Abstract
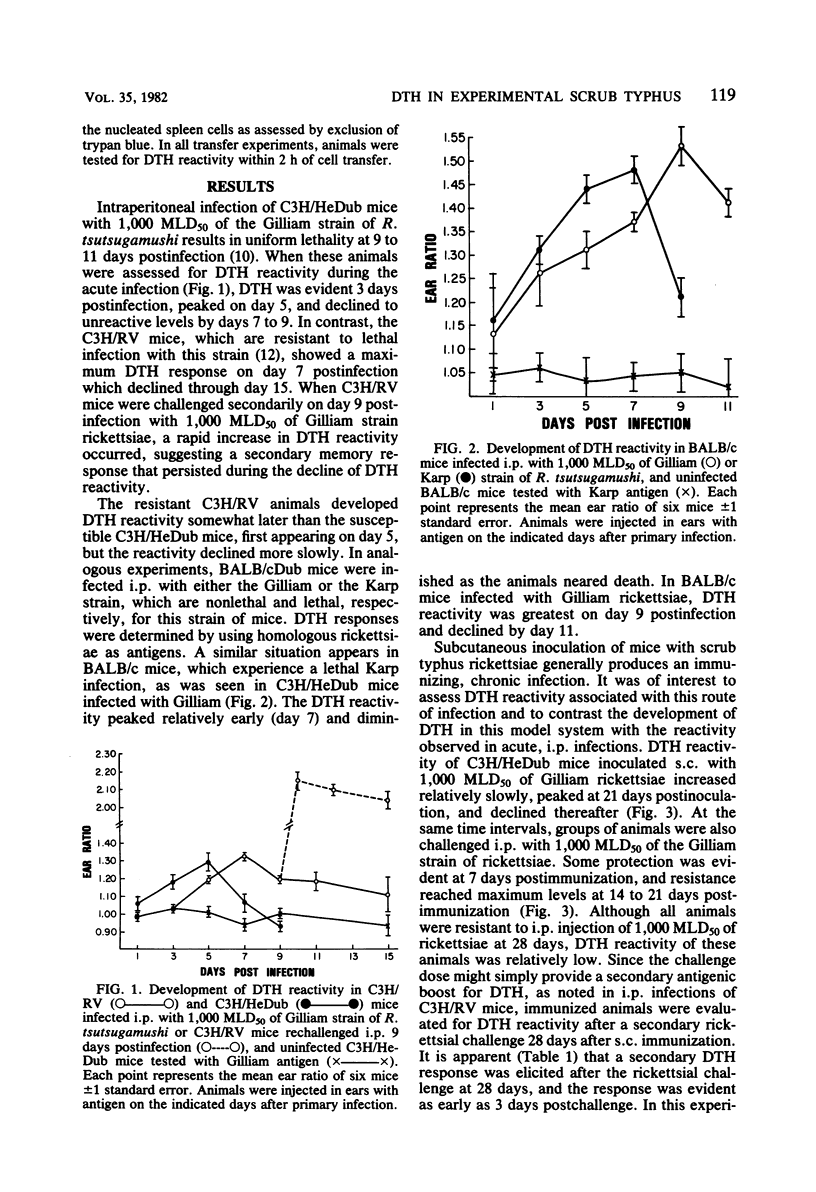
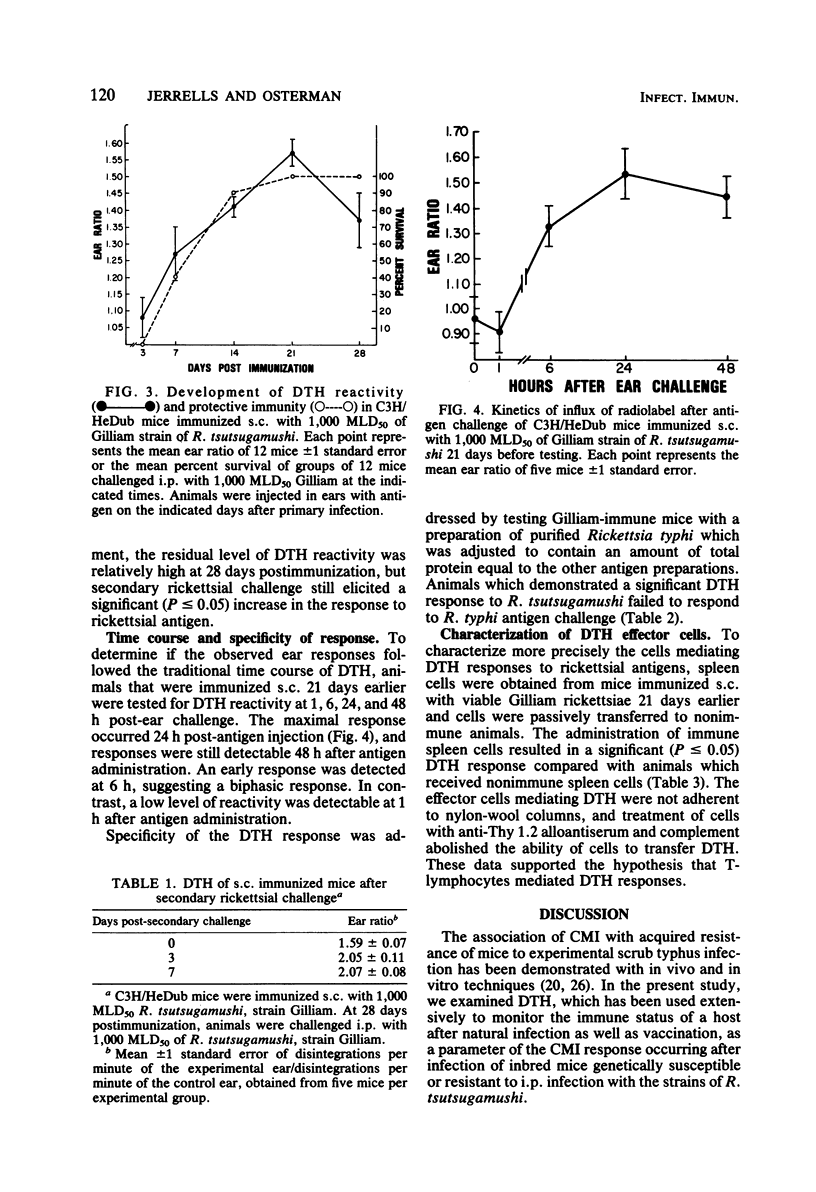
Delayed-type hypersensitivity responses of inbred mice during the course of lethal and chronic infections with strains of Rickettsia tsutsugamushi were evaluated by using the influx of radiolabeled cells into antigen-injected ears. Congenic strains of C3H mice, which previously have been shown to be resistant (C3H/RV) or sensitive (C3H/HeDub) to lethal intraperitoneal infection with the Gilliam strain of rickettsiae, both expressed delayed-type hypersensitivity early in the course of infection (5 to 7 days). The sensitive C3H/HeDub mice, however, exhibited a marked decline in reactivity just before death. In contrast, reactivity of C3H/RV mice remained high through day 9 and declined slowly through day 15 after infection. Similar results were obtained when BALB/c mice were infected with either the Karp or the Gilliam strain of rickettsiae, which produce a lethal or nonlethal infection, respectively, in this strain of mice. Rechallenge of C3H/RV mice elicited a rapid increase in reactivity, suggesting a secondary memory response. To analyze delayed-type hypersensitivity during chronic infection, C3H/HeDub mice were immunized by subcutaneous infection with the Gilliam strain of R. tsutsugamushi, and both delayed-type hypersensitivity reactivity and resistance to intraperitoneal challenge were examined. Delayed-type hypersensitivity reactivity developed slowly and peaked at 21 days postimmunization, which correlated with resistance to intraperitoneal challenge. Delayed-type hypersensitivity reactivity declined thereafter, but resistance to intraperitoneal challenge remained through 28 days postimmunization. Delayed-type hypersensitivity reactivity increased after secondary challenge at 28 days, again suggesting antigen memory generated by primary immunization. Transfer of delayed-type hypersensitivity reactivity was accomplished by using immune thymus-derived splenic lymphocytes isolated with nylon-wool columns. Abrogation of the ability of immune spleen cells to transfer delayed-type hypersensitivity reactivity after treatment with anti-Thy 1.2 alloantiserum and complement further supported the view that delayed-type hypersensitivity responses to scrub typhus rickettsiae were mediated by thymus-derived lymphocytes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J., Curtis J. Development of delayed hypersensitivity responses in Mycobacterium lepraemurium infections in resistant and susceptible strains of mice. Immunology. 1979 Mar;36(3):563–567. [PMC free article] [PubMed] [Google Scholar]
