Abstract
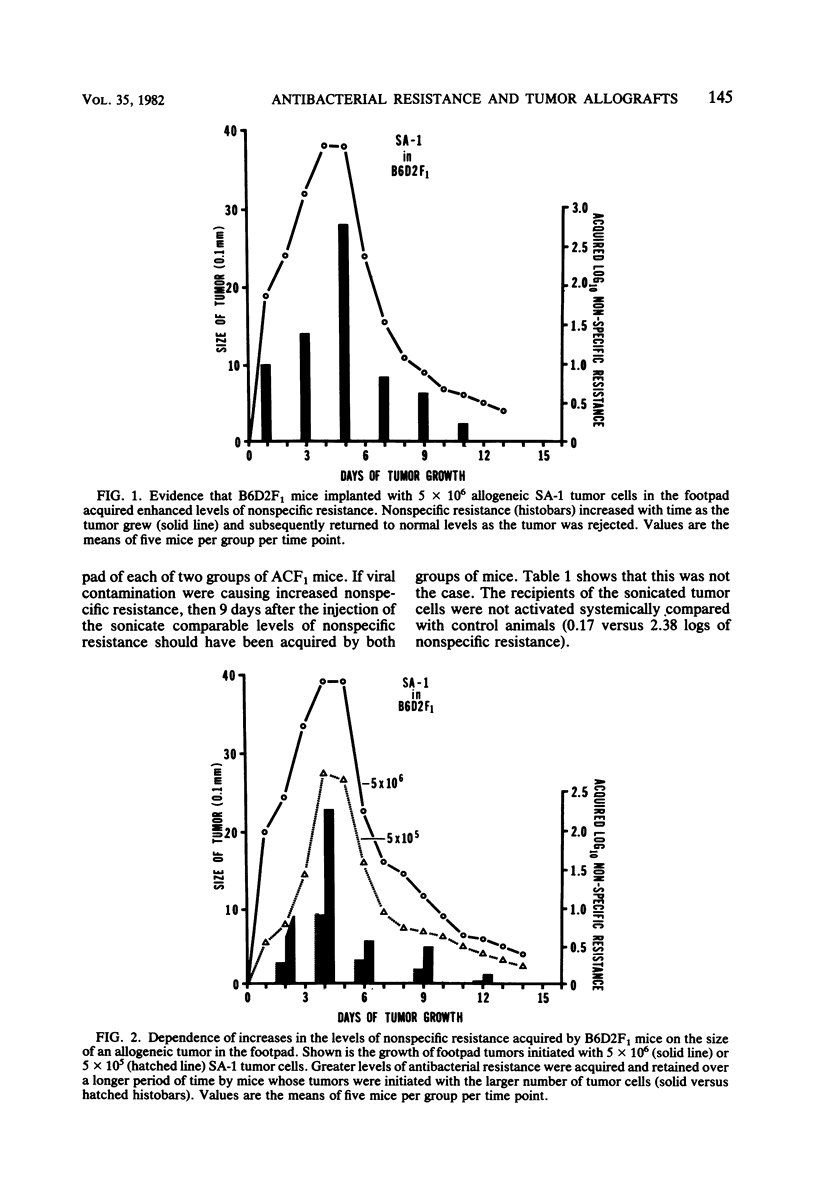
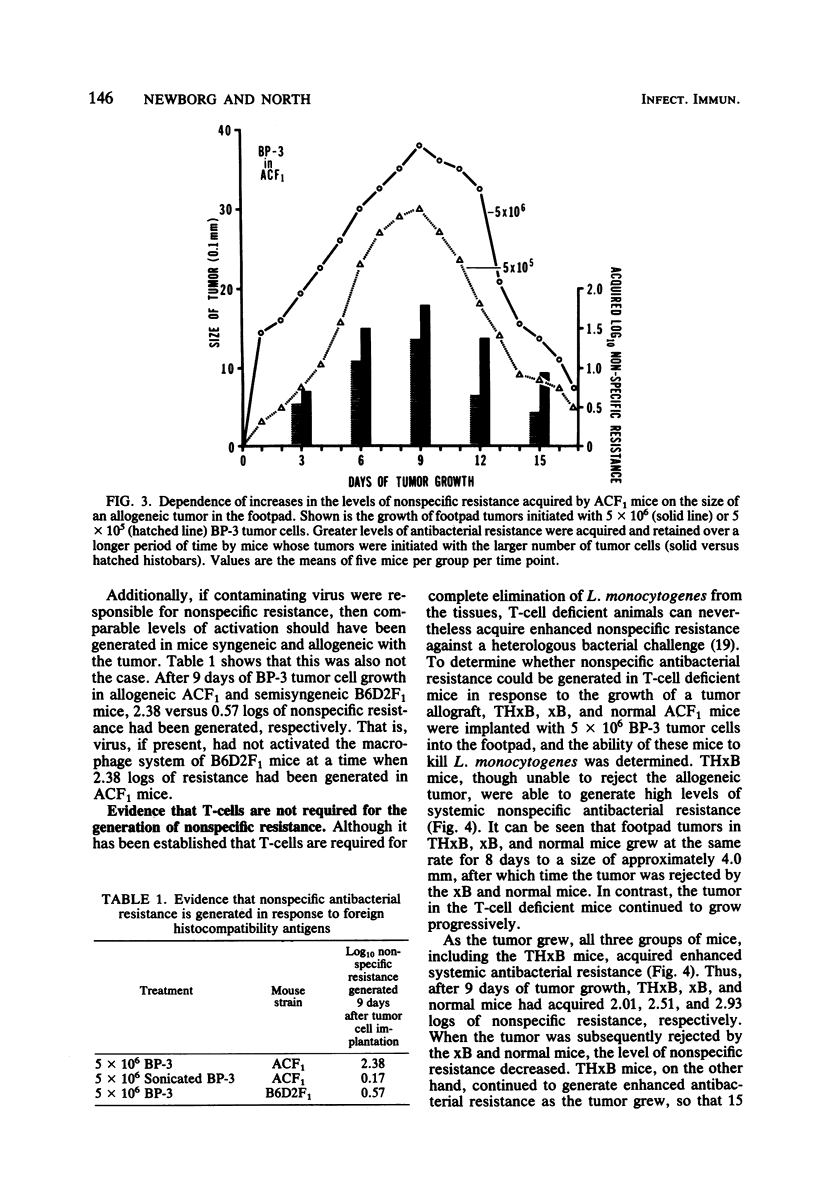
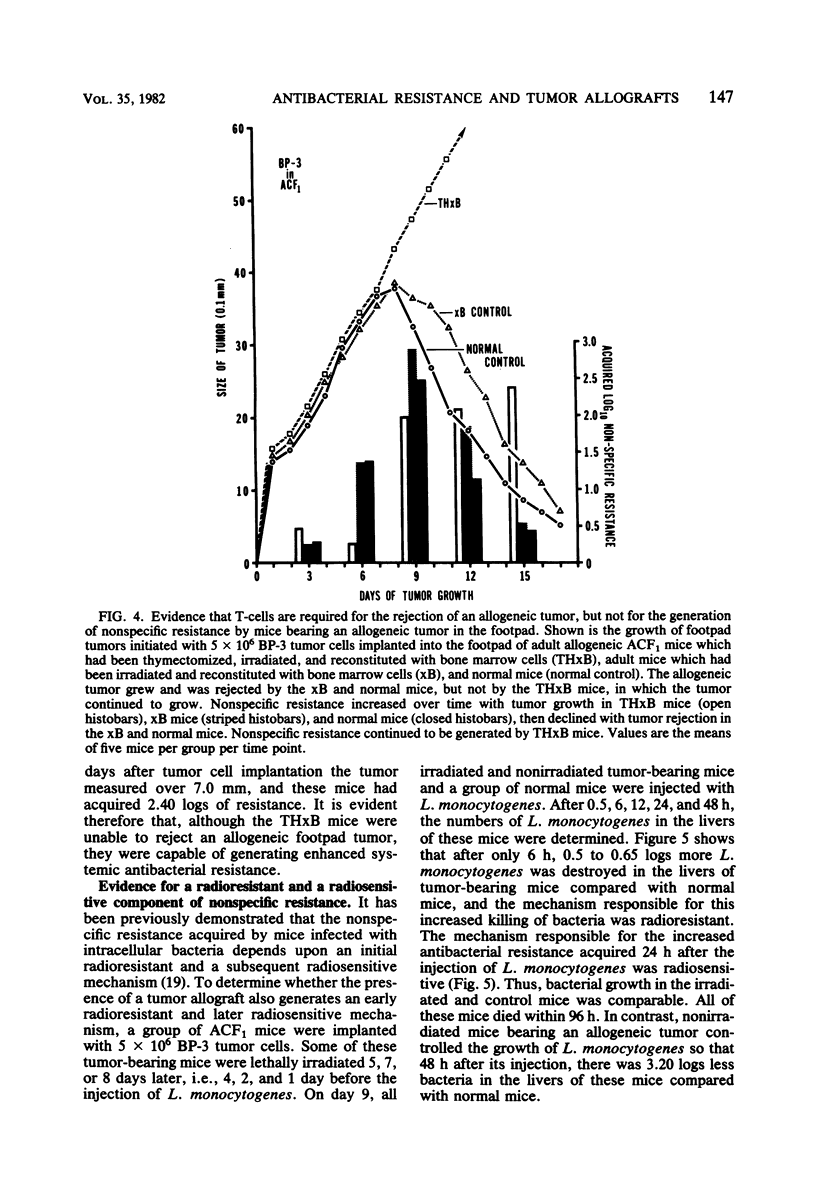
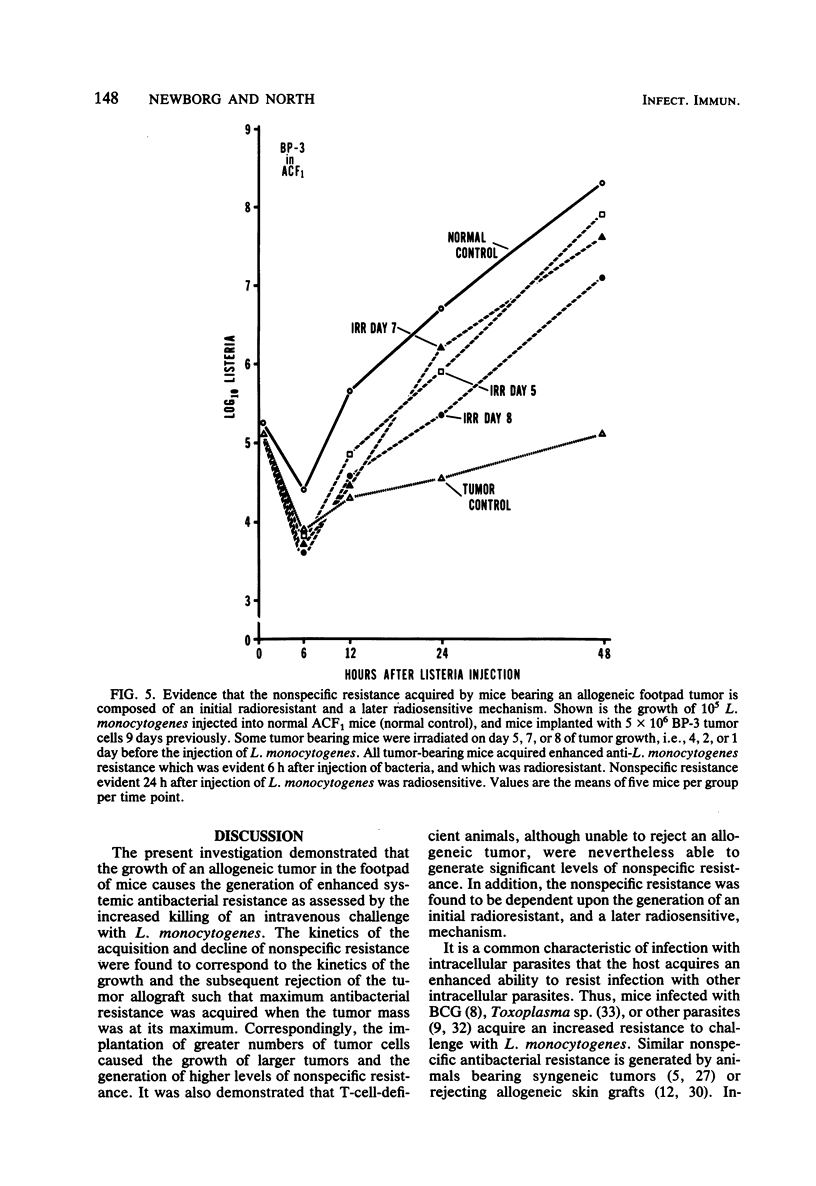
Enhanced systemic antibacterial resistance was acquired by ACF1 (H-2ad) and B6D2F1 (H-2bd) mice bearing allogenetic BP-3 (H-2b) and SA-1 (H-2a) footpad tumors, respectively. This effect was dose dependent, in that the implantation of greater numbers of tumor cells produced larger tumors and higher levels of nonspecific resistance. Although the rejection of the tumor was T-cell dependent, the generation of nonspecific resistance was not. Thus, T-cell-deficient mice bearing progressively growing tumor allografts generated significant levels of antibacterial resistance. Two mechanisms were found to be involved in the generation of nonspecific resistance: (i) an acquired radioresistant mechanism responsible for initially destroying a large proportion of injected bacteria and (ii) an acquired radiosensitive mechanism responsible for destroying a significant number of bacteria after 24 h.