Abstract
Rationale
The effects of adolescent marijuana use on the developing brain remain unclear, despite its prevalence. Arterial spin labeling (ASL) is a noninvasive imaging technique that characterizes neurovascular status and cerebral blood flow (CBF), potentially revealing contributors to neuropathological alterations. No studies to date have looked at CBF in adolescent marijuana users.
Objectives
This study examined CBF in adolescent marijuana users and matched healthy controls at baseline and after 4 weeks of monitored abstinence.
Methods
Heavy adolescent marijuana users (n=23, >200 lifetime marijuana use days) and demographically matched controls (n=23) with limited substance exposure underwent an ASL brain scan at an initial session and after 4 weeks of sequential urine toxicology to confirm abstinence.
Results
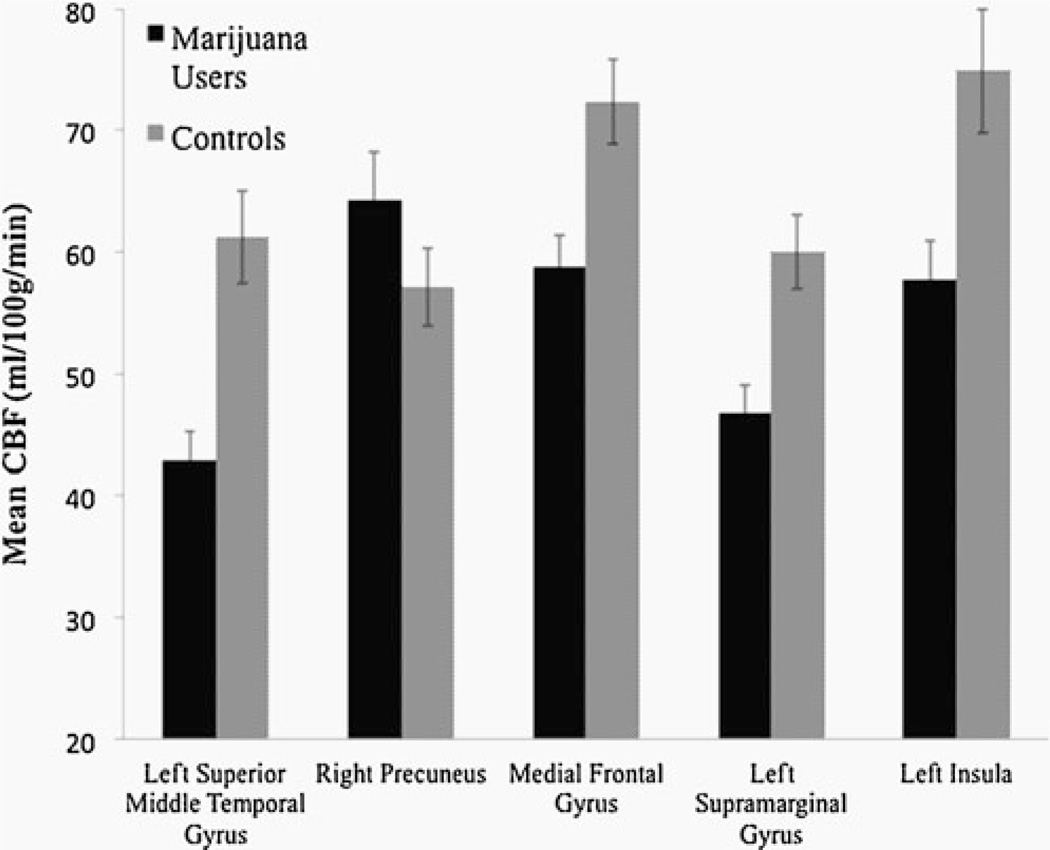
Marijuana users showed reduced CBF in four cortical regions including the left superior and middle temporal gyri, left insula, left and right medial frontal gyrus, and left supramarginal gyrus at baseline; users showed increased CBF in the right precuneus at baseline, as compared to controls (corrected p values<0.05). No between group differences were found at follow-up.
Conclusions
Marijuana use may influence CBF in otherwise healthy adolescents acutely; however, group differences were not observed after several weeks of abstinence. Neurovascular alterations may contribute to or underlie changes in brain activation, neuropsychological performance, and mood observed in young cannabis users with less than a month of abstinence.
Keywords: Marijuana, Adolescence, Neuroimaging, Arterial spin labeling, Cerebral blood flow
Introduction
Despite an increase in reported cannabis use in adolescence (Johnston et al. 2010), the structural and neurovascular effects of the drug on the brain are still not well understood. Some studies have shown cannabis use to be associated with alterations in brain tissue structure (Ashtari et al. 2009; Bava et al. 2010) and function (Abdullaev et al. 2010; Schweinsburg et al. 2011; Tapert et al. 2007), while others have shown fairly limited findings (Delisi et al. 2006; Jacobus et al. 2009a). The neurotoxic effects of cannabis use are still inconclusive, and only a few studies have investigated the effects of cannabis use on cerebral blood flow (CBF) in adults, which can aid in interpretation of blood oxygen level-dependent (BOLD) signal measured in functional magnetic resonance imaging (fMRI). Better quantification of CBF can help interpret neuronal signaling and cognitive functioning and any neurovascular pathology associated with cannabis use. To our knowledge, CBF has not yet been examined in adolescent cannabis users.
Arterial spin labeling (ASL) is a noninvasive method of quantifying CBF in local brain regions, providing better spatial and temporal resolution than other modalities that quantify blood flow such as positron emission tomography (PET). Functional applications of ASL have the capacity to improve signal localization as it measures the rate of arterial blood flow delivered to capillary beds, which is closer to the site of neural activity as opposed to venous blood flow changes reflected in fMRI studies (Buxton 2009). Alternative more invasive imaging modalities (e.g., 133Xenon inhalation method, PET, dynamic susceptibility contrast) have allowed researchers to investigate altered patterns of blood flow and blood volume in cannabis using adults as a result of acute intoxication and chronic use, yet ASL techniques (both resting state and functional) have not been widely utilized in the cannabis literature (Martin-Santos et al. 2010; van Hell et al. 2011).
Research findings on the acute effects of cannabis have been fairly consistent, with most studies reporting an increase in CBF after immediate exposure (Lundqvist et al. 2001). For example, several early adult studies found increased CBF after smoking or intravenous administration of cannabis relative to comparison subjects (Mathew and Wilson 1993; Mathewet al. 1989, 1992, 1997, 2002). Volkow et al. (1996) reported similar findings of increased glucose metabolism after tetrahydrocannabinol (THC) infusion. Brain regions identified across studies include both cortical and subcortical regions, particularly frontal brain regions, insula, basal ganglia, and cingulate. Only one study has utilized ASL to understand the acute effects of cannabis use. van Hell et al. (2011) delivered THC via vaporizer prior to a neuroimaging session. The authors found increased CBF in the cingulate cortex, superior frontal cortex, and insula; they also found decreased perfusion in the post-central gyrus and occipital gyrus.
