Abstract
The Nicotrol® (Pfizer, USA) nicotine inhaler reduces craving by mimicking the behavioural component of cigarettes and delivering controlled doses of nicotine, which binds to the beta-2 subunit-containing nicotinic acetylcholine receptors (β2*-nAChRs). Previous studies examined β2*-nAChR occupancy after administration of regular and low-nicotine cigarettes. Here, we measured occupancy of β2*-nAChRs after administration of nicotine via inhaler, and the relationship between occupancy and changes in craving for tobacco smoking and withdrawal symptoms. Tobacco smokers participated in [123I]5-IA-85380 SPECT studies with either a nicotine inhaler (n=9) or tobacco cigarette (n=4) challenge. [123I]5-IA was administered as a bolus plus constant infusion. After equilibrium was achieved, three 30-min baseline scans were collected, and subjects either used the nicotine inhaler or a regular cigarette, and up to six additional scans were obtained. Receptor occupancy was determined based on the Lassen plot method. Craving for tobacco smoking and withdrawal symptoms were evaluated pre- and post-challenge. Use of the nicotine inhaler produced an average 55.9±6.4% occupancy of β2*-nAChRs 2–5 h post-challenge, whereas use of a cigarette produced significantly higher receptor occupancy (F=10.6, p=0.009) with an average 67.6±14.1% occupancy 1.5–5 h post-challenge. There was a significant decrease in withdrawal symptoms post-nicotine inhaler use (F=6.13, p=0.04). These results demonstrate significant differences in occupancy of β2*-nAChRs by nicotine after use of the inhaler vs. a cigarette and confirm the ability of the nicotine inhaler to relieve withdrawal symptoms.
Keywords: β2*-nAChR occupancy, nicotine, nicotine inhaler, SPECT brain imaging
Introduction
Smoking is the leading cause of preventable disease in the USA. Recent data suggest that compared to non-smokers, smokers are 15 times more likely to develop lung cancer, and twice as likely to develop myocardial infarction (Meyers & Neuberger, 2008). Nearly 60% of current smokers attempt to quit each year, but the vast majority of these individuals relapse to smoking in <12 months (ACS, 2009). Therefore, effective treatments for smoking cessation are urgently required.
Nicotine is one of the most addictive components of the >4000 chemicals in tobacco smoke. Nicotine replacement therapies (NRTs), including chewing gum, transdermal patch, nasal spray, inhaler, sublingual tablet and lozenge are still the primary aids for smoking cessation and were developed to deliver nicotine without the harmful tars associated with tobacco smoke. Quit rates for smokers using these products range from 15–25% for 1 yr (Tønnesen, 2009). The dose of nicotine delivered to the brain depends upon the site and mode of nicotine administration (Gourlay & Benowitz, 1997) but all NRTs deliver nicotine more slowly, and at reduced levels, compared to a cigarette. Inhalation of nicotine via cigarette smoking is the most efficient method for delivery of a rapid nicotine bolus to the brain by transfer through pulmonary circulation and this pharmacokinetic profile probably contributes to tobacco addiction.
Nicotine binds to beta-2 subunit-containing nicotinic acetylcholine receptors (β2*-nAChRs; where * represents other subunits that may also be part of the receptor), and preclinical studies suggest that these receptors are critical for initiating the events leading to tobacco smoking addiction (Epping-Jordan et al. 1999; Maskos et al. 2005; Picciotto et al. 1998; Zoli et al. 1998). It has been shown previously that smoking a single cigarette occupies up to 88% of available β2*-nAChRs (Brody et al. 2006), and smoking a de-nicotinized (0.05 mg nicotine content) or low-nicotine (0.6 mg nicotine content) cigarette occupies up to 26% and 78% of available β2*-nAChRs, respectively (Brody et al. 2009). Further, it has been suggested that only after smoking at least one full cigarette (and having >80% β2*-nAChR occupancy by nicotine) was the craving for tobacco smoke significantly decreased (Brody et al. 2006). However, evaluation of the effectiveness of other NRTs, such as the nicotine inhaler, at targeting the β2*-nAChRs and the relationship to craving and withdrawal has not been examined.
We chose to study nicotine delivery via the Nicotrol® (Pfizer, USA) nicotine inhaler since it is easy to administer, is well tolerated, mimics the behavioural components associated with cigarette administration, and is a frequently used form of NRT. Several groups have evaluated the potential efficacy of the nicotine inhaler as a tobacco-smoke substitute but the results have been inconsistent. Regular use (6–11 inhalers per day) appears to relieve smoking cessation-associated craving symptoms, at least short-term (1 wk) (Lunell et al. 1995; Schneider et al. 1996). However, other studies found the inhaler inferior to other NRTs (including gum, lozenges, patch, nasal spray) in relieving craving symptoms over time (Schneider et al. 2005, 2008).
The aim of the current study was to measure β2*-nAChR occupancy by nicotine following administration of the Nicotrol nicotine inhaler using [123I]5-IA-85380 ([123I]5-IA) single photon emission computed tomography (SPECT) imaging and to examine the relationship between β2*-nAChR occupancy and craving and withdrawal symptoms associated with smoking cessation. [123I]5-IA measures β2*-nAChR availability in the brain, has excellent test–retest reproducibility (Staley et al. 2005), and has been previously used to measure receptor occupancy by nicotine after smoking to satiety (Esterlis et al. 2010). We hypothesized that occupancy of the β2*-nAChR by nicotine would be lower after one-time intensive use of the nicotine inhaler compared to smoking one cigarette [which has been estimated at 88% (Brody et al. 2006)] due to differences in the pharmacokinetic profile of delivery of nicotine. Specifically, after use of the nicotine inhaler the majority of nicotine becomes deposited in the oral cavity, oesophagus, and stomach, with only a small proportion reaching the lungs, whereas a significantly larger portion of nicotine reaches the lungs after regular cigarette use (Lunell et al. 1996). Therefore, since there is a positive association between the amount of nicotine reaching the lungs and the amount of nicotine reaching the brain, we hypothesized lower β2*-nAChR occupancy by nicotine after the nicotine inhaler vs. control cigarette use. We also hypothesized that the use of the nicotine inhaler would relieve the immediate withdrawal and craving symptoms, and the degree of relief would be associated with receptor occupancy by nicotine.