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. E., Warner N. L. Ionizing radiation and the immune response. Adv Immunol. 1976;24:215–335. doi: 10.1016/s0065-2776(08)60331-4. [DOI] [PubMed] [Google Scholar]
- Axline S. G., Cohn Z. A. In vitro induction of lysosomal enzymes by phagocytosis. J Exp Med. 1970 Jun 1;131(6):1239–1260. doi: 10.1084/jem.131.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BASES R. E., KRAKOFF I. H. ENHANCED RETICULOENDOTHELIAL PHAGOCYTIC ACTIVITY IN MYELOPROLIFERATIVE DISEASES. J Reticuloendothel Soc. 1965 May;2:1–7. [PubMed] [Google Scholar]
- Beaman B. L., Gershwin M. E., Scates S. S., Ohsugi Y. Immunobiology of germfree mice infected with Nocardia asteroides. Infect Immun. 1980 Aug;29(2):733–743. doi: 10.1128/iai.29.2.733-743.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendt M. J., North R. J., Kirstein D. P. The immunological basis of endotoxin-induced tumor regression. Requirement for T-cell-mediated immunity. J Exp Med. 1978 Dec 1;148(6):1550–1559. doi: 10.1084/jem.148.6.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendt M. J., North R. J., Kirstein D. P. The immunological basis of endotoxin-induced tumor regression. Requirement for a pre-existing state of concomitant anti-tumor immunity. J Exp Med. 1978 Dec 1;148(6):1560–1569. doi: 10.1084/jem.148.6.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blamey R. W., Crosby D. L., Baker J. M. Reticuloendothelial activity during the growth of rat sarcomas. Cancer Res. 1969 Feb;29(2):335–337. [PubMed] [Google Scholar]
- Blanden R. V. Increased antibacterial resistance and immunodepression during graft-versus-host reactions in mice. Transplantation. 1969 Jun;7(6):484–497. doi: 10.1097/00007890-196906000-00005. [DOI] [PubMed] [Google Scholar]
- Blanden R. V., Lefford M. J., Mackaness G. B. The host response to Calmette-Guérin bacillus infection in mice. J Exp Med. 1969 May 1;129(5):1079–1107. doi: 10.1084/jem.129.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V., Mackaness G. B., Collins F. M. Mechanisms of acquired resistance in mouse typhoid. J Exp Med. 1966 Oct 1;124(4):585–600. doi: 10.1084/jem.124.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheers C., Waller R. Activated macrophages in congenitally athymic "nude mice" and in lethally irradiate mice. J Immunol. 1975 Sep;115(3):844–847. [PubMed] [Google Scholar]
- Emmerling P., Finger H., Bockemühl J. Listeria monocytogenes infection in nude mice. Infect Immun. 1975 Aug;12(2):437–439. doi: 10.1128/iai.12.2.437-439.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISHER B., FISHER E. R. TISSUE TRANSPLANTATION AND THE RETICULOENDOTHELIAL SYSTEM. I. EFFECT OF SKIN GRAFTS IN NORMAL ANIMALS. Transplantation. 1964 Mar;2:228–234. [PubMed] [Google Scholar]
- Godal T., Rees R. J., Lamvik J. O. Lymphocyte-mediated modification of blood-derived macrophage function in vitro; inhibition of growth of intracellular mycobacteria with lymphokines. Clin Exp Immunol. 1971 Apr;8(4):625–637. [PMC free article] [PubMed] [Google Scholar]
- Howard R. J., Najarian J. S. Cytomegalovirus-induced immune suppression. I. Humoral immunity. Clin Exp Immunol. 1974 Sep;18(1):109–118. [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magarey C. J., Baum M. Reticulo-endothelial activity in humans with cancer. