Abstract
Rare genetic variants, identified by in-detail resequencing of loci, may contribute to complex traits. We used the apolipoprotein A-I gene (APOA1), a major high-density lipoprotein (HDL) gene, and population-based resequencing to determine the spectrum of genetic variants, the phenotypic characteristics of these variants, and how these results compared with results based on resequencing only the extremes of the apolipoprotein A-I (apoA-I) distribution. First, we resequenced APOA1 in 10,330 population-based participants in the Copenhagen City Heart Study. The spectrum and distribution of genetic variants was determined as a function of the number of individuals resequenced. Second, apoA-I and HDL cholesterol phenotypes were determined for nonsynonymous (NS) and synonymous (S) variants and were validated in the Copenhagen General Population Study (n = 45,239). Third, observed phenotypes were compared with those predicted using an extreme phenotype approach based on the apoA-I distribution. Our results are as follows: First, population-based resequencing of APOA1 identified 40 variants of which only 7 (18%) had minor allele frequencies >1%, and most were exceedingly rare. Second, 0.27% of individuals in the general population were heterozygous for NS variants which were associated with substantial reductions in apoA-I (up to 39 mg/dL) and/or HDL cholesterol (up to 0.9 mmol/L) and, surprisingly, 0.41% were heterozygous for variants predisposing to amyloidosis. NS variants associated with a hazard ratio of 1.72 (1.09–2.70) for myocardial infarction (MI), largely driven by A164S, a variant not associated with apoA-I or HDL cholesterol levels. Third, using the extreme apoA-I phenotype approach, NS variants correctly predicted the apoA-I phenotype observed in the population-based resequencing. However, using the extreme approach, between 79% (screening 0–1st percentile) and 21% (screening 0–20th percentile) of all variants were not identified; among these were variants previously associated with amyloidosis. Population-based resequencing of APOA1 identified a majority of rare NS variants associated with reduced apoA-1 and HDL cholesterol levels and/or predisposing to amyloidosis. In addition, NS variants associated with increased risk of MI.
Author Summary
Rare genetic variants, identified by in-detail resequencing of loci, may contribute to complex traits. We used the apolipoprotein A-I gene (APOA1), a major high-density lipoprotein (HDL) gene, and population-based resequencing to determine the spectrum of genetic variants, the phenotypic characteristics of these variants, and how these results compared with results based on resequencing only the extremes of the apolipoprotein A-I (apoA-I) distribution. By resequencing APOA1 in >10,000 Danes and genotyping an additional >45,000, we show that population-based resequencing of APOA1 identifies a majority of rare genetic variants that together are relatively frequent: 0.27% of the population are heterozygous for nonsynonymous (NS) variants in APOA1 that associate with substantial reductions in apoA-I and HDL cholesterol, and 0.41% are heterozygous for variants predisposing to amyloidosis. NS variants associated with a hazard ratio of 1.72 (1.09–2.70) for myocardial infarction (MI), largely driven by A164S, a variant not associated with apoA-I or HDL cholesterol levels. Resequencing only the extremes of the apoA-I distribution, between 79% and 21% of all variants are not identified; among these are variants previously associated with amyloidosis. These results provide direct evidence that rare NS variants in APOA1 contribute to low apoA-I and HDL cholesterol levels, to susceptibility to amyloidosis, and to risk of MI in the general population.
Introduction
Genome-wide association studies have identified multiple loci associated with complex traits and diseases, but until now common genetic variants (minor allele frequency >5%) at these loci only explain small proportions of the heritability [1], [2]. For example, the estimated heritability of high density lipoprotein (HDL) cholesterol in twin-studies is 50% [3], but the common alleles together or in combination explain less than 5–10% of the variation in plasma levels of HDL cholesterol [4]. Rare genetic variants (minor allele frequency <1%), which are identified by in-detail screening or resequencing of loci, may contribute to unravel this unexplained heritability [1], [2].
Apolipoprotein A-I (apoA-I) is the major protein component of HDL in plasma, and is a cofactor for lecithin∶cholesterol acyltransferase (LCAT), playing a key role in the so-called reverse cholesterol transport, i.e. the transport of cholesterol from peripheral tissues to the liver for excretion [3]. APOA1 (MIM 107680) encodes a 267 amino acid prepropeptide, which is sequentially cleaved to yield the mature 243 amino acid protein. Mutations in apoA-I may associate with low levels of plasma HDL cholesterol and apoA-I due to defective LCAT activation or to amyloidosis, or to amyloidosis with only minor or no effects on apoA-I and HDL cholesterol levels [5]–[11]. However, at present we lack comprehensive information on the spectrum of genetic variants in this pleiotropic gene in the general population, on the phenotypic characteristics of such variants in individuals in the general population, and whether additional information is gained from resequencing a sample of the entire general population, rather than using an extreme phenotype approach, previously used by us and others [12]–[16].
In the first part of this study, the aim was to determine the spectrum and distribution of genetic variants in APOA1 using a population-based resequencing approach. In the second part of the study, the aim was to determine the association of nonsynonymous (NS) and synonymous (S) variants in APOA1 in the general population with plasma levels of apoA-I and HDL cholesterol, and with risk of myocardial infarction (MI). In the third part of the study, we compared results using an extreme apoA-I phenotype approach with results from the population-based resequencing. For these purposes, we resequenced APOA1 in 10,330 participants in the Copenhagen City Heart Study (CCHS), and used the Copenhagen General Population Study (CGPS; n = 45,239 participants) to validate phenotypic results.
Materials and Methods
Subjects
Studies were approved by institutional review boards and Danish ethical committees (Nos. KF-100.2039/91, KF-01-144/01, H-KF-01-144/01) and conducted according to the Declaration of Helsinki. Informed consent was obtained from all participants. All participants were white and of Danish descent. No participants appeared in more than one of the two studies, permitting independent confirmation of the findings in each group.
The Copenhagen City Heart Study (CCHS)
The CCHS is a prospective study of the general population initiated in 1976–1978 with follow-up examinations in 1981–1983, 1991–1994, and 2001–2003 [17]–[19]. Individuals were selected based on the National Danish Civil Registration System to reflect the adult Danish population aged 20–80+ years. Data were obtained from a questionnaire, a physical examination, and from blood samples. Blood samples for DNA extraction were available on 10,330 participants attending the 1991–1994 and/or 2001–2003 examinations. Of these, 1,034 experienced an MI.
The Copenhagen General Population Study (CGPS)
The CGPS is a prospective study initiated in 2003 with ongoing enrollment [18], [19]. Participants were recruited and examined exactly as in the CCHS. At the time of genotyping, 45,239 had been included. Of these, 1,647 experienced an MI.
Myocardial infarction
In both studies of the general population, diagnoses of MI (WHO International Classification of Diseases; ICD8:410, ICD10:I21-I22) were collected from 1977 through May 10th 2011, and verified by reviewing all hospital admissions and diagnoses entered in the National Danish Patient Registry, and all causes of death entered in the National Danish Causes of Death Registry. A diagnosis of MI followed the changing definitions over time [20], [21].
Study designs
As shown in flowchart (Figure 1).
Figure 1. Study design.
MAF = minor allele frequency.
Part I: Resequencing APOA1 in the general population
In the first part of this study, the aim was to determine the spectrum and distribution of genetic variation in APOA1 in the general population. To this end, we resequenced the translated regions (exons 2–4) and exon-intron boundaries of APOA1 in all 10,330 participants in the CCHS. We added data for the core promoter and the untranslated exon 1 (711 bp), for intervening sequences 2 (94 bp) and 3 (159 bp), and for the untranslated 3′end (175 bp) of the gene from a previous resequencing effort using the extreme phenotype approach, and including participants in the 1991–94 examination of the CCHS [12]. Thus, a total of 1,139 basepairs in non-coding regions were resequenced in 180 individuals only, likely resulting in an underestimation of very rare non-coding variants in these regions. We determined 1) the cumulative number of genetic variants identified as a function of the number of individuals resequenced, stratified by minor allele frequencies (MAFs) (MAFs>1%, 0.005%<MAF<1%, MAF = 0.005% = singletons), and 2) the number of different genetic variants identified as a function of the number of individuals with these variants, corresponding to the exact MAFs.