Methods
Participants
Thirteen [inhaler group (n=9), cigarette group (n=4)] medically and neurologically healthy tobacco smokers provided written informed consent to participate in this study, which was conducted at Yale University and the West Haven VACHS, and was approved by the Human Investigational Review Committees at both institutions. Eligibility was evaluated via structured interview, behavioural assessments, physical examination, laboratory blood tests, urine drug screen, and an electrocardiogram. All subjects met the following criteria : (1) aged 18–50 yr; (2) English speakers, at least 6th-grade education; (3) able to give voluntary written informed consent; (4) no current medical condition such as neurological, cardiovascular, endocrine, renal, liver, or thyroid pathology; (5) had not used regularly any prescription, herbal or illegal psychotropic medications (e.g. antidepressants, antipsychotics, anxiolytics, ecstasy) for at least 1 yr ; (6) consumed <21 drinks/week and no more than 5 drinks per occasion; (7) had not used marijuana in the past 30 d and had not met criteria for dependence in the last 5 yr ; (8) had no DSM-IV diagnosis in last 10 yr (other than nicotine dependence; no depression or anxiety disorders in the past 2 yr) ; (9) not pregnant or breastfeeding; (10) did not have a pacemaker or other ferromagnetic material in body; (11) not claustrophobic; (12) smoke 10–40 cigarettes/d ; (13) had plasma cotinine levels >50 ng/ ml at intake; (14) had carbon monoxide (CO) levels ≥8 ppm at intake ; and (15) had a Fagerström Test for Nicotine Dependence (FTND) score ≥2.
Assessments
The severity of nicotine dependence was assessed using the FTND (Heatherton et al. 1991) at intake only. Nicotine withdrawal symptoms were assessed using the Minnesota Withdrawal Questionnaire (MWQ; Hatsukami et al. 1984), and craving was assessed using the Tiffany Smoking Urges Questionnaire (Tiffany & Drobes, 1991) on SPECT scan day pre- and post-nicotine inhaler challenge. Two factors of Tiffany Smoking Urges Questionnaire were employed here: desire (positive symptoms associated with wanting a cigarette ; e.g. ‘ I have an urge to smoke’) and relief (withdrawal relief expected if cigarette is smoked; e.g. ‘Nothing would be better than smoking a cigarette right now’).
Smoking cessation
Subjects were helped to abstain from smoking for 7 d prior to their SPECT scan day using brief behavioural counselling based on Clinical Practice Guidelines and contingency management (Roll et al. 1996; Stitzer & Bigelow, 1985; Stitzer et al. 1986). They were asked to abstain from smoking for approximately 7 d since this had been previously identified as the time period necessary for nicotine and its metabolites to clear from the body and brain (Benowitz & Jacob, 1997; Staley et al. 2005, 2006), to prevent interference with radiotracer binding. Subjects were not allowed to use any NRTs or other medications during this time. All smokers were met on 3–4 occasions during the abstinence period. Subjects were briefly counselled (10–15 min) on smoking cessation tailored to their life-style and provided with suggestions for quitting smoking. During the first few days, abstinence was confirmed via continuous reduction in breath CO and urine cotinine levels. At least 2 d prior to the SPECT scan, breath CO levels and urine cotinine levels had to be <10 ppm and <100 ng/ml, respectively. Subjects were compensated $10 for each day of smoking cessation. Participants who could not abstain from tobacco smoke or nicotine products for the duration of the study were withdrawn from further participation.
Use of nicotine inhaler
Subjects were instructed to puff on the inhaler as they would on a typical cigarette. A practice session (without nicotine cartridge) was administered prior to the SPECT scan day to acclimate subjects to the inhaler prior to the challenge session. It has been shown previously that depth of puff inhalation does not alter amount of nicotine delivery, since most of the delivery from the inhaler is to the oral cavity and not the lungs as in a cigarette (Bergstrom et al. 1995). Thus, subjects were allowed to puff as deeply as felt comfortable in order to avoid adverse events. Subjects were told to puff every 15 s for 20 min in order to deliver 80 puffs. Continuous puffing over a 20-min period or periodic use of 400 puffs has previously been shown to release on average 4 mg nicotine of which 2 mg is systemically absorbed (Pfizer, 2002; Schneider et al. 2001).
Use of regular cigarette
Subjects in the cigarette group were part of a preliminary study. They were instructed to smoke one cigarette after the baseline SPECT scans via the puff device (CreSS from Plowshare Technologies, USA), which provides measures of depth of puff inhalation and volume. They puffed on the cigarette once every 15 s for as many puffs as was required to finish the cigarette.
Magnetic resonance imaging (MRI) imaging and [123I]5-IA SPECT
MRI
Each subject participated in one MRI scan prior to SPECT scanning for subsequent anatomic SPECT volume of interest (VOI) placement. The MRI was performed on a Signa 1.5 T system (General Electric Co., USA). Axial images were acquired parallel to the anteroposterior commissural line with an echo time of 5 ms, repetition time of 24 ms, matrix 256×192, number of excitations 1, field of view of 24 cm, and 128 contiguous slices with a thickness of 1.3 mm.