Findings on the long-term effects of chronic cannabis use have been more variable, but generally suggest lower or stabilized CBF after a period of abstinence in heavy users. Chronic adult users assessed after a 5- to 8-day period of monitored abstinence showed initial global decreases in CBF with subsequent increases after 9–60 days of abstinence, suggesting a return to baseline CBF in chronic users after 2 weeks of abstinence (Tunving et al. 1986). Experienced adult cannabis smokers who refrained from smoking for 12 h also demonstrated decreased CBF at baseline (Mathew et al. 1989). Similarly, depressed levels of cerebellar metabolism were reported by Volkow et al. (1996) in chronic adult users after 3 days of abstinence when compared to controls. Sneider et al. (2008) found that cannabis users demonstrated significantly higher blood volumes in the right frontal area, the left temporal area, and the cerebellum when compared to controls after 6–36 h of abstinence (2006); however, this group reported relative stabilization of cerebral blood volume after a month of abstinence, particularly in frontal brain regions. Conversely, Herning et al. (2005) found that, after 3 days of abstinence, chronic users displayed increased blood flow velocity in the anterior and middle cerebral arteries when compared to controls, and these differences persisted after a month of monitored abstinence. There have been no studies to date that have used ASL to look at vascular changes over time in abstinent adolescent users.
Given the quantity of BOLD imaging studies in the literature in adolescents and young adults (for reviews, see Jacobus et al. 2009b; Martin-Santos et al. 2010), further examination of alteration in resting CBF is greatly needed. It is unclear which general vascular alterations may lead to alteration in cognitive status, or simply represent more isolated vasodilation properties of the cannabis compound. This is particularly important in cannabis research since the frontal lobe, hippocampal/temporal regions, basal ganglia, and cingulate cortices are susceptible to cannabis exposure due to their high density of cannabinoid receptors and implicated in cognitive functions such as memory and attention (Herkenham et al. 1990). Studies on chronic cannabis users have found correlations between performance on cognitive tasks and several measures of neurovasculature (Block et al. 2002; O’Leary et al. 2002; Bolla et al. 2005; Eldreth et al. 2004).
The neural underpinnings of higher-order cognitive functioning are being established in adolescence, a period of ongoing cerebral maturation in frontal regions of the brain. The previous studies are limited in their examination of adult users (van Hell et al. 2011), and currently, there has been no evaluation of the cerebrovascular effects of heavy cannabis use in adolescents using ASL imaging. Here, we examined the effects of chronic cannabis use on CBF levels in 15–18 year olds at baseline and again after 28 days of monitored abstinence. We hypothesized that cannabis users would demonstrate lower CBF levels at baseline when compared to age matched controls, and CBF would correlate with marijuana and other substance use severity, cognition, and mood variables. Furthermore, we predicted that CBF levels for users would stabilize after abstinence, particularly in areas important for cognition. Our goal was to prospectively evaluate the effects of chronic use on resting neurovasculature measures during a developmentally critical period.
Methods
Participants
Twenty-three adolescent heavy cannabis users and 23 demographically matched control teens with minimal substance use were recruited from local schools (see Table 1). Comprehensive screening interviews were administered to parents and/or guardians; adolescents were required to have a parent or legal guardian provide consent to participate in the study and to provide assent for their own participation, in accordance with University of California, San Diego Human Research Protections Program procedures. Exclusionary criteria included history of a Diagnostic and Statistical Manual for Mental Disorder-Fourth Edition. (DSM-IV; American Psychiatry Association 1994) Axis 1 disorder other than alcohol or cannabis use disorder, use of psychoactive medications, learning disability or mental retardation, neurological condition (e.g., migraine), or traumatic brain injury with loss of consciousness >2 min; prenatal alcohol or drug exposure; premature birth; left handedness; and non-fluency in English.
Table 1.
Participant characteristics (at baseline, unless otherwise noted)
| Controls (n=23) M (SD) or % |
Marijuana users (n=23) M (SD) or % |
|
|---|---|---|
| Age | 17.5 (0.8) | 17.7 (0.7) |
| Percent male | 83% | 74% |
| Percent Caucasian | 74% | 83% |
| Grade point average | 3.7 (0.6) | 3.4 (0.7) |
| Annual household income | $142K (70) | $197K (194) |
| Vocabulary T-score | 58.2 (7.9) | 55.1 (9.3) |
| Family history of substance use disorder | 22% | 17% |
| Spielberger state anxiety T-score at baseline | 35.0 (4.3) | 37.5 (6.1) |
| Spielberger state anxiety T-score at follow-up | 34.9 (6.5) | 36.4 (8.0) |
| Beck depression inventory total at baseline* | 1.7 (2.4) | 4.7 (4.6) |
| Beck depression inventory total at follow-up | 1.4 (2.2) | 2.0 (2.7) |
| Lifetime marijuana use days* | 0.5 (1.1) | 398.6 (181.5) |
| Past month marijuana use days at baseline* | 0.1(0.2) | 17.9 (9.2) |
| Lifetime alcohol use days* | 6.3 (15.9) | 114.3 (88.3) |
| Past month alcohol use days at baseline* | 0.2 (0.8) | 3.5 (2.5) |
| Past month, alcohol use days at follow-up | 0.2 (0.7) | 1.0 (1.6) |
| Past month, cigarettes smoked at baseline* | 0.0 (0.0) | 7.4 (20.4) |
| Past month, cigarettes smoked at follow-up* | 0.0 (0.0) | 7.3 (20.8) |
| Past month, other drug use days at baseline* | 0.0 (0.0) | 0.5 (0.7) |
| Past month, other drug use days at follow-up* | 0.0 (0.0) | 0.2 (0.6) |
| Lifetime amphetamine use* | 0.0 (0.0) | 0.7 (1.3) |
| Lifetime hallucinogen use* | 0.0 (0.0) | 1.7 (3.3) |
| Lifetime cocaine use* | 0.0 (0.0) | 0.3 (0.6) |
| Lifetime opiate use* | 0.0 (0.0) | 1.0 (1.7) |
| Lifetime ecstasy use* | 0.0 (0.0) | 0.7 (1.3) |
At follow-up, marijuana users had at least 4 weeks of monitored abstinence; other drug use excludes alcohol, marijuana, and nicotine.