Part II: Phenotype of heterozygotes for nonsynonymous (NS) and synonymous (S) variants in APOA1 in the general population
In the second part of the study, the aim was to determine the association of NS and S variants in APOA1 in the general population with plasma levels of apoA-I and HDL cholesterol, and with risk of MI. The association of NS and S variants with lipid and lipoprotein phenotypes was determined in the CCHS as a whole; for variants which occurred in more than two individuals in the CCHS, we validated the effect in the CGPS. The association between NS and S variants in APOA1 and risk of MI was determined prospectively in the CCHS and CGPS combined. Finally, the results were compared with in silico prediction using PANTHER (www.pantherdb.org/), SIFT (http://sift.jcvi.org/), PolyPhen (http://genetics.bwh.harvard.edu/pph2/), and Pmut (http://mmb2.pcb.ub.es:8080/PMut/).
Part III: Extreme phenotype approach versus population-based resequencing
Using an extreme phenotype approach based on the apoA-I distribution in the CCHS, we compared the results using this approach with results from population-based resequencing. We first compared the number of NS and S variants identified in the extreme low and high percentiles (0–1%, 0–5%, 0–10%, 0–20%) of the distribution. We then predicted an assumed apoA-I phenotype based on the distribution of NS and S variants identified exclusively in the lowest and highest 0–1 percentiles up to 0–20 percentiles of apoA-I, an extreme phenotype approach previously used by us and others for a number of different genes [12]–[16]. We tested the validity of this prediction for all NS and S variants identified, by comparing with the apoA-I and HDL cholesterol phenotype determined in the CCHS as a whole.
Gene screening and genotyping
We screened the translated region of APOA1 in all 10,330 participants in the CCHS using four PCR fragments covering 119 bp upstream of exon 2, exons 2–4, and exon-intron boundaries (APOA1 consensus sequence NC_000011.9) (Table S1). Mutational analysis was performed using a LightScanner (Idaho Technology Inc., Salt Lake City, UT, USA), followed by sequencing on an ABI 3730 DNA Analyzer (Applied Biosystems Inc., Foster City, CA, USA). NS and S variants identified in more than two individuals in the CCHS (K12K, S25S, S36A, F71Y, K107del, L144R, A164S, A190A), were genotyped in the CGPS using an ABI PRISM 7900HT Sequence Detection System (Applied Biosystems Inc., Foster City, CA, USA) and TaqMan-based assays.
Lipids, lipoproteins, and apolipoproteins
Colorimetric and turbidimetric assays were used to measure nonfasting plasma levels of apoA-I, HDL cholesterol, total cholesterol, triglycerides, and apoB (Boehringer Mannheim, Mannheim, Germany and Konelab, Helsinki, Finland). LDL cholesterol was calculated using the Friedewald equation [22] when plasma triglycerides were ≤4.0 mmol/L, and otherwise measured directly (Thermo Fisher Scientific, Waltham, MA, USA).
Other covariates
Body mass index was measured weight (kg) divided by measured height squared (m2). Lipid-lowering therapy was self-reported. Physical inactivity, drinking, smoking, hypertension and diabetes were dichotomized and defined as physical inactivity (less than 2–4 hours per week of light physical activity at leisure time), drinking (more than 1 drink per week), current smoking, hypertension (systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg, and/or use of antihypertensive therapy), and diabetes (self-reported disease, current use of anti-diabetic medication, and/or nonfasting plasma glucose >11.0 mmol/L).
Statistical analysis
We used Stata/S.E. 10.1. Two-sided p<0.05 was significant. χ2-tests evaluated Hardy-Weinberg equilibrium. To adjust for the effect of gender and age on absolute levels within studies, and for differences in absolute levels between studies, plasma levels of apoA-I and HDL cholesterol were converted to percentiles by gender and by age (in 10-year age groups) within each study, allowing for direct comparisons between percentiles for mutations both within and between studies (CCHS and CGPS). Mean apoA-I and HDL cholesterol percentiles in individuals with a specific mutation were compared with the mean percentile ( = 50th percentile of the normalized distribution) within the CCHS or CGPS as a whole using a z-test [23]. Mann-Whitney U-test and Fisher's exact test compared, respectively, continuous and categorical variables between heterozygotes for different mutations and noncarriers. Number of NS and S variants identified among individuals with extremely low or high plasma levels of apoA-I were compared using Fisher's exact test. Risk of MI for heterozygotes for all NS (S36A, F71Y, K107del, L144R, A164S) and for all S (K12K, S25S, A190A) mutations genotyped in both studies, was determined prospectively in the CCHS and CGPS combined, using Cox proportional hazards regression models with age as time scale and delayed entry (left truncation) in 1977. Fify-one individuals with a previous MI were excluded from the risk analyses. Hazard ratios were adjusted for age and sex, or multifactorially for age, sex, diabetes, hypertension and smoking.
Results
Study designs are shown as a flowchart in Figure 1.
Part I: Resequencing APOA1 in the general population
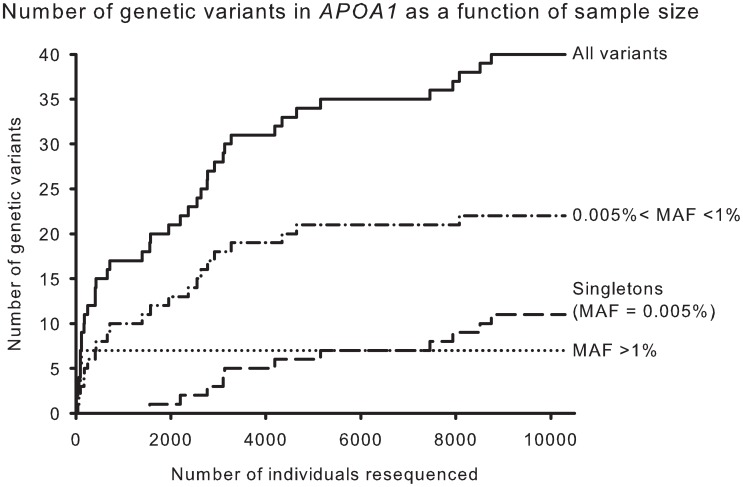
Resequencing APOA1 in the CCHS identified a total of 40 genetic variants (Figure 2 and Table S2). Only seven variants (18%) had MAFs >1% (all in Hardy-Weinberg equilibrium, P-values: 0.12 to 0.82). Of these, none were in the coding region of APOA1, that is in exons 1–4 coding for the 267 amino acid prepropeptide. The cumulative number of genetic variants in APOA1 as a function of the number of individuals resequenced showed that by resequencing fewer than 100 individuals all seven variants in APOA1 with a MAF >1% were identified (Figure 2). In contrast, the number of genetic variants identified with a 0.005%<MAF<0.1% increased almost logarithmically reaching a plateau around 5,000 individuals, while the number of singletons (minor allele frequency = 0.005%) increased almost linearly.
Figure 2. Cumulative number of genetic variants in APOA1 as a function of number of individuals resequenced.
Results are shown for all variants (solid) and for variants stratified by minor allele frequencies (MAFs), >1% (dotted), 0.005<MAF<1% (dash-dot), and MAF = 0.005% (long dash).
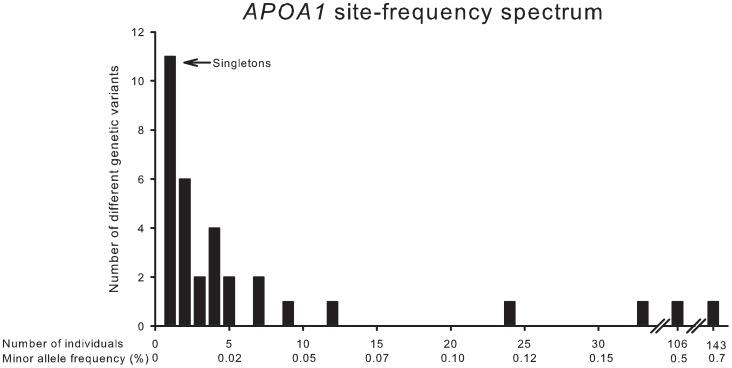
In agreement with this, the number of different genetic variants identified in APOA1 as a function of the number of individuals with each variant, corresponding to the MAF, showed that seventeen of 40 variants (43%) were exceedingly rare and identified in only one or two individuals in the CCHS (Figure 3).
Figure 3. APOA1 site-frequency spectrum.
Number of different genetic variants identified in APOA1 by resequencing as a function of individuals with each variant, and the corresponding minor allele frequencies.