SPECT
All emission scans were obtained on a Philips PRISM 3000 XP (USA) SPECT camera. The PRISM 3000 XP is a three-headed camera equipped with a low-energy, ultra-high resolution fanbeam collimator (photopeak window, 159 keV ±10%; matrix 128×128) with a uniform sensitivity across the field of view. A 57Co-distributed source was measured with each experiment to control for day-to-day variation in camera sensitivity. The axial resolution (full-width at half maximum) is 12.2 mm, measured with a 123I line source in water in a cylindrical phantom. [123I]5-IA was synthesized as previously described (Zoghbi et al. 2001) and administered using a bolus plus constant infusion paradigm with a ratio of 7.0 h (calculated as bolus in MBq divided by infusion rate in MBq/h) and total injected dose over the full SPECT scan day (accounting for decay) of 342.7±49.2 MBq for the inhaler group and 334.7±34.7 MBq for the cigarette group. The [123I]5-IA infusion lasted the duration of the scan day in order to maintain radiotracer level at equilibrium in the blood and brain (see Fig. 1 for overview of scan day procedures). Approximately 6 h following injection of [123I]5-IA, one 15-min scan (for inhomogeneous attenuation correction) and three 30-min equilibrium scans were obtained. Blood samples were collected in the middle of the second scan to quantify total parent and free fraction of parent tracer in plasma (fp, free fraction) (Zoghbi et al. 2001). Subjects were then removed from the camera, an arterial line was placed, and subjects were instructed to puff on the Nicotrol nicotine inhaler (10 mg available nicotine) every 15 s for 20 min or to puff on the cigarette every 15 s for the duration of the cigarette. Since initial nicotine levels in arterial blood are higher than in venous samples (Henningfield et al. 1993), arterial blood samples were collected for 30 min after the start of the nicotine challenge, starting at the time of inhalation of first puff, to determine nicotine and cotinine levels, after which time venous samples were collected hourly until study completion. Thereafter, two sets of three [123I]5-IA SPECT scans were acquired over 4 h following inhaler use (all but one subject participated in all scans) to evaluate occupancy of the β2*-nAChRs by nicotine throughout the brain. Blood samples were collected in the middle of each post-smoking scan session, again to quantify total parent and fp, in order to correct for individual differences in metabolism and protein binding of the radiotracer [123I]5-IA.
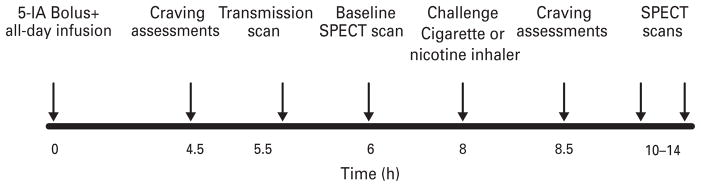
Fig. 1.
SPECT scan day procedures over a 14-h period. Subjects were infused with the radiotracer for the duration of the study. They completed craving and withdrawal assessments post-[123I]5-IA infusion start. At 5.5 h post-infusion STEP scan was obtained, followed by three SPECT emissions at 6–8 h post-infusion start. Then a nicotine challenge (inhaler or cigarette) was administered, subjects again completed questionnaires, and were placed in the camera 10–14 h post-infusion initiation for an additional two sets of three SPECT emissions.
Measurement of plasma concentrations of nicotine
Nicotine concentrations in plasma were assayed using reversed-phase HPLC. The procedure (Hariharan et al. 1988) was modified from the published assay by substitution of an aqueous micro-back-extraction clean-up step in place of solvent evaporation. Between-day coefficients of variation in routine use at nicotine concentrations of 20 and 4 ng/ml (the lower limit of quantitation) were 6.8% and 13.8%, respectively.
Image analysis
SPECT emission images were analysed as described previously (Staley et al. 2005). Specifically, SPECT emission images were reconstructed using a filtered back-projection algorithm with a ramp filter on a 128×128 matrix to obtain 50 slices with a pixel size of 2.06×2.06×3.56 mm in the x, y, and z axes. A three-dimensional (3D) Butterworth filter (order 10, cut-off frequency 0.24 cycle/pixel) was applied post-hoc. A co-registered MR image was used to guide the placement of standard 2D ROI templates using MEDx software (Medical Numerics Inc., USA). A 3D VOI was generated for each region and transferred to the co-registered SPECT image to determine regional radioactive densities. The chosen regions were those known to contain β2*-nAChRs and included the frontal, parietal, anterior cingulate, temporal, and occipital cortices, the thalamus, the striatum (an average of caudate and putamen) and the cerebellum. Regional [123I]5-IA uptake was determined by VT/fp, where VT is volume of distribution and fp is free plasma fraction. This measure was previously designated as VT, but the nomenclature was changed based on Innis et al. (2007). Each case was analysed by two raters, and the mean of the two raters was used. Inter-rater variability for VT/fp was <10% across all regions, which was computed as percent difference between two raters with the following equation:
Determination of receptor occupancy
VT/fP data, computed directly from the tissue and plasma radioactivity measurements from the pre-challenge baseline and post-challenge scans, were analysed by use of Lassen plots for assessment of occupancy of targets for which a brain tissue reference region is not available. This has been validated by a recent study (Cunningham et al. 2010) and used in our recent publication (Esterlis et al. 2010) with the equation for the line [y=rx+b], where y=[VT/ fp(baseline) – VT/fp(post-nicotine)], and x=VT(baseline), and linear regression of the fit to different brain regions provides receptor occupancy (r). This approach assumes that there is uniform receptor occupancy and non-displaceable binding across brain regions. Receptor occupancy was derived for each subject across all brain regions for each post-challenge scan (compared to the pre-challenge baseline), and the final result represents the average across all post-challenge scans for each subject.
Statistical analysis
All statistical analyses were performed using SPSS version 16.0 (SPSS Inc., USA). Repeated-measures analyses of variance were used to examine differences in withdrawal and craving scores, as well as receptor availability before and after the each challenge. Analysis of variance was used to compare receptor occupancy of the two challenge conditions. Non-parametric correlational analyses (Spearman’s ρ correlation coefficient) were used to examine the relationship between receptor occupancy and smoking variables and plasma nicotine levels. Statistical significance was set at p≤0.05, two-tailed.
Results
Nicotine inhaler challenge
Participants
Healthy tobacco smokers were four men and five women aged 36.1±12.9 yr who smoked an average of 15.2±4.1 cigarettes/d for 15.2±12.2 yr and were moderately dependent on nicotine (FTND score 3.4±2.1). Smoking status was verified by urine cotinine (516.7±285.8 ng/ml) and breath CO (15.9± 7.1 ppm) levels at intake. Participants abstained from smoking for 5.4±0.9 d prior to their SPECT scan day, and this was verified by negligible urine cotinine (13.3±10 ng/ml) and CO (3.8±3.4 ppm) levels.