- Arredondo B., Pérez H. Alterations of the immune response associated with chronic experimental leishmaniasis. Infect Immun. 1979 Jul;25(1):16–22. doi: 10.1128/iai.25.1.16-22.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheers C., Pavlov H., Riglar C., Madraso E. Macrophage activation during experimental murine brucellosis. III. Do macrophages exert feedback control during brucellosis? Cell Immunol. 1980 Jan;49(1):168–177. doi: 10.1016/0008-8749(80)90066-0. [DOI] [PubMed] [Google Scholar]
- Clark C., Wheelock M. In vivo cyclophosphamide-mediated augmentation and aqueous HGG-induced suppression of murine delayed hypersensitivity to HGG are reflected by in vitro HGG-stimulated proliferation of lymph node cells from equivalent mice. Cell Immunol. 1980 Jul 1;52(2):404–413. doi: 10.1016/0008-8749(80)90361-5. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Watson S. R. Suppressor T-cells in BCG-infected mice. Infect Immun. 1979 Aug;25(2):491–496. doi: 10.1128/iai.25.2.491-496.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisemann C. S., Osterman J. V. Proteins of typhus and spotted fever group rickettsiae. Infect Immun. 1976 Jul;14(1):155–162. doi: 10.1128/iai.14.1.155-162.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg G. H., Jr, Osterman J. V. Experimental scrub typhus immunogens: gamma-irradiated and formalinized rickettsiae. Infect Immun. 1977 Jan;15(1):124–131. doi: 10.1128/iai.15.1.124-131.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing E. P., Jr, Takeuchi A., Shirai A., Osterman J. V. Experimental infection of mouse peritoneal mesothelium with scrub typhus rickettsiae: an ultrastructural study. Infect Immun. 1978 Mar;19(3):1068–1075. doi: 10.1128/iai.19.3.1068-1075.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves M. G., Osterman J. V. Host defenses in experimental scrub typhus: genetics of natural resistance to infection. Infect Immun. 1978 Feb;19(2):583–588. doi: 10.1128/iai.19.2.583-588.