Part II: Phenotype of heterozygotes for nonsynonymous and synonymous variants in the general population
Observed phenotype
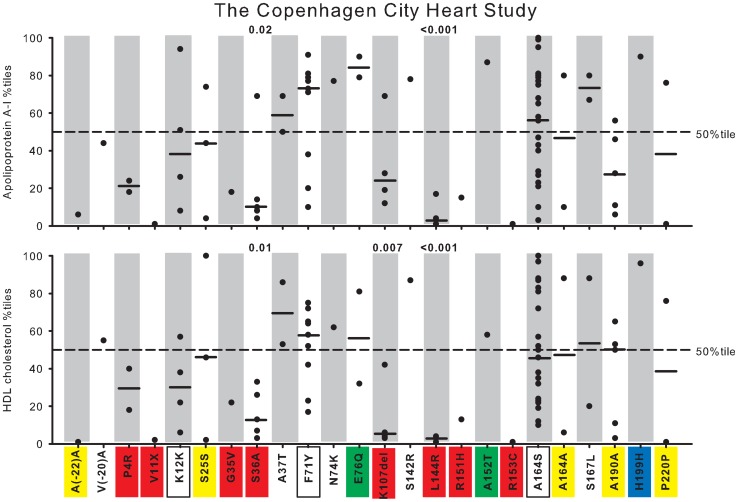
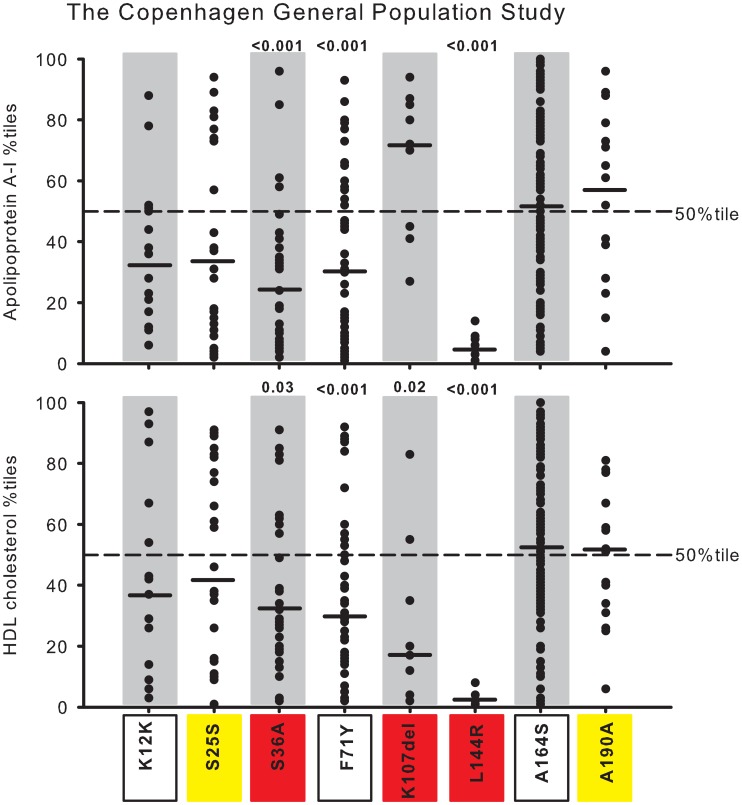
In Figure 4, Figure 5, and Figure S1, plasma levels of apoA-I and HDL cholesterol, for the individual mutations, are shown as percentiles for heterozygotes versus percentiles in the population as a whole. This corrects for the effect of gender and age on absolute plasma levels within and between studies, and for differences in absolute plasma levels of apoA-I and HDL cholesterol between studies, thus allowing for direct comparisons.
Figure 4. Plasma apoA-I and HDL cholesterol in percentiles for nonsynonymous and synonymous variants in APOA1.
Data are from the Copenhagen City Heart Study (CCHS). Each dot represents an individual with a given genetic variant. Percentiles are corrected for gender and age. P-values according to z-test comparing mean apoA-I or HDL cholesterol percentiles in heterozygotes with individuals in the CCHS as a whole. Solid black lines = median percentiles. Dashed line = 50th percentile. Red, nonsynonymous variants identified exclusively in the lowest 20 percentile of the apoA-I distribution in the CCHS using the extreme phenotype approach; yellow, synonymous variants identified exclusively in the lowest 20 percentile; green, nonsynonymous variants identified exclusively in the highest 20 percentile; blue, synonymous variants identified exclusively in the highest 20 percentile; white boxes, nonsynonymous and synonymous variants identified in both the lowest and highest 20 percentiles.
Figure 5. Plasma apoA-I and HDL cholesterol in percentiles for nonsynonymous and synonymous variants in APOA1.
Data are from the Copenhagen General Population Study (CGPS). Each dot represents an individual with a given genetic variant. Percentiles are corrected for gender and age. P-values according to z-test comparing mean apoA-I or HDL cholesterol percentiles in heterozygotes with individuals in the CGPS as a whole. Solid black lines = median percentiles. Dashed line = 50th percentile. Red, nonsynonymous variants identified exclusively in the lowest 20 percentile of the apoA-I distribution in the CCHS using the extreme phenotype approach; yellow, synonymous variants identified exclusively in the lowest 20 percentile; white boxes, nonsynonymous and synonymous variants identified in both the lowest and highest 20 percentiles.
Seventeen NS (9 new) and seven S (6 new) variants in APOA1 were identified in a total of 80 individuals (0.77%) by the population-based resequencing of the CCHS (Table S2). Two NS variants, S36A and L144R, associated with reduced apoA-I and HDL cholesterol with median levels of apoA-I at, respectively, the 10th and 3rd percentiles, and with corresponding HDL cholesterol levels at the 14th and 2nd percentiles, compared with the apoA-I and HDL cholesterol distributions in the CCHS as a whole (P-values: 0.02 to <0.001) (Figure 4, in red). K107del associated with a median HDL cholesterol level at the 5th percentile (P = 0.007) (Figure 4, in red), without a corresponding reduction in apoA-I, while P4R appeared to associate with both low apoA-I and HDL cholesterol levels (Figure 4, in red). None of the other variants associated with apoA-I or HDL cholesterol levels, although the singletons V11X, G35V, R151H and R153C all were within the lowest apoA-I and HDL cholesterol percentiles (Figure 4, in red), and the singleton A152T was in the highest apoA-I percentiles (Figure 4, in green).
Validation of observed phenotype
To further validate the associations between APOA1 genotype and plasma levels of apoA-I and HDL cholesterol observed in the CCHS, we genotyped all eight NS and S variants identified in more than two individuals in the CCHS (MAF >0.01%; K12K, S25S, S36A, F71Y, K107del, L144R, A164S, A190A) in the 45,239 participants in the CGPS. In this study, S36A associated with median apoA-I and HDL cholesterol levels at, respectively, the 24th and 29th percentile, and L144R associated with corresponding median levels at the 5th and 2nd percentiles (P-values 0.03 to <0.001; Figure 5, in red), confirming the results from the CCHS (Table S3, CCHS versus CGPS comparing relative percentile reductions: P-values from 0.90 to 0.07). We also confirmed that the deletion of one amino acid at position 107 (K107del) associated with a median HDL cholesterol level at the 17th percentile (P<0.05), without an effect on apoA-I levels, although apoA-I levels were higher in the CGPS (Figure 5, in red) (Table S3, CCHS versus CGPS: P-values 0.31 for HDL cholesterol and 0.03 for apoA-I). In addition, F71Y associated with lower than average apoA-I (median 31st percentile) and HDL cholesterol levels (median 29th percentile) in the (well-powered) CGPS (P-values <0.001), but not in the (less-powered) CCHS (compare Figure 4 and Figure 5, white and boxed; Table S3, CCHS versus CGPS: P-values 0.02 and 0.04). The NS variant A164S and the S variants K12K (both white and boxed), S25S (yellow) and A190A (yellow) did not associate with apoA-I or HDL cholesterol levels, also in agreement with results from the CCHS (Figure 4 and Figure 5 and Table S3, CCHS versus CGPS: P-values from 0.09 to 0.94). A comparison of the median differences between the CCHS and the CGPS in relative percentile values (50th percentile in population minus percentile for heterozygotes) and in absolute plasma levels of apoA-I and HDL cholesterol (noncarriers minus heterozygotes) for the individual mutations are shown in Table S3.
Potential gender differences for apoA-I and HDL cholesterol percentiles among heterozygotes for the individual mutations in CCHS and CGPS combined are shown in Figure S1. Although the effect of some variants appeared to differ between genders, there were no consistent patterns of gender heterogeneity.
Characteristics of heterozygotes for each of the four mutations associated with reductions in plasma levels of apoA-I and/or HDL cholesterol levels are shown for the CCHS and CGPS in Table 1. Comparing heterozygotes with noncarriers, only plasma levels of apoA-I and HDL cholesterol (mirrored in total cholesterol for L144R) were consistently different in both studies, suggesting that the effect of genotypes on these two parameters were unconfounded by other measured characteristics. In the CCHS and CGPS, the absolute reductions in plasma levels of apoA-I ranged from 14 mg/dL (F71Y) to 39 mg/dL (L144R), and in HDL cholesterol from 0.3 mmol/L to 0.9 mmo/L for the same variants (Table 1 and Table S3). For comparison, we have previously shown that the combination of two common variants in APOA1, −560A>C (MAF = 3.5%, tagging the haplotype-560A>C, −151C>T, *181A>G) and −310G>A (MAF = 16%), associated with minor increases in plasma levels of apoA-I and HDL cholesterol of up to, respectively, 9 mg/dL and 0.1 mmol/L in the 1% of individuals heterozygous for both variants [12].
Table 1. Characteristics of APOA1 S36A, F71Y, K107del, and L144R heterozygotes and noncarriers in the Copenhagen City Heart Study (n = 10,330) and the Copenhagen General Population study (n = 45,239).
| Copenhagen City Heart Study | Copenhagen General Population study | |||||||||
| Noncarriers | Heterozygotes | Noncarriers | Heterozygotes | |||||||
| S36A | F71Y | K107del | L144R | S36A | F71Y | K107del | L144R | |||
| n = 10,308 | n = 5 | n = 9 | n = 4 | n = 4 | n = 45,141 | n = 29 | n = 48 | n = 9 | n = 12 | |
| Age (years) | 59 (45–69) | 67 (38–37) | 62 (59–68) | 66 (52–70) | 47 (44–62) | 57 (47–67) | 65 (56–71)b | 52 (45–66) | 50 (41–57) | 57 (41–61) |
| Sex (F/M) | 5,738/4,570 | 2/3 | 8/1 | 2/2 | 3/1 | 24,847/20,294 | 23/6b | 32/16 | 4/5 | 8/4 |
| Apolipoprotein A-I (mg/dL) | 140 (123–161) | 106 (106–120)b | 162 (144–169) | 120 (108–148) | 101 (84–118)b | 156 (139–176) | 139 (131–162)a | 142 (130–162)b | 169 (151–172) | 117 (91–125)b |
| HDL cholesterol (mmol/L) | 1.5 (1.2–1.8) | 1.1 (1.0–1.2)a | 1.7 (1.5–1.8) | 1.0 (0.9–1.4)a | 0.8 (0.7–0.9)b | 1.6 (1.3–2.0) | 1.5 (1.3–1.8) | 1.3 (1.1–1.7)b | 1.1 (0.9–1.4)a | 0.7 (0.6–1.0)b |
| Total cholesterol (mmol/L) | 6.0 (5.1–6.9) | 5.0 (4.2–5.7) | 6.7 (6.2–6.9) | 6.9 (6.4–7.9) | 4.7 (4.2–5.4)a | 5.6 (4.9–6.3) | 5.8 (5.4–6.0) | 5.3 (4.4–6.3) | 5.7 (5.5–5.9) | 5.3 (4.1–5.5)a |
| LDL cholesterol (mmol/L) | 3.6 (2.9–4.4) | 3.0 (2.2–3.8) | 3.7 (3.6–4.5) | 4.5 (4.2–5.3)a | 3.2 (2.7–3.4) | 3.2 (2.6–3.8) | 3.3 (2.7–3.8) | 3.0 (2.4–3.9) | 3.3 (3.1–3.8) | 3.4 (3.0–3.7) |
| Triglycerides (mmol/L) | 1.5 (1.1–2.2) | 1.8 (1.8–1.9) | 1.7 (1.4–1.8) | 2.2 (1.5–3.3) | 2.2 (1.2–3.0) | 1.4 (1.0–2.1) | 1.4 (1.2–1.8) | 1.6 (0.8–2.4) | 1.3 (0.8–2.8) | 2.0 (1.1–2.5) |
| Apolipoprotein B (mg/dL) | 86 (71–103) | 70 (64–90) | 93 (89–107) | 102 (96–119) | 78 (69–107) | 107 (88–131) | 112 (96–128) | 99 (83–145) | 120 (102–144) | 118 (99–142) |
| Body mass index (kg/m2) | 25(22–28) | 27(26–28) | 26(22–28) | 28(25–32) | 27(24–29) | 26 (23–29) | 25 (22–28) | 25 (23–27) | 24 (22–26) | 26 (24–28) |
| Lipid lowering therapy (%) | 1 | 0 | 0 | 0 | 0 | 10 | 10 | 13 | 11 | 17 |
| Physical inactivity (%) | 64 | 80 | 56 | 25 | 75 | 52 | 48 | 58 | 67 | 42 |
| Drinking (%) | 57 | 80 | 56 | 25 | 100 | 32 | 28 | 26 | 56 | 25 |
| Smoking (%) | 47 | 20 | 67 | 50 | 25 | 22 | 17 | 28 | 22 | 2 |
| Hypertension (%) | 52 | 80 | 67 | 25 | 50 | 61 | 78 | 56 | 25 | 58 |
| Diabetes (%) | 4 | 20 | 0 | 0 | 0 | 4 | 0 | 4 | 0 | 0 |
Values are median (interquartile range) or percentage. Mann-Whitney U-test or Fishers exact test was used for continuous and categorical traits, respectively. Lipid-lowering therapy was self-reported. Physical inactivity, drinking, smoking, hypertension and diabetes were dichotomized and defined as physical inactivity (less than 2–4 hours per week of light physical activity at leisure time), drinking (more than 1 drink per week), current smoking, hypertension (systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg, and/or use of antihypertensive therapy), and diabetes (self-reported disease, current use of anti-diabetic medication, and/or nonfasting plasma glucose >11.0 mmol/L). LDL cholesterol levels were calculated using the Friedewald equation if triglycerides were ≤4 mmol/L, but measured directly at higher triglyceride levels.
P<0.05;
P<0.01.
Finally, collapsing all NS and S variants genotyped in the CCHS (n = 24) (Table S4, top), or in both the CCHS and CGPS (n = 8) (Table S4, bottom), showed that both NS and S variants were associated with significant reductions in plasma levels of apoA-I and/or HDL cholesterol in the CGPS, and that these effects were more pronounced when A164S, a relatively frequent variant without effect on apoA-I or HDL cholesterol [13], was excluded from the analysis. Combining the two studies confirmed that S variants associated with reductions in apoA-I (P = 0.03), and showed that variants suspected of (A164S) or previously associated with amyloidosis (S36A, F71Y, K107del) tended to have higher levels of apoA-I and HDL cholesterol than variants associated with reduced LCAT activation (L144R) (Figure S2; P-values <0.01).
In summary, results for seven of the eight most common NS and S variants in the CCHS were confirmed by genotyping in the CGPS. In this very large study, an additional variant, F71Y, associated with low apoA-I and HDL cholesterol levels. Thus, taken together approximately 0.27% of individuals in the general population carry NS mutations in APOAI, which associate with substantial reductions in apoA-I and/or HDL cholesterol levels compared to noncarriers. Combining NS and S variants in both the CCHS and CGPS, suggested that S variants also were associated with reductions in apoA-I levels.
Contribution of rare and common variants to variation in plasma levels of apoA-I and HDL cholesterol
The correlation between plasma levels of HDL cholesterol and apoA-I was R = 0.83 in the CCHS, that is, 69% (R2 = 0.69) of the total variability in HDL cholesterol levels were explained by the linear relationship with apoA-I levels. Results were similar in the CGPS.
In the CCHS, common variants in the promoter and coding regions of APOA1 (−560A>C MAF = 3.5%, tagging the haplotype-560A>C, −151C>T, *181A>G, and −310G>A MAF = 16%) contributed 0.4% (R2 = 0.004) to the total variability in plasma levels of apoA-I, and 0.2% to the total variability in HDL cholesterol; the corresponding contributions from rare variants were 10-fold lower, respectively, 0.03% and 0.04%. Contributions were similar in the CGPS.
Risk of myocardial infarction
Risk of MI was determined for heterozygotes for S (n = 68) and NS (n = 252) variants in APOA1 in the CCHS and CGPS combined (Figure 6). Hazard ratios for MI were 1.03 (95% CI:0.39–2.75) for heterozygotes for S variants, 1.72 (1.09–2.70) for heterozygotes for all NS variants, 1.41 (0.70–2.82) for NS variants without A164S, and 2.04 (1.13–3.69) for A164S alone, validating previous results for this variant based on the CCHS alone [13]. Results were similar after multifactorial adjustment for age, sex, diabetes, hypertension, and smoking.
Figure 6. HDL cholesterol levels and risk of myocardial infarction for heterozygotes for synonymous (S) or nonsynonymous (NS) variants in APOA1.
Hazard ratios by Cox proportional hazard regression models with age as time scale and delayed entry (left truncation) in 1977 based on the combined study of Copenhagen City Heart Study and the Copenhagen General Population. *P<0.01 comparing heterozygotes with noncarriers by Mann-Whitney U-test.
Distribution, evolutionary conservation, and in silico prediction of functional effects
NS and S variants appeared evenly distributed throughout the protein, although no NS variants were identified more C-terminal than S167L (Figure 7A). Almost all NS variants were highly conserved (except G35V), and all variants identified exclusively in the lower 20th percentile of apoA-I, including variants associated with low apoA-I and/or HDL cholesterol (P4R, S36A, L144R, F71Y – no prediction for K107del) in the CCHS and/or the CGPS, were predicted to affect function by at least three of four in silico programs (Figure 7B). In addition two singletons, R151H and R153C, in, respectively, the lowest 20th and 1st percentiles of the apoA-I distribution in the CCHS, were predicted to affect function by all four programs.
Figure 7. Nonsynonymous and synonymous variants in APOA1.
A. The positions of all nonsynonymous and synonymous variants identified by resequencing APOA1 in the Copenhagen City Heart Study (CCHS, n = 10,330) have been superimposed on the secondary structure of apoA-I. B. Evolutionary sequence conservation and predicted functional effects of nonsynonymous genetic variants in APOA1. Variants are divided into four groups, depending on whether they were identified exclusively in the lowest 20 percentile, the highest 20 percentile, or in both the lowest and highest 20 percentiles of the apoA-I distribution, using the extreme phenotype approach. The fourth group includes variants exclusively identified using the population-based resequencing approach. The truncation, APOA1 V11X, is not included in the figure. The alignment includes human (H. sapiens), chimpanzee (P. troglodytes), dog (C. lupus familiaris), cow (B. taurus), mouse (M. musculus), chicken (G. gallus), and zebrafish (D. Rerio). Alignment by HomoloGene (www.ncbi.nlm.nih.gov/homologene/). anot possible to model. PANTHER: + = P-deleterious>0.5; − = P-deleterious<0.5 (www.pantherdb.org/). SIFT: + = affect protein function; − = tolerated (http://sift.jcvi.org/). PolyPhen: + = probably or possibly damaging; − = benign (http://genetics.bwh.harvard.edu/pph2/). PMut: + = pathological; − = neutral (http://mmb2.pcb.ub.es:8080/PMut/). Figure 7A was adapted from [6].
Part III: Extreme phenotype approach versus population-based resequencing
Number of nonsynonymous and synonymous variants in APOA1 by apoA-I percentiles
Comparing the number of NS and S variants identified exclusively in the extreme percentiles of the apoA-I distribution (0–1, 0–5, 0–10, 0–20, 0–50 percentiles) in the CCHS, revealed a predominance of variants in the low percentile versus the high percentile groups (Table 2; P-values: 0.004 to 0.02; comparing lowest versus highest 0–5 to 0–20 percentiles), suggesting that most variants were loss of function variants associated with low levels of apoA-I. Nineteen of a total of 24 variants (79%) (Table 2), were identified by resequencing individuals in the lowest and highest 20 percentiles of apoA-I, whereas resequencing the 0–1, 0–5 and 0–10 percentiles identified, respectively, five (21%), eight (33%), and twelve variants (50%).
Table 2. Number of nonsynonymous and synonymous variants identified in APOA1 by apoA-I percentiles.
| Extreme phenotype screened, %tile | Number of genetic variants | |||||||
| Total | Exclusively low apoA-I | Exclusively high apoA-I | Both low and high apoA-I | |||||
| NS | S | NS | S | NS | S | P-valuea | ||
| 0–1 | 5 | 3 | 1 | 1 | 0 | 0 | 0 | 0.39 |
| 0–5 | 8 | 4 | 3 | 0 | 0 | 1 | 0 | 0.02 |
| 0–10 | 12 | 4 | 5 | 0 | 0 | 2 | 1 | 0.004 |
| 0–20 | 19 | 8 | 5 | 2 | 1 | 2 | 1 | 0.02 |
| b0–50 | 24 | 7 | 1 | 5 | 1 | 5 | 5 | 0.79 |
P-values by Fisher's exact test comparing nonsynonymous (NS) and synonymous (S) variants combined and identified exclusively in the low apoA-I or in the high apoA-I group. Values represent the cumulative number of genetic variants identified in the lowest and highest 0–1 percentile, 0–5 percentile and 0–10 percentile, 0–20 percentile and 0–50 percentile of the apoA-I distribution in the Copenhagen City Heart Study.
The upper and lower 0–50 percentiles combined correspond to the total number of nonsynonymous and synonymous variants identified in the Copenhagen City Heart Study.
Predicted phenotype using extreme phenotype approach
To determine whether the apoA-I and HDL cholesterol phenotype predicted from the distribution of the variants in the extreme 20 percentile groups (exclusively low, exclusively high or both) corresponded to the phenotype observed in the CCHS as a whole, we compared the results from the extreme phenotype approach with results observed from the population-based resequencing of the CCHS (compare Figure 4 with Figure 8 – same color code).
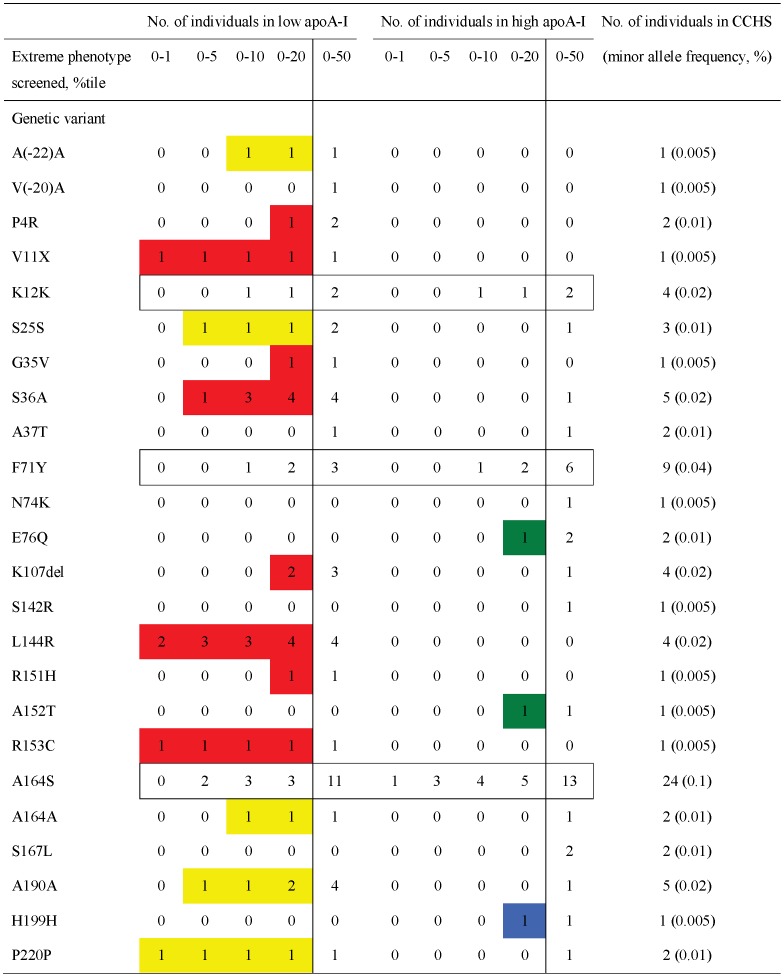
Figure 8. Cumulative number of individuals with a specific nonsynonymous or synonymous variant in APOA1 by apoA-I percentiles in the Copenhagen City Heart Study.
Red, nonsynonymous variants identified exclusively in the lowest 20 percentile of the apoA-I distribution using the extreme phenotyping approach; yellow, synonymous variants identified exclusively in the lowest 20 percentile; green, nonsynonymous variants found exclusively in the highest 20 percentile, blue, synonymous variants identified exclusively in the highest 20 percentile; white boxes, nonsynonymous and synonymous variants identified in both the lowest and highest 20 percentiles. In plain white, variants only identified by population-based resequencing, corresponding to the 20–80 percentile.
Based on the extreme phenotype approach and a 20 percentile cut-off, thirteen variants (Figure 8, NS in red and S in yellow) identified in 32 (0.31%) individuals in the CCHS were predicted to associate with low apoA-I and HDL cholesterol levels, three variants (Figure 8, NS in green and S in blue) identified in four (0.04%) individuals were predicted to associate with high apoA-I and HDL cholesterol, whereas the remaining variants identified in both extreme percentile groups (Figure 8, white boxed, K12K, F71Y, A164S) in 37 (0.36%) individuals were not predicted to affect apoA-I or HDL cholesterol levels. Five NS variants (V(-20)A, A37T, N74K, S142R, S167L, Figure 8 in plain white) in seven individuals (0.07%) were only identified by the population-based resequencing approach.
Comparing these results with results from the population-based resequencing showed that NS variants associated with low apoA-I and HDL cholesterol levels or with no effect in the CCHS were correctly predicted using the extreme 20 percentile approach (compare Figure 4 with Figure 8, variants in red and boxed white). However, the number of these variants identified depended on the extreme percentiles resequenced (Figure 8, compare 0–1 versus 0–20 percentiles). Synonymous variants predicted to associate with low apoA-I and HDL cholesterol were not correctly predicted (compare Figure 8 with Figure 4, variants in yellow). NS and S variants predicted to associate with high apoA-I and HDL cholesterol levels were mostly rare, and could therefore not be validated (Figure 4 and Figure 8, variants in green and blue). Using the extreme phenotype approach, between 19 (79%; 0–1 percentile) and 5 (21%; 0–20 percentile) of all variants were not identified at all depending on the extreme percentile resequenced, among these variants known to, or suspected of associating with amyloidosis (F71Y, A164S) [13], [24], and with risk of MI and early death (A164S) [13].
Discussion
Using population-based resequencing of APOA1 in 10,330 individuals allowed description of the spectrum and distribution of genetic variants in this gene. Our results showed that the vast majority of variants, including variants associated with apoA-I and HDL cholesterol phenotype, were individually rare, though collectively relatively common. These results are in complete agreement with results from two previous population-based resequencing studies of three other genes affecting, respectively, triglycerides and diabetes related traits [25], [26].
Novel findings, compared to previous population-based screenings of apoA-I using isoelectric focusing [27], [28], include: First, the number of NS variants identified and the number of heterozygotes for these variants were, respectively, 5–6 fold and 10–20 fold increased. Second, we showed that 0.27% of the population were heterozygous for variants associated with substantial reductions in apoA-I and HDL cholesterol levels, and 0.41% were heterozygous for variants previously associated with amyloidosis, although none had been diagnosed with this disease. In addition, S variants, not identified by isoelectric focusing, also associated with reductions in apoA-I levels in the CCHS and CGPS combined (n>55,000). Third, heterozygosity for NS variants in APOA1 associated with a 2-fold increased risk of MI, largely driven by A164S, a variant not associated with apoA-I and HDL cholesterol levels. Finally, while these rare variants might have some effects on the extremes of the population distribution of apoA-I and HDL cholesterol and on levels in the individual, the contribution of both rare and common variants in APOA1 to the total variation in plasma levels of apoA-1 and HDL cholesterol were very modest, respectively, 0.03% and 0.3%, in agreement with the very large number of genes found to associate with apoA-I and/or HDL cholesterol levels in genomewide association studies [29]. The effect of common variants on plasma levels were 10-fold higher than for rare variants, suggesting that rare variants in this gene do not contribute in any major way to the missing heritability on a population level.
An advantage of population-based resequencing is that the genetic variants identified can be tested against multiple phenotypes. This becomes especially important, if the gene under study has pleiotropic effects, i.e. affects multiple phenotypic traits, as is the case for APOA1: mutations in APOA1 have been associated with an inability to activate LCAT and with hereditary amyloidosis [5]–[7]. While mutations that poorly activate LCAT associate with low apoA-I and HDL cholesterol levels due to the rapid removal of lipid-poor discoidal HDL from the circulation [30], mutations that cause amyloidosis may [5], [6] or may not [9]–[11] associate with low apoA-I and/or HDL cholesterol, most likely depending on the severity of the mutation [8]. Of the seventeen nonsynonymous variants identified in the present study, only seven have been reported by others (P4R, V11X, S36A, A37T, F71Y, K107del, L144R) [11], [13], [24], [27], [31]–[41]. Of these variants, five (P4R, V11X, S36A, K107del, L144R) associated with low apoA-I and/or HDL cholesterol levels in the CCHS and in other studies [13], [32]–[34], [40], [41]. L144R is unable to activate LCAT [13], while S36A, F71Y, K107del, have been associated with amyloidosis [24], [38]. A164S, a variant without any association with HDL cholesterol or apoA-I levels in the CCHS and CGPS, was associated with an increased risk of IHD, MI, and premature death, and with reduced survival after diagnosis of IHD in the CCHS, most likely due to an attenuated form of cardiac amyloidosis [13]. Thus, four variants in APOA1 identified in 42 individuals (0.41% of the population) have either previously been associated with or suspected of causing amyloidosis. Only two of these variants, S36A and K107del, associated with low HDL cholesterol in the CCHS. This highlights two points: 1) The inherent weakness of the extreme approach when a gene has pleiotropic effects. Using this approach in the CCHS, both F71Y, a known amyloidosis mutation [24], and A164S, a suspected amyloidosis mutation associated with increased risk of ischemic heart disease and early mortality [13], would have been assumed to be nonfunctional; 2) That variants in APOA1 associated with amyloidosis are relatively common in the general population.
Comparing the results from the population-based resequencing approach with the results obtained using only the extremes of the population distribution of apoA-I in the CCHS showed that NS variants were overrepresented in the lower percentiles of the apoA-I distribution, especially those predicted in silico to be more pathological, and correctly predicted association with low apoA-I and/or HDL cholesterol levels. Thus, 0.27% of the total population were heterozygous for one of nine different variants associated with substantial reductions in apoA-I and/or HDL cholesterol levels of up to, respectively, 39 mg/dL and 0.9 mmol/L.
As previously shown in the CCHS [13] and validated in the present study including the CGPS, NS mutations in APOA1 may associate with increased risk of MI, without associating with reduced apoA-1 and HDL cholesterol levels. The most likley explanation for this is that these mutations represent less severe amyloidosis mutations manifesting clinically as MI, instead of the more severe restrictive cardiomyopathy [42]. Accordingly, we found that the combined NS mutations associated with an increased risk of MI, the main contributor to this increased risk was A164S, a presumed amyloidogenic mutation not associated with apoA-1 or HDL cholesterol levels in either the CCHS or the CGPS.
Our study suggests that the extreme phenotype approach used in a number of previous studies [12]–[16] is a powerful analytical strategy to capture the effects of both common and rare genetic variants on a specific a priori specified complex trait, provided the gene in question does not have pleiotropic effects. However, the success of this strategy depends on using a gene-near phenotype, preferably the direct gene product, on the size of the underlying study, the cut-off point for the extreme percentiles screened, and the frequencies and effect sizes of the identified variants.
In conclusion, population-based resequencing of APOA1 identified a majority of rare NS variants associated with reduced apoA-I and HDL cholesterol levels and/or predisposing to amyloidosis. In addition, NS variants associated with increased risk of MI.