Plasma nicotine levels
Nicotine concentration values for each subject are shown in Table 1. [Note that subject no. 6 in the inhaler group had significantly lower (>2 S.D. from the mean) blood nicotine values compared to the group mean and was therefore excluded from further analyses.] For the rest of the sample the average time to maximum nicotine concentration in plasma (Tmax) was 27.8±19.8 min; and the average peak concentration of nicotine (Cmax) was 8.0±4.1 ng/ml (Table 1, Fig. 2).
Table 1.
Plasma nicotine, receptor occupancy, and smoking variables associated with nicotine inhaler challenge
| Subject no. | Average % receptor occupancy | Plasma nicotine
|
Change in withdrawal and craving symptomsa
|
|||
|---|---|---|---|---|---|---|
| Tmax (min) | Cmax (ng/ml) | MWQ | Desireb | Reliefb | ||
| Inhaler challenge | ||||||
| 1 | 52.92 | 20.00 | 7.80 | 0 | 5 | 1 |
| 2 | 45.59 | 60.00 | 5.70 | −1 | 9 | 0 |
| 3 | 56.56 | 30.00 | 5.60 | 9 | 10 | 18 |
| 4 | 63.89 | 20.00 | 10.10 | 3 | 0 | −2 |
| 5 | 65.35 | 20.00 | 16.40 | −1 | 2 | 0 |
| 6c | 34.97 | 0.30 | 4.20 | 0 | −8 | −3 |
| 7 | 56.29 | 20.00 | 12.00 | 6 | 4 | 4 |
| 8 | 53.47 | 20.00 | 5.80 | 9 | −3 | 16 |
| 9 | 52.79 | 20.00 | 4.30 | 11 | −1 | 4 |
| Average | 53.0±10.1 | 27.8±19.8 | 8.0±4.1 | 4.0±4.8 | 2.0±5.7 | 4.2±7.6 |
| Cigarette challenge | ||||||
| 1 | 66.77 | 4.00 | 64.00 | −2 | ||
| 2 | 73.84 | 6.00 | 89.00 | 4 | ||
| 3 | 69.49 | 4.00 | 105.70 | 0 | ||
| 4 | 60.27 | 4.00 | 115.20 | 3 | ||
| Average | 67.6±5.7 | 4.5±1.0 | 93.5±22.4 | 1.3±2.8 | ||
Tmax, Time to maximum nicotine detection in blood; Cmax, peak nicotine concentration in blood; MWQ, Minnesota Withdrawal Questionnaire.
Change=scorepre-challenge – scorepost-challenge.
Desire and relief scores not obtained for the regular cigarette condition, which was a part of a different study.
Denotes outlier in the nicotine inhaler sample: plasma nicotine levels significantly lower than in the rest of the sample. Excluded from statistical analyses.
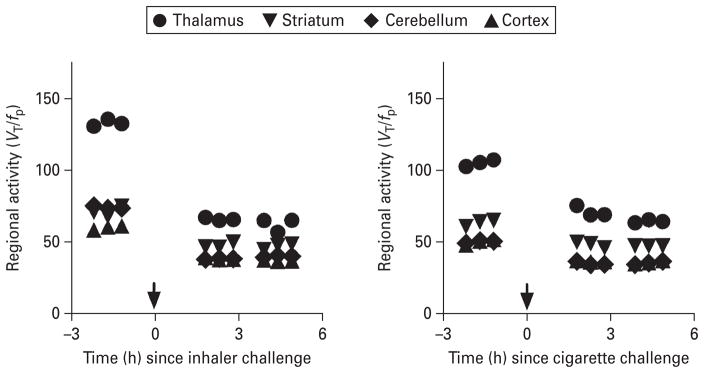
Fig. 2.
Time–activity curves before and after Nicotrol nicotine inhaler (left : 30-yr-old Hispanic man, 276.8 MBq injected total) and regular cigarette use (right : 22-yr-old Asian woman, 301.6 MBq injected total) abstinent for 1 wk prior to scan and imaged using [123I]5-IA SPECT. Equilibrium, defined as <5% change per hour, was reached prior to challenge. Three equilibrium scans were obtained, the nicotine inhaler or cigarette were administered, and the subject then participated in up to six post-inhaler scans starting 2 h after administration of nicotine inhaler.
Receptor occupancy by nicotine
Equilibrium, defined as ≤5% change in receptor availability per hour (VT/fp.h), was achieved between 6 and 8 h after injection (Fig. 1; representative subject – average percent change was 1.3%/h thalamus, 5.0%/h striatum, 4.2%/h cortex, 2.0%/h cerebellum). Subjects were placed back in the camera at 108.2 (±22.1) min post-initiation of nicotine inhaler challenge. There was minimal change in radiotracer binding during the 2–5 h post-inhaler use (Figs 2, 3), suggesting a pseudo-equilibrium state was achieved at a time similar to our previous report (Esterlis et al. 2010). The decrease in β2*-nAChR availability (which corresponds to receptor occupancy by nicotine) was dramatic, with peak occupancy of 60.0±9.5% reached at 3 h post-challenge. The range of occupancy was 45.6–63.9% across subjects and scans with an average occupancy across all scans (2–5 h post-challenge) of 55.9±6.4% (Table 1).
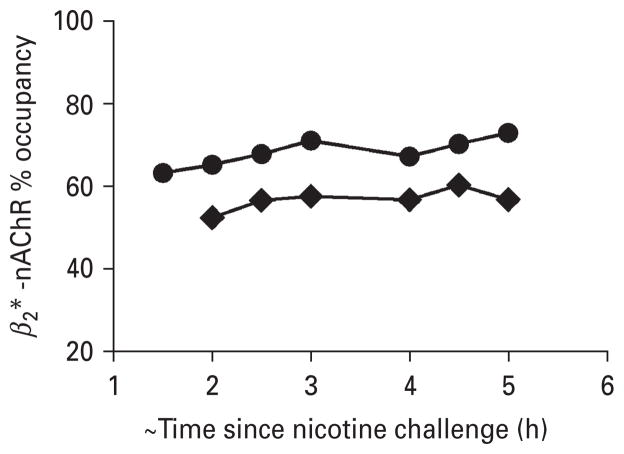
Fig. 3.
β2*-nAChR occupancy in each subject over the 4 h of scan time after the nicotine challenge. Subjects were placed in the camera on average 108.2 (±22.1) min post-initiation of nicotine inhaler challenge (–◆–) and 91.8 (±20.3) min post-initiation of cigarette challenge (–●–). Average receptor occupancy across subjects and scans was 56.7±6.7% for nicotine inhaler, which is less than after smoking one regular cigarette (66.4±5.7 %).
Withdrawal and craving variables
Prior to the inhaler challenge on SPECT scan day, subjects reported moderate levels of withdrawal (8.0± 6.7), and craving [desire (28.3±11.4), relief (18.3± 11.9)] symptoms. Change in withdrawal and craving symptoms was calculated as: scorepre-challenge – scorepost-challenge. There was a significant reduction in withdrawal symptoms after use of the inhaler (50% decrease; F=5.51, p=0.05) (Table 1). There were not significant changes in craving measures after use of the inhaler.
Relationship between receptor occupancy and smoking variables
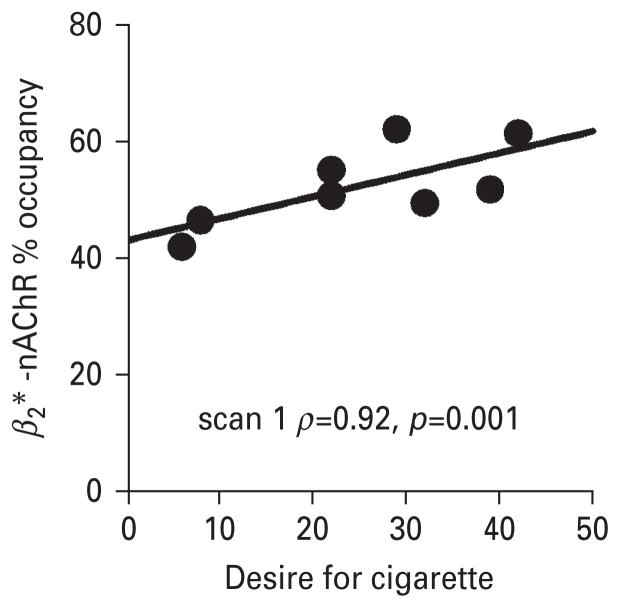
We examined potential associations between β2*-nAChR occupancy and withdrawal and craving scores in relation to the nicotine inhaler challenge. We found a significant positive association between receptor occupancy and desire for cigarettes both before and after nicotine inhaler administration [before cigarette : scan 1 (ρ=0.92, p=0.001) ; scan 2 (ρ=0.90, p=0.002) ; after cigarette : scan 1 (ρ=0.85, p=0.007) ; scan 2 (ρ=0.89, p=0.003)] (Fig. 4), such that, high desire to smoke a cigarette was associated with higher β2*-nAChR occupancy. However, there were no significant associations between receptor occupancy by nicotine and pre-post inhaler administration change in withdrawal symptoms.
Fig. 4.
Subjects with a greater desire to smoke a cigarette after use of the nicotine inhaler had higher β2*-nAChR occupancy by nicotine. Individual subject data are shown in the scatter plot and the regression line represents correlation results for scan 1 (ρ=0.92, p=0.001).
Regular cigarette control group
Participants
Healthy tobacco smokers were two men and two women aged 28.8±7.3 yr who smoked an average of 14.0±4.2 cigarettes/d for 12.4±7.9 yr and were moderately dependent on nicotine (FTND score 3.2±2.6). They did not significantly differ on any demographic and smoking variables compared to the inhaler group. Smoking status was verified by urine cotinine (550.0±346.4 ng/ml) and breath CO (12.4±3.7 ppm) levels at intake. Participants abstained from smoking for 6.6±2.1 d prior to their SPECT scan day, and this was verified by negligible urine cotinine (38±24.6 ng/ml) and CO (4.4±4.0 ppm) levels.
Plasma nicotine levels
Tmax and Cmax values are listed in Table 1 for each subject. The average Tmax to nicotine concentration in plasma was significantly faster (4±1.4 min) and the average Cmax was significantly higher (78.1±39.5 ng/ml) in the cigarette group compared to the inhaler group (Table 1, Fig. 2).
Receptor occupancy by nicotine
Subjects were placed back in the camera at 91.8 (±20.3) min post-initiation of cigarette challenge. Similar to the nicotine inhaler results, there was minimal change in radiotracer binding during the 2–5 h post-cigarette use (Figs 2, 3). The decrease in β2*-nAChR availability (which corresponds to receptor occupancy by nicotine) was significantly higher than in the inhaler group (F=10.6, p=0.009), with peak average occupancy of 65.7±12.3% reached at 3 h postchallenge. The range of occupancy in the cigarette group was 41.4–73.8% across subjects and scans with an average occupancy across all scans of 62.35±12.7%.
Withdrawal and craving variables
Prior to the cigarette challenge on SPECT scan day, subjects reported moderate levels of withdrawal symptoms (6.7±5.9) (craving measures were not obtained from this subgroup because it was part of a preliminary study). In contrast to the inhaler group, change in withdrawal pre- to post-challenge was not significant.
Relationship between receptor occupancy and smoking variables
We examined potential associations between β2*-nAChR occupancy and withdrawal following smoking challenge and found a significant positive association with withdrawal reduction only (ρ =0.95, p=0.05).
Discussion
The present study evaluated the efficacy of the Nicotrol nicotine inhaler at delivering nicotine to β2*-nAChRs for which nicotine has a high affinity. Average occupancy of β2*-nAChRs following administration of the Nicotrol nicotine inhaler is 56%, which is less than that achieved after smoking a regular cigarette in the present study. The maximum average nicotine concentration in the blood in the present study was less than that achieved after use of one cigarette (8 ng/ml vs. 78.1±39.5 ng/ml) but similar to those attained after use of the nicotine inhaler (Caldwell et al. 2009; Schneider et al. 2001), although previous studies sampled venous not arterial blood nicotine concentration and the nicotine dose from the inhaler varied across studies.
We chose to study the nicotine inhaler since it most closely mimics the sensory components of smoking tobacco, is well tolerated by tobacco smokers and is frequently used by smokers trying to quit smoking. We determined that intense use of the inhaler (80 puffs in 20 min) does not mimic smoking a regular nicotine 1.2–1.4 mg cigarette in its ability to deliver nicotine to the brain. Further, the nicotine inhaler was not effective for relieving craving symptoms. However, although our cigarette sample was not administered craving measures, a previous study showed that a minimum of one cigarette is required to relieve craving symptoms in smokers (Brody et al. 2006).
We also found a significant reduction in withdrawal symptoms associated with nicotine administration via the inhaler, which is consistent with previous research that showed use of the nicotine inhaler caused a reduction in withdrawal symptoms associated with smoking cessation (Lunell et al. 1995). However, we did not observe a significant reduction in craving variables. Previous reports (Lunell et al. 1995; Schneider et al. 1996) suggested that consistent daily use of the nicotine inhaler is required to significantly decrease cigarette craving, thus the one-time intensive administration of nicotine via the inhaler in the present study was not sufficient. Further, Brody and colleagues demonstrated that smoking at least one cigarette is required to relieve cigarette craving symptoms (Brody et al. 2006). Since the nicotine inhaler group did not achieve occupancy levels to the degree of the cigarette-smoking group, it is likely the inhaler may not be delivering enough nicotine to reduce craving for a cigarette. Thus, there may be some relief for the smoker who is trying to quit smoking when using the nicotine inhaler as a NRT, as it may be effective at alleviating some of the symptoms associated with withdrawal. However, use of the nicotine inhaler does not appear to be a sufficient method in the reduction of craving symptoms.
We observed a significant positive correlation between receptor occupancy and desire for cigarette before and after nicotine inhaler administration. This suggests that although the nicotine inhaler is able to provide nicotine to the receptors, it is not delivering a high enough dose to medicate the high craving for a cigarette. In fact, Brody and colleagues concluded that use of at least one regular cigarette (and thus greater receptor occupancy) is required to achieve significant reduction in craving, and this is not accomplished by a low-nicotine cigarette or a few puffs of a regular cigarette (Brody et al. 2006, 2009). This provides evidence that nicotine’s direct effect on β2*-nAChRs plays an important role in smoking addiction and craving relief. Importantly, there are more than 4000 other chemicals in cigarette smoke in addition to nicotine, including monoamine oxidase inhibitors (MAOI; harman and norharman) (Kahlil et al. 2000), which play a role in craving reduction. Additionally, smokers experience sensory and behavioural cues from cigarettes that may not be mimicked by the nicotine inhaler or any other NRT (Benowitz, 1999; Monchuk et al. 2007; Rose & Behm, 2004). Thus, the absence of a combination of these variables may prevent a reduction in cigarette craving with nicotine inhaler use.
In the present study we demonstrate that nicotine occupancy of β2*-nAChRs after use of the nicotine inhaler is prolonged but lower than that observed after smoking a regular cigarette. Although the sample size of the present study is small, the low variability in receptor occupancy between subjects in each group suggests this is a valid measure of the occupancy of β2*-nAChRs. However, smokers tend to smoke every 1–2 h during waking hours, which is much more frequent than would be expected by the long time-course of receptor occupancy. One possible explanation for frequent smoking is that it may be an attempt to avoid free unbound receptors. If at least 80% receptor occupancy by nicotine is related to alleviation of craving as shown previously (Brody et al. 2006), then it is possible that smokers may continue to smoke to occupy all free receptors and to either alleviate the desire for a cigarette or to prevent the desire from occurring in the first place. In addition, as suggested by several groups, tobacco smokers may continually seek the euphoric effects provided by the surge in dopamine release related to the use of nicotine and tobacco products (Nestler, 2005; Rice & Cragg, 2004; Zhang & Sulzer, 2004). They may also continue to smoke frequently to obtain relief of mood symptoms, or enhancement of relief associated with gamma-aminobutyric acid release during tobacco-smoke administration (Brody et al. 2006; Mansvelder et al. 2002; Rice & Cragg, 2004; Sofuoglu et al. 2005; Stolerman & Shoaib, 1991). Further, we have previously suggested that the slow kinetics of the radiotracer might not accurately model the correct time-period for maximal occupancy of β2*-nAChRs by nicotine (Esterlis et al. 2010) since [123I]5-IA is characterized by a slow dissociation of the receptor–ligand complex and slow clearance from brain (Endres & Carson, 1998; Fujita et al. 2010; Mukhin et al. 2000; Vaupel et al. 1998). This means that radioligand binding to the receptor does not instantaneously match the quantity of available receptors. Nicotine delivered through tobacco smoke reaches the brain within ~30 s of inhalation. Therefore, nicotine may have reached higher occupancy in the brain prior to the dissociation of the radioligand from the receptor, better explaining more frequent smoking intervals.
In conclusion, this is the first study to evaluate β2*-nAChR occupancy by nicotine after use of the nicotine inhaler and to examine related withdrawal and craving symptoms in tobacco smokers. We showed that the nicotine delivered via the inhaler occupies fewer β2*-nAChRs than smoking one regular or low-nicotine cigarette as previously calculated, but more than smoking one denicotinized cigarette. We also suggest that nicotine inhaler administration, even intensive puffing in a short period of time, does not deliver enough nicotine to reduce craving for tobacco smoke in smokers with high symptoms of cigarette desire.
Acknowledgments
We thank Louis Amici for radiochemistry. We are grateful to Richard E. Carson for intellectual contribution. We also thank Gina Nicoletti, Andrea Perez, and Jane Bartosik for technical support. Funding was provided by RO1DA015577, R21 DA020788, P50AA15632, KO2DA021863, KO1 DA020651 and VA Career Development Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Addiction and Alcoholism, the National Institute on Drug Abuse, or the National Institutes of Health.