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrells T. R., Mallavia L. P., Hinrichs D. J. Detection of long-term cellular immunity to Coxiella burneti as assayed by lymphocyte transformation. Infect Immun. 1975 Feb;11(2):280–286. doi: 10.1128/iai.11.2.280-286.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrells T. R., Osterman J. V. Host defenses in experimental scrub typhus: inflammatory response of congenic C3H mice differing at the Ric gene. Infect Immun. 1981 Mar;31(3):1014–1022. doi: 10.1128/iai.31.3.1014-1022.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lane F. C., Unanue E. R. Requirement of thymus (T) lymphocytes for resistance to listeriosis. J Exp Med. 1972 May 1;135(5):1104–1112. doi: 10.1084/jem.135.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefford M. J. The measurement of tuberculin hypersensitivity in rats. Int Arch Allergy Appl Immunol. 1974;47(4):570–585. doi: 10.1159/000231251. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., Russell S. M. Delayed-type hypersensitivity to influenza virus. Induction of antigen-specific suppressor T cells for delayed-type hypersensitivity to hemagglutinin during influenza virus infection in mice. J Exp Med. 1980 Apr 1;151(4):799–814. doi: 10.1084/jem.151.4.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The relationship of delayed hypersensitivity to acquired cellular resistance. Br Med Bull. 1967 Jan;23(1):52–54. doi: 10.1093/oxfordjournals.bmb.a070516. [DOI] [PubMed] [Google Scholar]
- Mitsuyama M., Nomoto K., Akeda H., Takeya K. Enhanced elimination of Listeria monocytogenes at the site of delayed footpad reaction. Infect Immun. 1980 Oct;30(1):1–4. doi: 10.1128/iai.30.1.1-4.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacy C. A., Osterman J. V. Host defenses in experimental scrub typhus: role of normal and activated macrophages. Infect Immun. 1979 Nov;26(2):744–750. doi: 10.1128/iai.26.2.744-750.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura R. M., Tokunaga T. Induction of suppressor T cells in delayed-type hypersensitivity to Mycobacterium bovis BCG in low-responder mice. Infect Immun. 1980 May;28(2):331–335. doi: 10.1128/iai.28.2.331-335.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oaks S. C., Jr, Osterman J. V., Hetrick F. M. Plaque assay and cloning of scrub typhus rickettsiae in irradiated L-929 cells. J Clin Microbiol. 