Supporting Information
Plasma apoA-I and HDL cholesterol in percentiles for nonsynonymous and synonymous variants in APOA1 stratified by gender. Data from the Copenhagen City Heart Study (CCHS) and the Copenhagen General Population Study (CGPS) combined. Each dot represents an individual with a given genetic variant. Percentiles corrected for age within each study, and stratified by gender. Solid black lines = median percentiles. Dashed line = 50th percentile. P-values by Mann-Whitney U-test comparing females and males. Red, nonsynonymous variants identified exclusively in the lowest 20 percentile of the apoA-I distribution in the CCHS using the extreme phenotype approach; yellow, synonymous variants identified exclusively in the lowest 20 percentile; white boxes, nonsynonymous and synonymous variants identified in both the lowest and highest 20 percentiles.
(EPS)
Plasma levels of apoA-I and HDL cholesterol in percentiles for all synonymous variants in APOA1, and for nonsynonymous variants suspected of (A164S) or known to associate with amyloidosis (S36A-F71Y-L144R), or with reduced ability to activate LCAT (L144R). Data are from the Copenhagen City Heart Study (CCHS) and the Copenhagen General Population Study (CGPS) combined. Each dot represents an individual with a given genetic variant. Percentiles are corrected for gender and age within each study. Solid black lines = median percentiles. Dashed line = 50th percentile. Percentiles between groups of variants are compared by Mann-Whitney U-test. P-values comparing median percentiles with 50th percentile in the CCHS and CGPS as a whole by z-test and shown directly on figure. *P<0.05 and **P<0.01.
(EPS)
PCR primers and fragment lengths for high-resolution melting analysis using a LightScanner followed by sequencing of APOA1 in the Copenhagen City Heart Study.
(PDF)
Genetic variation identified in the core promoter, coding region and exon-intron boundaries of APOA1 in the Copenhagen City Heart Study (n = 10,330).
(PDF)
Differences (Δ) in relative percentiles (50th percentile in population minus heterozygote percentile) and in absolute levels (noncarriers minus heterozygotes) of apolipoprotein A-I and HDL cholesterol for APOA1 variants in the Copenhagen City Heart Study and in the Copenhagen General Population Study.
(PDF)
Lipid and lipoprotein levels in noncarriers and heterozygotes for synonymous(S) and nonsynonymous(NS) variants in the Copenhagen City Heart Study (left, top and bottom) and the Copenhagen General Population Study (right, bottom).
(PDF)
Acknowledgments
We thank technicians Karin Møller Hansen and Mette Refstrup for their persistent attention to the details of the high resolution melting analysis, genotyping, and sequencing. We are indebted to the staff and participants of the Copenhagen City Heart Study and the Copenhagen General Population Study for their important contributions.
Funding Statement
This work was supported by a Specific Targeted Research Project grant from the European Union, Sixth Framework Programme Priority (FP-2005-LIFESCIHEALTH-6) contract 037631, the Danish Medical Research Council (Copenhagen), the Research Fund at Rigshospitalet, Copenhagen University Hospital (Copenhagen), Chief Physician Johan Boserup and Lise Boserup's Fund (Copenhagen), Ingeborg and Leo Dannin's Grant (Copenhagen), Henry Hansen's and Wife's Grant (Copenhagen), and a grant from the Order of Odd Fellows (Copenhagen). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, et al. (2009) Finding the missing heritability of complex diseases. Nature 461: 747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cirulli ET, Goldstein DB (2010) Uncovering the roles of rare variants in common disease through whole-genome sequencing. Nat Rev Genet 11: 415–425. [DOI] [PubMed] [Google Scholar]
- 3.Tall AR, Breslow JL, Rubin EM (2001) Genetic disorders affecting plasma high-density lipoproteins. In: Schriver C, Beaudet A, Sly W, Valle D, editors. The metabolic and molecular bases of inherited diseases. New York, NY: McGraw-Hill. pp. 2915–2936
- 4. Boes E, Coassin S, Kollerits B, Heid IM, Kronenberg F (2009) Genetic-epidemiological evidence on genes associated with HDL cholesterol levels: A systematic in-depth review. Exp Gerontol 44: 136–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sorci-Thomas MG, Thomas MJ (2002) The effects of altered apolipoprotein A-I structure on plasma HDL concentration. Trends Cardiovasc Med 12: 121–128. [DOI] [PubMed] [Google Scholar]
- 6. Zannis VI, Chroni A, Krieger M (2006) Role of apoA-I, ABCA1, LCAT, and SR-BI in the biogenesis of HDL. J Mol Med 84: 276–294. [DOI] [PubMed] [Google Scholar]
- 7. Benson MD (2005) Ostertag revisited: the inherited systemic amyloidoses without neuropathy. Amyloid 12: 75–87. [DOI] [PubMed] [Google Scholar]
- 8. Obici L, Palladini G, Giorgetti S, Bellotti V, Gregorini G, et al. (2004) Liver biopsy discloses a new apolipoprotein A-I hereditary amyloidosis in several unrelated Italian families. Gastroenterology 126: 1416–1422. [DOI] [PubMed] [Google Scholar]
- 9. de Sousa MM, Vital C, Ostler D, Fernandes R, Pouget-Abadie J, et al. (2000) Apolipoprotein AI and transthyretin as components of amyloid fibrils in a kindred with apoAI Leu178His amyloidosis. Am J Pathol 156: 1911–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Persey MR, Booth DR, Booth SE, Zyl-Smit R, Adams BK, et al. (1998) Hereditary nephropathic systemic amyloidosis caused by a novel variant apolipoprotein A-I. Kidney Int 53: 276–281. [DOI] [PubMed] [Google Scholar]
- 11. Rall SC Jr, Weisgraber KH, Mahley RW, Ogawa Y, Fielding CJ, et al. (1984) Abnormal lecithin:cholesterol acyltransferase activation by a human apolipoprotein A-I variant in which a single lysine residue is deleted. J Biol Chem 259: 10063–10070. [PubMed] [Google Scholar]
- 12. Haase CL, Tybjærg-Hansen A, Grande P, Frikke-Schmidt R (2010) Genetically elevated apolipoprotein A-I, high-density lipoprotein cholesterol levels, and risk of ischemic heart disease. J Clin Endocrinol Metab 95: E500–E510. [DOI] [PubMed] [Google Scholar]
- 13. Haase CL, Frikke-Schmidt R, Nordestgaard BG, Kateifides AK, Kardassis D, et al. (2011) Mutation in APOA1 predicts increased risk of ischaemic heart disease and total mortality without low HDL cholesterol levels. J Int Med 270: 136–146. [DOI] [PubMed] [Google Scholar]
- 14. Frikke-Schmidt R, Nordestgaard BG, Jensen GB, Tybjærg-Hansen A (2004) Genetic variation in ABC transporter A1 contributes to HDL cholesterol in the general population. J Clin Invest 114: 1343–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cohen JC, Kiss RS, Pertsemlidis A, Marcel YL, McPherson R, et al. (2004) Multiple rare alleles contribute to low plasma levels of HDL cholesterol. Science 305: 869–872. [DOI] [PubMed] [Google Scholar]
- 16. Cohen JC, Pertsemlidis A, Fahmi S, Esmail S, Vega GL, et al. (2006) Multiple rare variants in NPC1L1 associated with reduced sterol absorption and plasma low-density lipoprotein levels. Proc Natl Acad Sci USA 103: 1810–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schnohr P, Jensen JS, Scharling H, Nordestgaard BG (2002) Coronary heart disease risk factors ranked by importance for the individual and community. A 21 year follow-up of 12000 men and women from The Copenhagen City Heart Study. Eur Heart J 23: 620–626. [DOI] [PubMed] [Google Scholar]
- 18. Frikke-Schmidt R, Nordestgaard BG, Stene MCA, Sethi AA, Remaley AT, et al. (2008) Association of loss-of-function mutations in the ABCA1 gene with high-density lipoprotein cholesterol levels and risk of ischemic heart disease. JAMA 299: 2524–2532. [DOI] [PubMed] [Google Scholar]
- 19. Zacho J, Tybjærg-Hansen A, Jensen JS, Grande P, Sillesen H, et al. (2008) Genetically elevated C-reactive protein and ischemic vascular disease. N Engl J Med 359: 1897–1908. [DOI] [PubMed] [Google Scholar]
- 20. Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, et al. (2007) Universal definition of myocardial infarction. Eur Heart J 28: 2525–2538. [DOI] [PubMed] [Google Scholar]