Footnotes
Statement of Interest
None
References
- ACS. Smoking Cessation Aids Use Increases, Success Rate Declines. American Cancer Society; 2009. ( http://www.cancer.org/index) [Google Scholar]
- Benowitz N. Nicotine addiction. Primary Care. 1999;26:611–631. doi: 10.1016/s0095-4543(05)70120-2. [DOI] [PubMed] [Google Scholar]
- Benowitz N, Jacob P. Individual differences in nicotine kinetics and metabolism in humans. NIDA Research Monographs. 1997;173:48–64. [PubMed] [Google Scholar]
- Bergstrom M, Nordberg A, Lunell E, Aton G, et al. Regional disposition of inhaled 11C-vapor in the human airways as visualized by positron emission tomography. Clinical Pharmacological Therapy. 1995;57:309–317. doi: 10.1016/0009-9236(95)90156-6. [DOI] [PubMed] [Google Scholar]
- Brody A, Mandelkern M, Costello M, Abrams A, et al. Brain nicotinic cetylcholine receptor occupancy: effect of smoking a denicotinized cigarette. International Journal of Neuropsychopharmacology. 2009;12:305–316. doi: 10.1017/S146114570800922X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody A, Mandelkern M, London E, Olmstead R, et al. Cigarette smoking saturates brain alpha 4 beta 2 nicotinic acetylcholine receptors. Archives of General Psychiatry. 2006;63:907–915. doi: 10.1001/archpsyc.63.8.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell B, Dickson S, Burgess C, Siebers R, et al. A pilot study of nicotine delivery to smokers from a metered-dose inhaler. Nicotine and Tobacco Research. 2009;11:342–347. doi: 10.1093/ntr/ntp027. [DOI] [PubMed] [Google Scholar]
- Cunningham V, Rabiner E, Slifstein M, Laruelle M, Gunn RN. Measuring drug occupancy in the absence of a reference region: the Lassen plot revisited. Journal of Cerebral Blood Flow and Metabolism. 2010;30:46–50. doi: 10.1038/jcbfm.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres C, Carson R. Assessment of dynamic neurotransmitter changes with bolus or infusion delivery of neuroreceptor ligands. Journal of Cerebral Blood Flow and Metabolism. 1998;18:1196–1210. doi: 10.1097/00004647-199811000-00006. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan M, Picciotto M, Changeux J, Pich EM. Assessment of nicotinic acetylcholine receptor subunit contributions to nicotine self-administration in mutant mice. Psychopharmacology. 1999;147:25–26. doi: 10.1007/s002130051135. [DOI] [PubMed] [Google Scholar]
- Esterlis I, Cosgrove K, et al. Quantification of smoking induced occupancy of β2-nicotinic acetylcholine receptors : estimation of nondisplaceable binding. Journal of Nuclear Medicine. 2010;51:1226–1233. doi: 10.2967/jnumed.109.072447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Tamagnan G, Zoghbi SS, Al-Tikriti MS, et al. Measurement of alpha4beta2 nicotinic acetylcholine receptors with [123I]5-I-A-85380 SPECT. Journal of Nuclear Medicine. 2010;41:1552–1560. [PubMed] [Google Scholar]
- Gourlay S, Benowitz N. Arteriovenous differences in plasma concentration of nicotine and catecholamines and related cardiovascular effects after smoking, nicotine nasal spray and intravenous nicotine. Clinical Pharmacological Therapy. 1997;62:453–463. doi: 10.1016/S0009-9236(97)90124-7. [DOI] [PubMed] [Google Scholar]
- Hariharan M, VanNoord T, Greden JF. A highperformance liquid-chromatographic method for routine simultaneous determination of nicotine and cotinine in plasma. Clinical Chemistry. 1988;34:724–729. [PubMed] [Google Scholar]
- Hatsukami D, Hughes J, Pickens R, Svikis D. Tobacco withdrawal symptoms : An experimental analysis. Psychopharmacology. 1984;84:231–236. doi: 10.1007/BF00427451. [DOI] [PubMed] [Google Scholar]
- Heatherton T, Kozlowski L, Frecker R, Fagerstrom K. The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom tolerance questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Henningfield J, Stapletom J, Benowitz N, Grayson R, et al. Higher levels of nicotine in arterial than in venous blood after cigarette smoking. Drug and Alcohol Dependence. 1993;33:23–29. doi: 10.1016/0376-8716(93)90030-t. [DOI] [PubMed] [Google Scholar]
- Innis R, Cunningham V, Delforge J, Fujita M, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. Journal of Cerebral Blood Flow and Metabolism. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Kahlil A, Steyn S, Castagnoli N. Isolation and characterization of monoamine oxidase inhibitor from tobacco leaves. Chemistry Research and Toxicology. 2000;13:31–35. doi: 10.1021/tx990146f. [DOI] [PubMed] [Google Scholar]
- Lunell E, Bergström M, Antoni G, Långström B, Nordberg A. Nicotine deposition and body distribution from a nicotine inhaler and a cigarette studied with positron emission tomography. Clinical Pharmacology and Therapeutics. 1996;59:593–594. doi: 10.1016/S0009-9236(96)90188-5. [DOI] [PubMed] [Google Scholar]
- Lunell E, Molander K, Leischow S, Fagerström K. Effect of nicotine vapour inhalation on the relief of tobacco withdrawal symptoms. European Journal of Clinical Pharmacology. 1995;48:235–240. doi: 10.1007/BF00198304. [DOI] [PubMed] [Google Scholar]
- Mansvelder H, Keath J, McGehee D. Synaptic mechanisms underlie nicotine induced excitability of brain reward areas. Neuron. 2002;33:905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Maskos U, Molles B, Pons S, Besson M, et al. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature. 2005;436:103–107. doi: 10.1038/nature03694. [DOI] [PubMed] [Google Scholar]