p<0.05
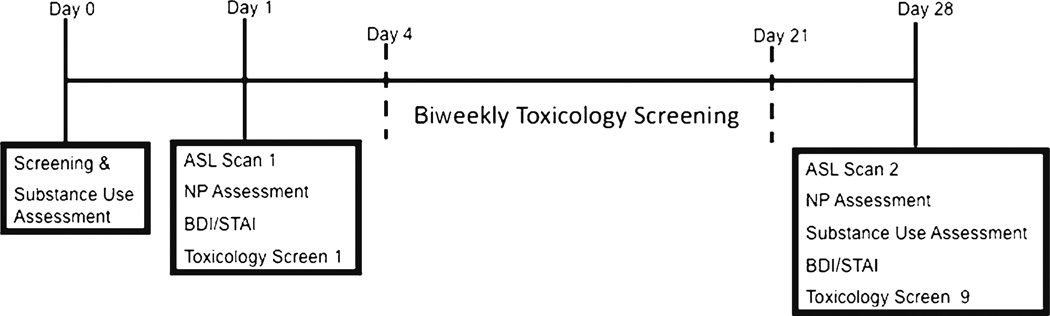
Teens were classified as controls (<four marijuana lifetime use days; CON) or marijuana users (>200 lifetime marijuana use days; MJ). All participants received neuroimaging at baseline, at which time marijuana users had 1–17 days (M=5.1, SD=3.8) of abstinence, and underwent twice weekly urine toxicology. Specifically, THCCOOH to creatinine ratios were examined in relation to published data on residual excretion rates (Smith et al. 2009) for confirmation of abstinence over the course of 4 weeks. Breathalyzer was also given to all participants at each urine toxicology screen appointment and at the follow-up scan 4 weeks later to confirm sobriety from alcohol (see Fig. 1 for timeline of procedures).
Fig. 1.
Timeline of study procedures. ASL Arterial spin labeling, BDI beck depression inventory, STAI state trait anxiety inventory, NP neuropsychological
Measures
Substance use
The Customary Drinking and Drug Use Record (Brown et al. 1998) was used to assess lifetime alcohol, marijuana, nicotine, and other illicit substance use at baseline. The Timeline Followback (Sobell and Sobell 1992) was used to assess past month substance use (e.g., alcohol, marijuana) prior to both baseline and follow-up scans.
Emotional functioning
The State Trait Anxiety Index (STAI; Spielberger et al. 1970) assessed state anxiety at baseline and follow-up scan session. The Beck Depression Inventory-Second Edition, 2nd edn. (BDI-II; Beck et al. 1961) assessed active depressive symptoms at both imaging appointments.
Cognitive functioning
The Wechsler Abbreviated Scale of Intelligence (WASI) Vocabulary and Block design subtest (Wechsler 1999) and the Wide Range Achievement Test-3 Reading subtest (Wilkinson 1993) provided an estimate of premorbid intellectual functioning. Attention and processing speed were measured using the Wechsler Adult Intelligence Scale Third Edition Digit Symbol and Digit Span subtests subtests (Wechsler 1997). The California Verbal Learning Test-Second Edition was used to assess verbal learning and memory (Delis et al. 2001).
Demographics
The Family History Assessment module (Rice et al. 1995) was used to assess family history of psychiatric disorders. Parental income and grade point average was collected during comprehensive clinical interview prior to imaging sessions.
Procedures
ASL acquisition and processing
All scans were acquired on a 3.0T General Electric magnetic resonance imager with an eight-channel receive coil at the University of California, San Diego. Subjects were asked to remain awake and still in the scanner to the best of their ability. Three imaging sequences (one perfusion-weighted acquisition and two calibration scans) were acquired to calculate absolute CBF. First, resting state blood flow was measured with pulsed ASL using a modified flow-sensitive alternating inversion recovery (FAIR) sequence (6 min and 40 sec) with QUIPSS-II saturation pulses and an interleaved (four-shot) spiral readout (Kim 1995; Liu and Wong 2005). ASL pulse sequence parameters include TE=3.3 ms, TR=2500 ms, field of view=22 cm, slice thickness=5.0 mm, image matrix=64×64, T11=600 ms, T12=1600 ms, tag thickness 10 cm, 20 axial slices, and 20 tag+control image pairs. Two calibration scans were acquired (36 and 32 s) including one acquisition with inversion pulses turned off to estimate the equilibrium magnetization of cerebral spinal fluid and a minimum contrast scan to account for coil and field inhomogeneities (Chalela et al. 2000; Wang et al. 2005). A high-resolution MRI anatomical spoiled gradient recalled (SPGR) acquisition scan sequence was also collected at each session with TE/TR=min full, field of view=24 cm, resolution=1 mm3, 170 continuous slices.
All imaging data were processed using Analysis of Functional NeuroImages (AFNI; Cox 1996), Functional Magnetic Resonance Imaging of the Brain's software package, FMRIB Software Library (FSL; Smith et al. 2004) and inhouse MatLab scripts. The sensitivity encoding (SENSE) reconstruction algorithm (Pruessmann et al. 1999) was first applied to reconstruct the image from each interleave and then the images from the four consecutive interleaves were summed together. CBF quantification was then estimated by MatLab scripts utilizing AFNI and FSL tools for surround subtraction of the tag-control time series. Estimated CBF values were converted to ml/100g/min using CSF as the reference signal (Wong et al. 1998). Each individual high-resolution anatomical dataset was spatially standardized, segmented, and skull-stripped and registered to the perfusion dataset. CBF data were smoothed using a 4.0-mm full-width, half-maximum Gaussian filter, registered to standardized space, and negative intensity voxels were replaced with zero. Each participant’s CBF data were then multiplied by their respective gray matter anatomical dataset in order to ensure only gray matter voxels were included in all subsequent analysis.
Statistical analysis
A two-way mixed model whole-brain analysis of variance (ANOVA) was applied (AFNI 3dANOVA3) to examine both within subject and between subject effects. The main effects of time, group, and their interaction were specified, in addition to a priori contrasts to examine the differences between group levels (i.e., CON and MJ) at each time point (i.e., baseline and 28-day follow-up) and within subject differences for each group as a follow-up to significant factors identified by two-way ANOVA. Monte Carlo simulation for type I error control (AFNI AlphaSim) with voxelwise alpha 0.05 yielded a minimum cluster volume threshold of 832 µl (13 contiguous voxels). Blood flow values from significant clusters were extracted for exploratory bivariate correlations between CBF and substance use, neurocognitive, mood state variables, and other substance use covariates as appropriate.
Results
Between group differences
Groups did not differ statistically on any demographic characteristics (p values>0.05, see Table 1). The marijuana users reported significantly higher levels of depressed mood at baseline (although only two participants were in the mildly clinically significant range). Groups differed on lifetime frequency and duration of alcohol, marijuana, tobacco, and other drug use, as anticipated (see Table 1).
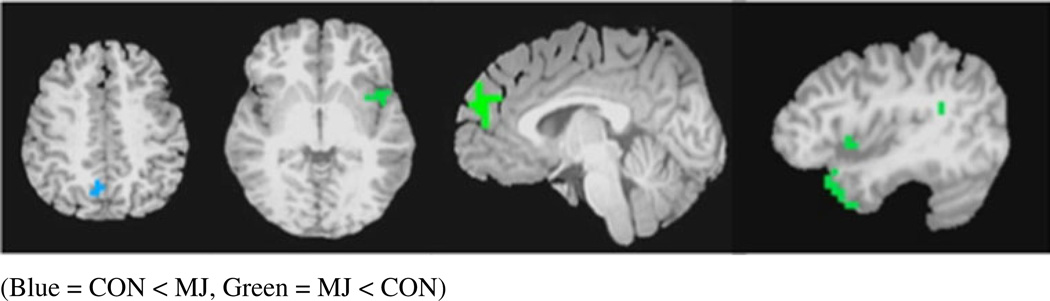
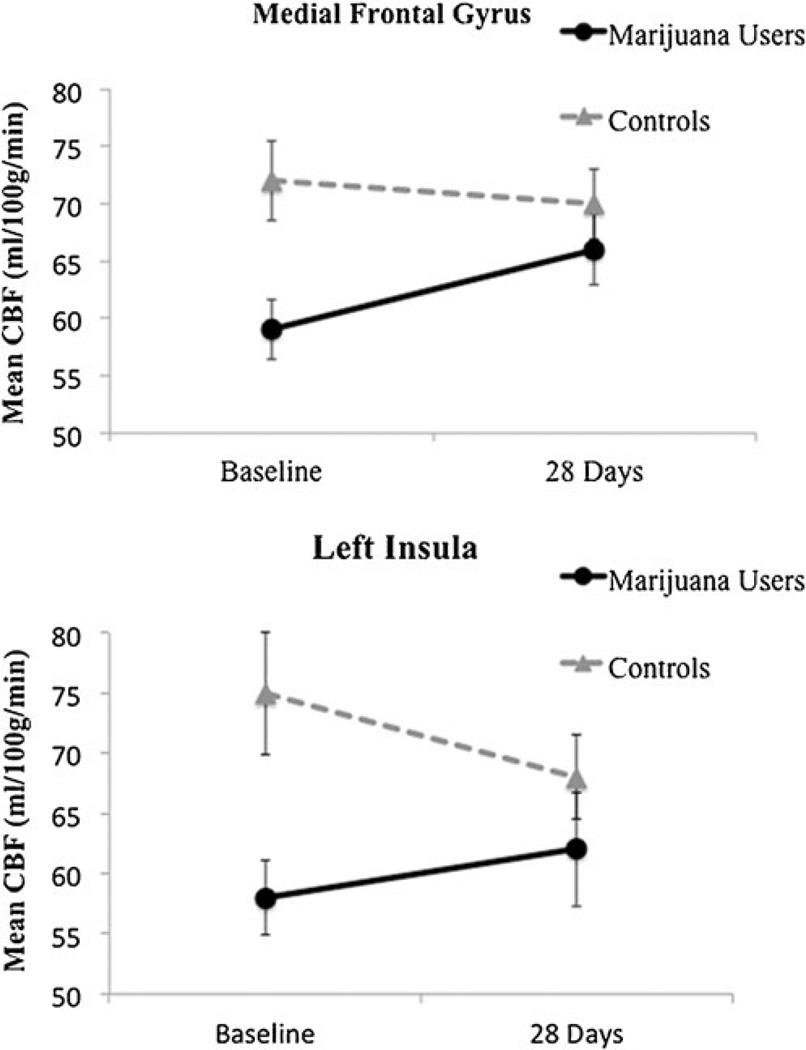
Whole-brain ANOVA revealed a main effect of group between users and controls (F(1,44)=4.08, p<0.05, corrected). Follow-up a priori contrasts (p values<0.05, corrected) between groups at both time points demonstrate differences in five clusters at baseline (see Fig. 2). In four clusters, MJ users showed significantly lower CBF values than controls at baseline in: (1) left superior and middle temporal gyri (t=4.0), (2) left insula (t=2.1), (3) left extending to right medial frontal gyrus (t=3.4), and 4) left supramarginal gyrus (t=4.4). One cluster in the right precuneus (t=−2.9) revealed higher CBF values in the MJ users than controls at baseline (see Figs. 2, 3 and 4 and Table 2). No between group differences were found at follow-up. The within subjects main effect of time and the group by time interaction on CBF were nonsignificant, and therefore follow-up contrasts were not explored.
Fig. 2.
Brain regions showing mean blood perfusion differences with standard errors between marijuana users and controls at baseline (all p values<0.05, corrected)
Fig. 3.
Anatomical locations of between-group CBF differences at baseline (from left), right precuneus, left insula (axial view), medial frontal gyrus, superior and middle gyrus, left insula (sagittal view), and supramarginal gyrus (p values<0.05, corrected). CON<MJ (blue), MJ<CON (green)
Fig. 4.
Mean blood perfusion differences and standard errors at baseline and after monitored abstinence, *p<0.05, corrected
Table 2.
Regions with baseline cerebral blood perfusion differences between groups (p<0.05, corrected)
| Anatomical region | Brodmann areas | Volume (µl) | Talairach coordinates | Controls | Marijuana users | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | Mean CBF (ml/100 g/min) |
Mean CBF (ml/100 g/min) |
|||
| Left superior/ middle temporal gyrus | 38, 47, 21 | 2048 | 34.0 | −15.0 | −36.0 | 61.2 | 43.0 |
| Right precuneus | 7, 31 | 1024 | −14.0 | 61.0 | 24.0 | 57.1 | 64.3 |
| Right and left medial frontal gyrus | 9 | 960 | −2.0 | −51.0 | 32.0 | 72.4 | 58.8 |
| Left supramarginal gyrus | 13, 40 | 960 | 58.0 | 49.0 | 28.0 | 60.0 | 46.7 |
| Left insula | 13, 22 | 832 | 46.0 | −3.0 | −4.0 | 75.9 | 57.8 |
To determine if principal findings remained significant after controlling for other substance use, the analyses were reexamined in PASW Statistics v. 18.0 (SPSS) controlling for between group other substance use differences identified in Table 1 (e.g., alcohol, nicotine, amphetamines, etc.), and relationships remained significant (p values<0.01)
Correlations with substance use severity
Bivariate correlations were examined in the MJ user group between CBF values at baseline and alcohol, nicotine, and all other substance use severity variables (see Table 1) to identify potential covariates for dose-dependent CBF relationships with marijuana use; if any significant relationships were found with other substance use variables, they were included as covariates. Within the MJ user group (n=23), more days per month of marijuana use in the past month was associated with lower baseline CBF in the left insula (r=−0.43, p=0.04). Unexpectedly, more days since last marijuana use at baseline was related to reduced CBF in the medial frontal cluster (r=−0.43, p=0.04). However, in the medial frontal cluster, more heavy drinking days in the month before baseline was associated with increased CBF values (r=0.55, p<0.01). Therefore, the marijuana use severity and CBF association was reevaluated controlling for heavy drinking. Marijuana use severity (days since last marijuana use) was not found to be a significant predictor of CBF in the medial frontal cluster after controlling for heavy drinking days in the past month (p=0.12). In the right precuneus, more total drinking days in the past month (r=0.58, p<0.01) and more cigarettes smoked in the month prior to baseline (r=0.58, p<0.01) were linked to higher CBF for this region, but marijuana use was not related to CBF here.
