. 2000;44:105–120. doi: 10.1007/s004840000060. [DOI] [PubMed] [Google Scholar]
- 42.Spies C, Cutolo M, Straub R, Burmester G, Buttgereit F. More Night Than Day – Circadian Rhythms in Polymyalgia Rheumatica and Ankylosing Spondylitis. The Journal of Rheumatology. 2010;37:894–899. doi: 10.3899/jrheum.091283. [DOI] [PubMed] [Google Scholar]
- 43.Strian F, Lautenbacher S, Galfe G, Holzl R. Diurnal variations in pain perception and thermal sensitivity. Pain. 1989;36:125–131. doi: 10.1016/0304-3959(89)90120-6. [DOI] [PubMed] [Google Scholar]
- 44.Sutton Y, McBride K, Pye S. An ultrasound mini-balance for measurement of therapy level ultrasound. Physics in Medicine and Biology. 2006;51:3397–3404. doi: 10.1088/0031-9155/51/14/008. [DOI] [PubMed] [Google Scholar]
- 45.Ward J. High-intensity focused ultrasound for therapeutic tissue ablation in surgical oncology. Surgical Oncology Clinics of North America. 2011;20:489–407. doi: 10.1016/j.soc.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 46.Wright A, Davies I. The recording of brain evoked potentials resulting from intra-articular focused ultrasonic stimulation: a new experimental model for investigating joint pain in humans. Neuroscience Letters. 1989;97:145–150. doi: 10.1016/0304-3940(89)90154-7. [DOI] [PubMed] [Google Scholar]
- 47.Wright A, Davies I, Riddell J. Intra-articular ultrasonic stimulation and intracutaneous electrical stimulation: evoked potential and visual analogue scale data. Pain. 1993;52:149–155. doi: 10.1016/0304-3959(93)90126-A. [DOI] [PubMed] [Google Scholar]
- 48.Wright A, Graven-Nielsen T, Davies I, Arendt-Nielsen L. Temporal summation of pain from skin, muscle and joint following nociceptive ultrasonic stimulation in humans. Experimental Brain Research. 2002;144:475–482. doi: 10.1007/s00221-002-1062-4. [DOI] [PubMed] [Google Scholar]