1977 Jul;6(1):76–80. doi: 10.1128/jcm.6.1.76-80.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P. J., Lefford M. J. Antigen specific lymphocyte transformation, delayed hypersensitivity and protective immunity. I. Kinetics of the response. Cell Immunol. 1978 May;37(2):315–326. doi: 10.1016/0008-8749(78)90200-9. [DOI] [PubMed] [Google Scholar]
- Plant J., Glynn A. A., Wilson B. M. Protective effects of a supernatant factor from Salmonella typhimurium on Salmonella typhimurium infection of inbred mice. Infect Immun. 1978 Oct;22(1):125–131. doi: 10.1128/iai.22.1.125-131.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riglar C., Cheers C. Macrophage activation during experimental murine brucellosis. II. Inhibition of in vitro lymphocyte proliferation by brucella-activated macrophages. Cell Immunol. 1980 Jan;49(1):154–167. doi: 10.1016/0008-8749(80)90065-9. [DOI] [PubMed] [Google Scholar]
- Shirai A., Catanzaro P. J., Phillips S. M., Osterman J. V. Host defenses in experimental scrub typhus: role of cellular immunity in heterologous protection. Infect Immun. 1976 Jul;14(1):39–46. doi: 10.1128/iai.14.1.39-46.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura S., Kojima A., Egashira Y. Regulatory mechanism of delayed-type hypersensitivity in mice. II. Effect of suppressor cells on the development of memory cells for delayed-type hypersensitivity. Cell Immunol. 1980 May;51(2):250–261. doi: 10.1016/0008-8749(80)90257-9. [DOI] [PubMed] [Google Scholar]
- Vadas M. A., Miller J. F., Gamble J., Whitelaw A. A radioisotopic method to measure delayed type hypersensitivity in the mouse. I. Studies in sensitized and normal mice. Int Arch Allergy Appl Immunol. 1975;49(5):670–692. doi: 10.1159/000231449. [DOI] [PubMed] [Google Scholar]
- Wisseman C. L., Jr, el Batawi Y., Wood W. H., Jr, Noriega A. R. Gross and microscopic skin reactions to killed typhus Rickettsiae in human beings. J Immunol. 1967 Jan;98(1):194–209. [PubMed] [Google Scholar]
- Youdim S., Stutman O., Good R. A. Studies of delayed hypersensitivity to L. Monocytogenes in mice: nature of cells involved in passive transfers. Cell Immunol. 1973 Jan;6(1):98–109. doi: 10.1016/0008-8749(73)90010-5. [DOI] [PubMed] [Google Scholar]