Correlations with emotional and cognitive functioning
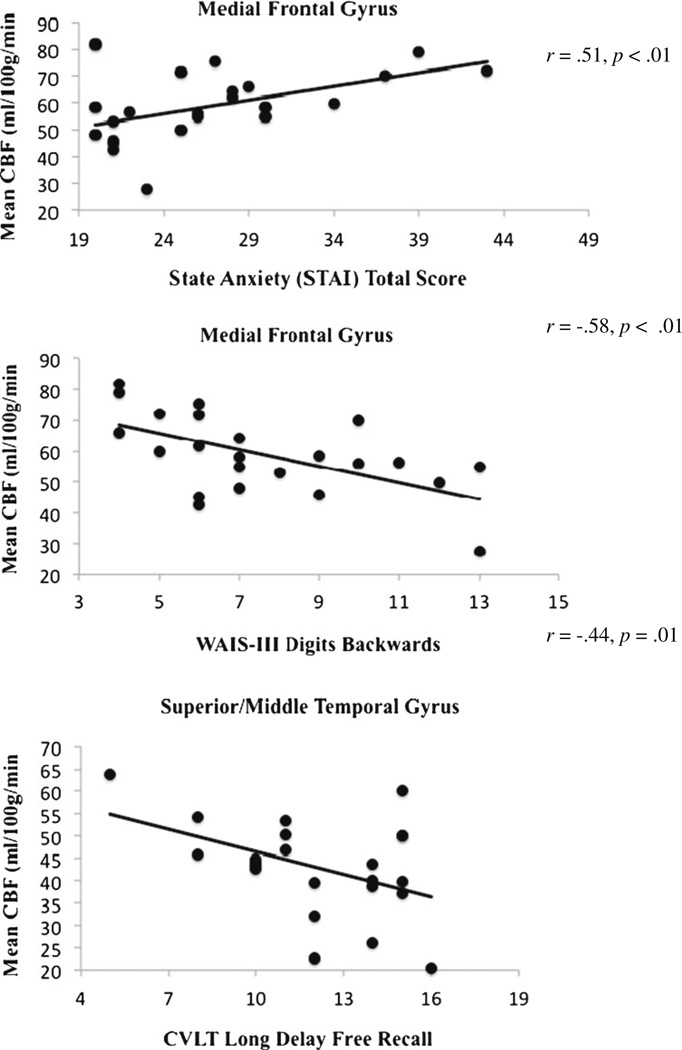
In the MJ group, increased CBF at time 1 in the medial frontal cluster was related to increased anxiety state at time 1 (r=0.51, p<0.01). It was also related to decreased performance on trial 1 learning on the CVLT-II at baseline (r=−0.53, p<0.01) and to decreased performance on the digits backwards component of the WAIS-III digit span subtest (r=−0.58, p<0.01); however, the relationship with CVLT-II trial 1 at baseline was no longer significant after controlling for anxiety (p=0.052). In the left superior medial temporal gyrus, increased CBF at time 1 was related to fewer words recalled on CVLT-II long delay free recall (r=−0.44, p=0.01). No relationships were observed between CBF and executive functioning, nonverbal memory, structural verbal memory, or visuospatial processing (see Fig. 5).
Fig. 5.
Correlations between CBF, mood, and cognitive status in adolescent marijuana users (n=23) at baseline (1–17 days of abstinence)
Discussion
We identified significantly different CBF values for adolescent marijuana users compared to controls after just a few days of abstinence. Regions in which marijuana users demonstrated decreased CBF at baseline were the medial frontal gyrus, left insula, left supramarginal gyrus, and left temporal regions. Further, more days of marijuana use in the month before scanning was linked to reduced baseline CBF in the insula for users. However, in the more posterior right precuneus, marijuana users showed increased CBF compared to controls at baseline, and this was linked to more past-month tobacco smoking and heavy drinking episodes among users. Importantly, no CBF differences were apparent between groups after 4 weeks of monitored abstinence, suggesting these neurovasculature abnormalities have the capacity to recover with abstinence in physically healthy adolescents.
The acute increases in CBF after exposure to cannabis are well documented across multiple imaging modalities (Lundqvist et al. 2001). Our findings are fairly similar to adult studies reporting decreased CBF or metabolic activity in recently abstinent marijuana users (Mathew et al. 1989; Sevy et al. 2008; Tunving et al. 1986; Volkow et al. 1996), which returns to baseline with more protracted abstinence (Sneider et al. 2008). A recent cross sectional adult study by van Hell et al. (2011) utilizing ASL also reported similar anatomical CBF perfusion changes in frontal, temporal, and limbic system brain regions (e.g., insula, anterior cingulate cortex) in response to marijuana use; the authors found perfusion increased in fronto-limbic brain regions and decreased in posterior (i.e., occipital gyrus) regions after acute administration of THC. Interestingly, our findings show the opposite pattern with shorter-term abstinence (compared to acute administration findings reported by van Hell), as we suggest decreased cortico-limbic neurovascular activity and increased posterior cortical vascular activity. These vascular perfusion differences may have implications not only for efficient cognitive functioning in both adolescent and adults but also for altered patterns of healthy neurodevelopment in adolescents, which may then lead to deleterious effects on neural tissue and neural signaling.
Cannabis is believed to have vasodilation properties; evidence from animal studies suggests decreased heart rate, blood pressure, and increased blood flow during cannabis consumption (Kunos et al. 2000; Randall et al. 2004). The human literature is not as clear regarding both the acute cardiovascular effects and the longer-term neurovascular effects of cannabis (and frequency and duration are likely to also play a role in these relationships; Jones 2002). However, previous findings along with the present seem to point to the possibility of decreased blood flow, while THC is being eliminated from the body (Block et al. 2000; Lundqvist et al. 2001; Mathew et al. 1989; Sevy et al. 2008; Tunving et al. 1986). This raises particular concern for adolescent neurodevelopment, as decreased perfusion may correspond to increased potential for neurophysiological complications and hindered development. Adequate blood flow is likely critical for ongoing subtle developmental processes which continue into late adolescence, including both synaptic pruning and myelination (Takahashi et al. 1999).