- Meyers D, Neuberger J. Cardiovascular effect of bans on smoking in public places. American Journal of Cardiology. 2008;102:1421–1424. doi: 10.1016/j.amjcard.2008.06.065. [DOI] [PubMed] [Google Scholar]
- Monchuk D, Rousu M, Shogren J, Nonnemaker J, et al. Decomposing the value of cigarettes using experimental auctions. Nicotine and Tobacco Research. 2007;9:93–99. doi: 10.1080/14622200601078392. [DOI] [PubMed] [Google Scholar]
- Mukhin A, Gundisch D, Horti A, Koren A, et al. 5-Iodo-A-85830, an α4β2 subtype-selective ligand for nicotinic acetylcholine receptors. Molecular Pharmacology. 2000;57:642–649. doi: 10.1124/mol.57.3.642. [DOI] [PubMed] [Google Scholar]
- Nestler E. Is there a common molecular pathway for addiction? Nature Neuroscience. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- Pfizer. Nicotrol® NS and Nicotrol® Inhaler. 2002. [Google Scholar]
- Picciotto M, Zoli M, Rimondin R, Lena C, et al. Acetycholine receptors containing the β2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Rice M, Cragg S. Nicotine amplifies reward-related dopamine signals in striatum. Nature Neuroscience. 2004;7:583–584. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- Roll J, Higgins S, Badger G. An experimental comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Journal of Applied Behavior Analysis. 1996;29:495–504. doi: 10.1901/jaba.1996.29-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J, Behm F. Extinguishing the rewarding value of smoke cues: Pharmacological and behavioral treatments. Nicotine and Tobacco Research. 2004;6:523–532. doi: 10.1080/14622200410001696501. [DOI] [PubMed] [Google Scholar]
- Schneider N, Cortner C, Gould J, Olmstead MKR. Comparison of craving and withdrawal among four combination nicotine treatments. Human Psychopharmacology. 2008;23:513–517. doi: 10.1002/hup.947. [DOI] [PubMed] [Google Scholar]
- Schneider N, Olmstead R, Franzon M, Lunell E. The Nicotine Inhaler : Clinical pharmacokinetics and comparison with other nicotine treatments. Clinical Pharmacokinetics. 2001;40:661–684. doi: 10.2165/00003088-200140090-00003. [DOI] [PubMed] [Google Scholar]
- Schneider N, Olmstead R, Nilsson F, Mody F, et al. Efficacy of a nicotine inhaler in smoking cessation : a double-blind, placebo-controlled trial. Addiction. 1996;191:1293–1306. [PubMed] [Google Scholar]
- Schneider N, Terrace S, Koury M, Patel S, et al. Comparison of three nicotine treatments : initial reactions and preferences with guided use. Psychopharmacology (Berlin) 2005;182:545–550. doi: 10.1007/s00213-005-0123-3. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Mouratidis M, Yoo S, Kosten KCT. Effects of tiagabine in combination with intravenous nicotine in overnight abstinent smokers. Psychopharmacology (Berlin) 2005;181:504–510. doi: 10.1007/s00213-005-0010-y. [DOI] [PubMed] [Google Scholar]
- Staley J, Dyck Cv, Weinzimmer D, Brenner E, et al. Iodine-123-5-IA-85380 SPECT measurement of nicotinic acetylcholine receptors in human brain by the constant infusion paradigm: feasibility and reproducibility. Journal of Nuclear Medicine. 2005;46:1466–1472. [PubMed] [Google Scholar]
- Staley J, Krishnan-Sarin S, Cosgrove K, Krantzler E, et al. Human tobacco smokers in early abstinence have higher levels of beta2-nicotinic acetylcholine receptors than nonsmokers. Journal of Neuroscience. 2006;26:8707–8714. doi: 10.1523/JNEUROSCI.0546-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitzer M, Bigelow G. Contingent reinforcement for reduced breath carbon monoxide levels : target-specific effects on cigarette smoking. Addictive Behavior. 1985;10:345–349. doi: 10.1016/0306-4603(85)90030-9. [DOI] [PubMed] [Google Scholar]
- Stitzer M, Rand C, Bigelow G, Mead A. Contingent payment procedures for smoking reduction and cessation. Journal of Applied Behavioral Analysis. 1986;19:197–202. doi: 10.1901/jaba.1986.19-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolerman I, Shoaib M. The neurobiology of tobacco addiction. Trends in Pharmacological Science. 1991;12:467–473. doi: 10.1016/0165-6147(91)90638-9. [DOI] [PubMed] [Google Scholar]
- Tiffany S, Drobes D. The development and initial validation of a questionnaire on smoking urges. British Journal of Addictions. 1991;86:1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- Tønnesen P. Smoking cessation : How compelling is the evidence? A review. Health Policy. 2009;91 (Suppl 1):S15–S25. doi: 10.1016/S0168-8510(09)70004-1. [DOI] [PubMed] [Google Scholar]
- Vaupel D, Mukhin A, Kimes A, Horti A, et al. In vivo studies with [125I]5-IA 85380, a nicotinic acetylcholine receptor radioligand. NeuroReport. 1998;9:2311–2317. doi: 10.1097/00001756-199807130-00030. [DOI] [PubMed] [Google Scholar]
- Zhang H, Sulzer D. Frequency-dependent modulation of dopamine release by nicotine. Nature Neuroscience. 2004;7:581–582. doi: 10.1038/nn1243. [DOI] [PubMed] [Google Scholar]
- Zoghbi S, Tamagnan G, Fujita M, Baldwin R, et al. Measurement of plasma metabolites of (S)-5-[123I]iodo-3-(2-azetidinylmethoxy) pyridine (5-IA-85380), a nicotinic acetylcholine receptor imaging agent, in non-human primates. Nuclear Medicine and Biology. 2001;28:91–96. doi: 10.1016/S0969-8051(00)00188-8. [DOI] [PubMed] [Google Scholar]
- Zoli M, Lena C, Picciotto M, Changeux J. Identification of four classes of brain nicotinic receptors using beta2 mutant mice. Journal of Neuroscience. 1998;18:4461–4472. doi: 10.1523/JNEUROSCI.18-12-04461.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]