- 21. The Joint European Society of Cardiology/American College of Cardiology Committee (2000) Myocardial infarction redefined - A consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the Redefinition of Myocardial Infarction. Eur Heart J 21: 1502–1513. [DOI] [PubMed] [Google Scholar]
- 22. Friedewald WT, Levy RI, Friedrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18: 499–502. [PubMed] [Google Scholar]
- 23. Tybjærg-Hansen A, Steffensen R, Meinertz H, Schnohr P, Nordestgaard BG (1998) Association of mutations in the apolipoprotein B gene with hypercholesterolemia and the risk of ischemic heart disease. N Engl J Med 338: 1577–1584. [DOI] [PubMed] [Google Scholar]
- 24. Rowczenio D, Dogan A, Theis JD, Vrana JA, Lachmann HJ, et al. (2011) Amyloidogenicity and clinical phenotype associated with five novel mutations in apolipoprotein A-I. Am J Pathol 179: 1978–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Romeo S, Pennacchio LA, Fu Y, Boerwinkle E, Tybjærg-Hansen A, et al. (2007) Population-based resequencing of ANGPTL4 uncovers variations that reduce triglycerides and increase HDL. Nat Genet 39: 513–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Coventry A, Bull-Otterson LM, Liu X, Clark AG, Maxwell TJ, et al. (2010) Deep resequencing reveals excess rare recent variants consistent with explosive population growth. Nat Commun 1: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Araki K, Sasaki J, Matsunaga A, Takada Y, Moriyama K, et al. (1994) Characterization of two new human apolipoprotein A-I variants: apolipoprotein A-I Tsushima (Trp-108→Arg) and A-I Hita (Ala-95→Asp). Biochim Biophys Acta 1214: 272–278. [PubMed] [Google Scholar]
- 28. von Eckardstein A, Funke H, Walter M, Altland K, Benninghoven A, et al. (1990) Structural analysis of human apolipoprotein A-I variants. Amino acid substitutions are nonrandomly distributed throughout the apolipoprotein A-I primary structure. J Biol Chem 265: 8610–8617. [PubMed] [Google Scholar]
- 29. Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, et al. (2010) Biological, clinical and population relevance of 95 loci for blood lipids. Nature 466: 707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zannis VI, Koukos G, Drosatos K, Vezeridis A, Zanni EE, et al. (2008) Discrete roles of apoA-I and apoE in the biogenesis of HDL species: Lessons learned from gene transfer studies in different mouse models. Ann Med 40 Suppl 1: 14–28. [DOI] [PubMed] [Google Scholar]
- 31. Menzel HJ, Assmann G, Rall SC Jr, Weisgraber KH, Mahley RW (1984) Human apolipoprotein A-I polymorphism. Identification of amino acid substitutions in three electrophoretic variants of the Münster-3 type. J Biol Chem 259: 3070–3076. [PubMed] [Google Scholar]
- 32. von Eckardstein A, Funke H, Henke A, Altland K, Benninghoven A, et al. (1989) Apolipoprotein A-I variants. Naturally occurring substitutions of proline residues affect plasma concentration of apolipoprotein A-I. J Clin Invest 84: 1722–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Miccoli R, Bertolotto A, Navalesi R, Odoguardi L, Boni A, et al. (1996) Compound heterozygosity for a structural apolipoprotein A-I variant, apo A-I(L141R)Pisa, and an apolipoprotein A-I null allele in patients with absence of HDL cholesterol, corneal opacifications, and coronary heart disease. Circulation 94: 1622–1628. [DOI] [PubMed] [Google Scholar]
- 34. Kiss RS, Kavaslar N, Okuhira Ki, Freeman MW, Walter S, et al. (2007) Genetic etiology of isolated low HDL syndrome: Incidence and heterogeneity of efflux defects. Arterioscler Thromb Vasc Biol 27: 1139–1145. [DOI] [PubMed] [Google Scholar]
- 35. Matsunaga T, Hiasa Y, Yanagi H, Maeda T, Hattori N, et al. (1991) Apolipoprotein A-I deficiency due to a codon 84 nonsense mutation of the apolipoprotein A-I gene. Proc Natl Acad Sci USA 88: 2793–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fullerton M, Buchanan V, Sonpar A, Taylor L, Smith D, et al. (2004) The effects of scale: variation in the APOA1/C3/A4/A5 gene cluster. Hum Genet V115: 36–56. [DOI] [PubMed] [Google Scholar]
- 37. Utermann G, Feussner G, Franceschini G, Haas J, Steinmetz A (1982) Genetic variants of group A apolipoproteins. Rapid methods for screening and characterization without ultracentrifugation. J Biol Chem 257: 501–507. [PubMed] [Google Scholar]
- 38. Amarzguioui M, Mucchiano G, Häggqvist B, Westermark P, Kavlie A, et al. (1998) Extensive intimal apolipoprotein A1-derived amyloid deposits in a patient with an apolipoprotein A1 mutation. Biochem Biophys Res Commun 242: 534–539. [DOI] [PubMed] [Google Scholar]
- 39. Tilly-Kiesi M, Packard CJ, Kahri J, Ehnholm C, Shepherd J, et al. (1997) In vivo metabolism of apo A-I and apo A-II in subjects with apo A-I(Lys107→0) associated with reduced HDL cholesterol and Lp(AI w AII) deficiency. Atherosclerosis 128: 213–222. [DOI] [PubMed] [Google Scholar]
- 40. Tilly-Kiesi M, Qiuping Z, Ehnholm S, Kahri J, Lahdenpera S, et al. (1995) ApoA-IHelsinki (Lys107→0) associated with reduced HDL cholesterol and LpA-I:A-II deficiency. Arterioscler Thromb Vasc Biol 15: 1294–1306. [DOI] [PubMed] [Google Scholar]
- 41. Recalde D, Velez-Carrasco W, Civeira F, Cenarro A, Gomez-Coronado D, et al. (2001) Enhanced fractional catabolic rate of apo A-I and apo A-II in heterozygous subjects for apoA-IZaragoza (L144R). Atherosclerosis 154: 613–623. [DOI] [PubMed] [Google Scholar]
- 42. Falk RH, Dubrey SW (2010) Amyloid heart disease. Prog Cardiovasc Dis 52: 347–361. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Plasma apoA-I and HDL cholesterol in percentiles for nonsynonymous and synonymous variants in APOA1 stratified by gender. Data from the Copenhagen City Heart Study (CCHS) and the Copenhagen General Population Study (CGPS) combined. Each dot represents an individual with a given genetic variant. Percentiles corrected for age within each study, and stratified by gender. Solid black lines = median percentiles. Dashed line = 50th percentile. P-values by Mann-Whitney U-test comparing females and males. Red, nonsynonymous variants identified exclusively in the lowest 20 percentile of the apoA-I distribution in the CCHS using the extreme phenotype approach; yellow, synonymous variants identified exclusively in the lowest 20 percentile; white boxes, nonsynonymous and synonymous variants identified in both the lowest and highest 20 percentiles.
(EPS)
Plasma levels of apoA-I and HDL cholesterol in percentiles for all synonymous variants in APOA1, and for nonsynonymous variants suspected of (A164S) or known to associate with amyloidosis (S36A-F71Y-L144R), or with reduced ability to activate LCAT (L144R). Data are from the Copenhagen City Heart Study (CCHS) and the Copenhagen General Population Study (CGPS) combined. Each dot represents an individual with a given genetic variant. Percentiles are corrected for gender and age within each study. Solid black lines = median percentiles. Dashed line = 50th percentile. Percentiles between groups of variants are compared by Mann-Whitney U-test. P-values comparing median percentiles with 50th percentile in the CCHS and CGPS as a whole by z-test and shown directly on figure. *P<0.05 and **P<0.01.
(EPS)
PCR primers and fragment lengths for high-resolution melting analysis using a LightScanner followed by sequencing of APOA1 in the Copenhagen City Heart Study.
(PDF)
Genetic variation identified in the core promoter, coding region and exon-intron boundaries of APOA1 in the Copenhagen City Heart Study (n = 10,330).
(PDF)
Differences (Δ) in relative percentiles (50th percentile in population minus heterozygote percentile) and in absolute levels (noncarriers minus heterozygotes) of apolipoprotein A-I and HDL cholesterol for APOA1 variants in the Copenhagen City Heart Study and in the Copenhagen General Population Study.
(PDF)
Lipid and lipoprotein levels in noncarriers and heterozygotes for synonymous(S) and nonsynonymous(NS) variants in the Copenhagen City Heart Study (left, top and bottom) and the Copenhagen General Population Study (right, bottom).
(PDF)