Further, changes in hemodynamics may have an impact on our interpretation of BOLD imaging fMRI studies, although studies have suggested BOLD imaging to be independent of neurovascular alterations associated with drugs of abuse (Murphy et al. 2006). Nevertheless, 4 weeks of abstinence prior to functional neuroimaginag (as requested for this project) may increase confidence that differences between substance users and controls represent neural signaling compared to general vascular alterations, although this is speculative and further research is clearly warranted. Better understanding of dynamic CBF changes in adolescent cannabis users may help inform our discussion on the neural and/or vascular underpinning of structural and functional brain changes (both increases and decreases). For example, neurovascular changes may influence sensory perception (e.g., insular cortex), decision-making and cognitive control (e.g., medial-frontal brain regions), and other higher-order cognitive processing (e.g., supramarginal gyrus, superior temporal gyrus) associated with marijuana use and reflected in BOLD imaging (Becker et al. 2010; Filbey et al. 2009; Jager et al. 2010; Schweinsburg et al. 2011) and traditional neurocognitive evaluation (Hanson et al. 2010; Pope et al. 2001). We also found relationships between CBF and cognitive status in the user group, as higher CBF was largely related to poorer cognitive performance. It is possible that individuals with higher CBF values have more recent use and therefore perform worse on cognitive evaluation, particularly on memory tasks and more demanding attentional processing tasks (i.e., our findings of poorer performance on long delay free recall and digit backwards); while we did not see direct evidence of this theory in our data set (recency of marijuana use and/or alcohol use was not related to neuropsychological test performance reported here), this may be consistent with our finding of more days since last use related to decreased CBF values in the user group.
It is important to note that the relationship between vascular activity and marijuana use is likely modulated by both nicotine and alcohol, particularly given their common comorbidity in adolescent populations (Johnston et al. 2010), and our sample differed on alcohol and nicotine consumption as anticipated. The independent effects these substances have on CBF must also be considered (Demir et al. 2002; Domino et al. 2000; Zubieta et al. 2001). For example, we saw heavy drinking and severity of cigarette use related to increased CBF in the user group, future research should work to disentangle the effects of these substances on adolescent neurovascular dynamics and neurodevelopment. Further, while our contrasts identified differences at time 1 only, we emphasize that we did not see a significant within-subject main effect of time or time by group interaction (despite few weaker trends, p values>0.07) in any brain regions in our principal analyses; therefore, we must be cautious about inferring any changes in CBF following abstinence from cannabis use. While within-subject changes in CBF, per se, were not substantiated here, regression to the mean is an important consideration in longitudinal research examining blood flow changes over time in both adult and adolescent cannabis users. Given that effect sizes are relatively modest and conducting multiple correlational analyses increases type I error, replication would be helpful in working to better understand the true impact of cannabis use on CBF and cognitive processing.
Although group differences in anxiety state were not found, it is possible that differences between groups could reflect differences in emotional functioning to some degree. While weekly urine toxicology was conducted to confirm abstinence, duration and frequency of substance use variables was based on self-report. Further, it is possible that these findings do not generalize to those excluded from study, including those individuals with more severe psychopathology or neurologic injury. Differences in methodology must also be considered when comparing vascular changes related to cannabis use, not only neuroimaging approaches but also limitations related to controlling for default cognitive networks likely to be active during resting states.
Future work in our laboratory will focus on identifying relationships between alterations in CBF and neurocognitive measures, in addition to other multimodal imaging techniques, in both cannabis users and healthy adolescents. Given significant research focus on functional and structural brain changes in adolescent substance use, and imaging indices as predictors of cognitive outcome, understanding the role vascular changes have in these processes will allow us to better understand the physiological underpinnings of neuropathological changes. Noninvasive measures of CBF may provide an important biomarker of impact on neural networks and cognitive development in adolescence.
Acknowledgment
We extend our appreciation to Anthony Scarlett and Rachel Thayer for their assistance with data collection and to participating families and schools. This research was made possible by funding from the National Institute on Drug Abuse (P20 DA024194, R01 DA021182, F31 DA026263), the National Institute on Alcohol Abuse and Alcoholism (7R01 AA13419), and the National Institute of Mental Health (R01 MH084796).
Footnotes
Conflict of Interest None
Contributor Information
Joanna Jacobus, Psychology Service (116B), VA San Diego Healthcare System, 3350 La Jolla Village Drive, San Diego, CA 92161, USA; Department of Psychiatry, University of California, San Diego, 9500 Gilman Drive (0603), La Jolla, CA 92093-0603, USA.
Diane Goldenberg, Department of Psychiatry, University of California, San Diego, 9500 Gilman Drive (0603), La Jolla, CA 92093-0603, USA.
Christina E. Wierenga, Psychology Service (116B), VA San Diego Healthcare System, 3350 La Jolla Village Drive, San Diego, CA 92161, USA Department of Psychiatry, University of California, San Diego, 9500 Gilman Drive (0603), La Jolla, CA 92093-0603, USA.
Neil J. Tolentino, Psychology Service (116B), VA San Diego Healthcare System, 3350 La Jolla Village Drive, San Diego, CA 92161, USA
Thomas T. Liu, Department of Radiology, University of California, San Diego, 9500 Gilman Drive (0677), La Jolla, CA 92093-0603, USA
Susan F. Tapert, Email: stapert@ucsd.edu, Psychology Service (116B), VA San Diego Healthcare System, 3350 La Jolla Village Drive, San Diego, CA 92161, USA; Department of Psychiatry, University of California, San Diego, 9500 Gilman Drive (0603), La Jolla, CA 92093-0603, USA.
References
- Abdullaev Y, Posner MI, Nunnally R, Dishion TJ. Functional MRI evidence for inefficient attentional control in adolescent chronic cannabis use. Behav Brain Res. 2010;215:45–57. doi: 10.1016/j.bbr.2010.06.023. [DOI] [PubMed] [Google Scholar]
- Ashtari M, Cervellione K, Cottone J, Ardekani BA, Sevy S, Kumra S. Diffusion abnormalities in adolescents and young adults with a history of heavy cannabis use. J Psychiatr Res. 2009;43:189–204. doi: 10.1016/j.jpsychires.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, Jacobus J, Mahmood O, Yang TT, Tapert SF. Neurocognitive correlates of white matter quality in adolescent substance users. Brain Cogn. 2010;72:347–354. doi: 10.1016/j.bandc.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Becker B, Wagner D, Gouzoulis-Mayfrank E, Spuentrup E, Daumman J. The impact of early-onset cannabis use on functional brain correlates of working memory. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:837–845. doi: 10.1016/j.pnpbp.2010.03.032. [DOI] [PubMed] [Google Scholar]
- Block RI, O’Leary DS, Hichwa RD, Augustinack JC, Ponto LL, Ghoneim MM, Arndt S, Erhardt JC, Hurtig RR, Watkins GL, Hall JA, Nathan PE, Andreasen NC. Cerebellar hypoactivity in frequent marijuana users. NeuroReport. 2000;11:749–753. doi: 10.1097/00001756-200003200-00019. [DOI] [PubMed] [Google Scholar]
- Block RI, O’Leary DS, Hichwa RD, Augustinack JC, Boles PLL, Ghoneim MM, Arndt S, Hurtig RR, Watkins GL, Hall JA, Nathan PE, Andreasen NC. Effects of frequent marijuana use on memory-related regional cerebral blood flow. Pharmacol Biochem Behav. 2002;72:237–250. doi: 10.1016/s0091-3057(01)00771-7. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, Matochik JA, Cadet JL. Neural substrates of faulty decision-making in abstinent marijuana users. NeuroImage. 2005;26:480–492. doi: 10.1016/j.neuroimage.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the customary drinking and drug use record (CDDR): a measure of adolescent alcohol and drug involvement. J Stud Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Buxton RB. Introduction to functional magnetic resonance imaging: principles and techniques. Cambridge: University Press; 2009. [Google Scholar]
- Chalela JA, Alsop DC, Gonzalez-Atavales JB, Maldjian JA, Kasner SE, Detre JA. Magnetic resonance perfusion imaging in acute ischemic stroke using continuous arterial spin labeling. Stroke. 2000;31:680–687. doi: 10.1161/01.str.31.3.680. [DOI] [PubMed] [Google Scholar]
- Cox R. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):172–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California verbal learning test. 2nd edn. San Antonio: The Psychological Corporation; 2001. [Google Scholar]
- Delisi LE, Bertisch HC, Szulc KU, Majcher M, Brown K, Bappal A, Ardekani BA. A preliminary DTI study showing no brain structural change associated with adolescent cannabis use. Harm Reduct J. 2006;3:17. doi: 10.1186/1477-7517-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir B, Ulug B, Lay Ergun E, Erbas B. Regional cerebral blood flow and neuropsychological functioning in early and late onset alcoholism. Psychiatry Res. 2002;115:115–125. doi: 10.1016/s0925-4927(02)00071-9. [DOI] [PubMed] [Google Scholar]
- Domino EF, Minoshima S, Guthrie S, Ohl L, Ni L, Koeppe RA, Zubieta J. Nicotine effects on regional cerebral blood flow in awake resting tobacco smokers. Synapse. 2000;38:313–321. doi: 10.1002/1098-2396(20001201)38:3<313::AID-SYN10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Eldreth DA, Matochik JA, Cadet JL, Bolla KI. Abnormal brain activity in prefrontalbrain regions in abstinent marijuana users. NeuroImage. 2004;23:914–920. doi: 10.1016/j.neuroimage.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Schacht JP, Myers US, Chavez RS, Hutchinson KE. Marijuana craving in the brain. Proc Natl Acad Sci USA. 2009;106:13016–13021. doi: 10.1073/pnas.0903863106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson KL, Winward JL, Schweinsburg AD, Medina KL, Brown SA, Tapert SF. Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addict Behav. 2010;35:970–976. doi: 10.1016/j.addbeh.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proc Natl Acad Sci USA. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herning RI, Better WE, Tate K, Cadet JL. Cerebrovascular perfusion in marijuana users during a month of monitored abstinence. Neurology. 2005;64:488–493. doi: 10.1212/01.WNL.0000150882.69371.DD. [DOI] [PubMed] [Google Scholar]
- Jacobus J, McQueeny T, Bava S, Schweinsburg BC, Frank LR, Yang TT, Tapert SF. White matter integrity in adolescents with histories of marijuana use and binge drinking. Neurotoxicol Teratol. 2009a;31:349–355. doi: 10.1016/j.ntt.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Bava S, Cohen-Zion M, Mahmood O, Tapert SF. Functional consequences of marijuana use in adolescents. Pharmacol Biochem Behav. 2009b;92:559–565. doi: 10.1016/j.pbb.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager G, Block RI, Luijten M, Ramsey NF. Cannabis use and memory function in adolescent boys: a cross-sectional multicenter functional magnetic resonance imaging study. J Am Acad Child Adolesc Psychiatry. 2010;49:561–572. doi: 10.1016/j.jaac.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Secondary school students. Bethesda, MD: National Institute on Drug Abuse; Monitoring the Future national survey results on drug use, 1975–2009: Vol I. (NIH publication no. 107584). [Google Scholar]
- Jones RT. Cardiovascular system effects of marijuana. J Clin Pharmacol. 2002;42:58S–63S. doi: 10.1002/j.1552-4604.2002.tb06004.x. [DOI] [PubMed] [Google Scholar]
- Kim SG. Quantification of regional cerebral blood flow change by flow sensitive alternating inversion recovery (FAIR) technique: application to functional mapping. Magn Reson Med. 1995;34:293–301. doi: 10.1002/mrm.1910340303. [DOI] [PubMed] [Google Scholar]
- Kunos G, Jarai Z, Batkai S, Goparaju SK, Ishac EJN, Liu J, Wang L, Wagner JA. Endocannabinoids as cardiovascular modulators. Chem Phys Lipids. 2000;108:159–168. doi: 10.1016/s0009-3084(00)00194-8. [DOI] [PubMed] [Google Scholar]
- Liu TT, Wong EC. A signal processing model for arterial spin labeling functional MRI. NeuroImage. 2005;24:207–215. doi: 10.1016/j.neuroimage.2004.09.047. [DOI] [PubMed] [Google Scholar]
- Lundqvist T, Jonsson S, Warkentin S. Frontal lobe dysfunction in long-term cannabis users. Neurotoxicol Teratol. 2001;23:437–443. doi: 10.1016/s0892-0362(01)00165-9. [DOI] [PubMed] [Google Scholar]
- Mathew RJ, Wilson WH. Acute changes in cerebral blood flow after smoking marijuana. Life Sci. 1993;52:757–767. doi: 10.1016/0024-3205(93)90239-y. [DOI] [PubMed] [Google Scholar]
- Mathew RJ, Wilson WH, Tant SR. Acute changes in cerebral blood flow associated with marijuana smoking. Acta Psychiatr Scand. 1989;79:118–129. doi: 10.1111/j.1600-0447.1989.tb08579.x. [DOI] [PubMed] [Google Scholar]
- Mathew RJ, Wilson WH, Humphreys DF, Lowe JV, Wiethe KE. Regional cerebral blood flow after marijuana smoking. J Cereb Blood Flow Metab. 1992;12:750–758. doi: 10.1038/jcbfm.1992.106. [DOI] [PubMed] [Google Scholar]
- Mathew RJ, Wilson WH, Coleman RE, Turkington TG, DeGrado TR. Marijuana intoxication and brain activation in marijuana smokers. Life Sci. 1997;60:2075–2089. doi: 10.1016/s0024-3205(97)00195-1. [DOI] [PubMed] [Google Scholar]
- Mathew RJ, Wilson WH, Turkington TG, Hawk TC, Coleman RE, DeGrado TR, Provenzale J. Time course of tetrahydrocannabinol-induced changes in regional cerebral blood flow measured with positron emission tomography. Psychiatry Res. 2002;116:173–185. doi: 10.1016/s0925-4927(02)00069-0. [DOI] [PubMed] [Google Scholar]
- Martin-Santos R, Fagundo AB, Crippa JA, Atakan Z, Bhattacharyya S, Allen P, Fusar-Poli P, Borgwardt S, Seal M, Busatto GF, McGuire P. Neuroimaging in cannabis use: a systematic review of the literature. Psychol Med. 2010;40:383–398. doi: 10.1017/S0033291709990729. [DOI] [PubMed] [Google Scholar]
- Murphy K, Dixon V, LaGrave K, Kaufman J, Risinger R, Bloom A, Garavan H. A validation of event-related fMRI comparisons between users of cocaine, nicotine, or cannabis and control subjects. Am J Psychiatry. 2006;163:1245–1251. doi: 10.1176/ajp.2006.163.7.1245. [DOI] [PubMed] [Google Scholar]
- O’Leary DS, Block RI, Koeppel JA, Flaum M, Schultz SK, Andreasen NC, Ponto LB, Watkins GL, Hurtig RR, Hichwa RD. Effects of smoking marijuana on brain perfusion and cognition. Neuropsychopharmacology. 2002;26:802–816. doi: 10.1016/S0893-133X(01)00425-0. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Neuropsychological performance in long-term cannabis users. Arch Gen Psychiatry. 2001;58:909–915. doi: 10.1001/archpsyc.58.10.909. [DOI] [PubMed] [Google Scholar]
- Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999;42:952–962. [PubMed] [Google Scholar]
- Randall MD, Kendall DA, O’Sullivan S. The complexities of the cardiovascular actions of cannabinoids. Br J Pharmacol. 2004;142:20–26. doi: 10.1038/sj.bjp.0705725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Jr, Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinburg BC, Nagel BJ, Eyler LT, Tapert SF. Neural correlates of verbal learning in adolescent alcohol and marijuana users. Addiction. 2011;106:564–573. doi: 10.1111/j.1360-0443.2010.03197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevy S, Smith GS, Ma Y, Dhawan V, Chaly T, Kingsley PB, Kumra S, Abdelmessih S, Eidelberg D. Cerebral glucose metabolism and D2/D3 receptor availability in young adults with cannabis dependence measured with positron emission tomography. Psychopharmacology (Berl) 2008;197:549–556. doi: 10.1007/s00213-008-1075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Wollrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DR, Niazy RK, Saunders J, Bickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith ML, Barnes AJ, Huestis MA. Identifying new cannabis use with urine creatinine-normalized THCCOOH concentrations and time intervals between specimen collections. J Anal Toxicol. 2009;33:185–189. doi: 10.1093/jat/33.4.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneider JT, Pope HG, Silveri MM, Simpson NS, Gruber SA, Yurgelun-Todd DA. Differences in regional blood volume during a 28-day period of abstinence in chronic cannabis smokers. Neuropsychopharmacology. 2008;18:612–619. doi: 10.1016/j.euroneuro.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Totowa, NJ: Humana Press; 1992. Timeline follow back. A technique for assessing self-reported alcohol consumption. [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the state-trait anxiety inventory (self-evaluation questionnaire) Palo Alto, CA: Consulting Psychologists; 1970. [Google Scholar]
- Takahashi T, Shirane R, Sato S, Yoshimoto T. Developmental changes of cerebral blood flow and oxygen metabolism in children. AJNR. 1999;20:917–922. [PMC free article] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Drummond SP, Paulus MP, Brown SA, Yang TT, Frank LR. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology (Berl) 2007;194:173–183. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunving K, Thulin SO, Risberg J, Warkentin S. Regional cerebral blood flow in long-term heavy cannabis use. Psychiatry Res. 1986;17:15–21. doi: 10.1016/0165-1781(86)90037-5. [DOI] [PubMed] [Google Scholar]
- van Hell H, Bossong MG, Jager G, Kristo G, van Osch MJP, Zelaya F, Kahn RS, Ramsey NF. Evidence for involvement of the insula in the psychotropic effects of THC in humans: a double-blind, randomized pharmacological MRI study. Int J Neuropsychopharmacol. 2011;14:1377–1388. doi: 10.1017/S1461145711000526. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Gillespie H, Mullani N, Tancredi L, Grant C, Valentine A, Hollister L. Brain glucose metabolism in chronic marijuana users at baseline and during marijuana intoxication. Psychiatry Res. 1996;67:29–38. doi: 10.1016/0925-4927(96)02817-x. [DOI] [PubMed] [Google Scholar]
- Wang J, Qiu M, Constable RT. In vivo method for correcting transmit/receive nonuniformities with phased array coils. Magn Reson Med. 2005;53:666–674. doi: 10.1002/mrm.20377. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WAIS-III manual. New York, NY: Psychological Corporation; 1997. [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence-administration and scoring manual. London, UK: Psychological Corporation; 1999. [Google Scholar]
- Wilkinson GD. WRAT-3 Wide range achievement test administration manual. 3rd edn. Wilmington, DE: Western Psychological Services; 1993. [Google Scholar]
- Wong EC, Buston RB, Frank LR. Quantitative imaging of perfusion using a single subtraction (QUIPSS and QUIPSS II) Magn Reson Med. 1998;39:702–708. doi: 10.1002/mrm.1910390506. [DOI] [PubMed] [Google Scholar]
- Zubieta J, Lombardi U, Minoshima S, Guthrie S, Ni L, Ohl LE, Koeppe RA, Domino EF. Regional cerebral blood flow effects of nicotine in overnight abstinent smokers. Biol Psychiatry. 2001;49:906–913. doi: 10.1016/s0006-3223(00)01070-2. [DOI] [PubMed] [Google Scholar]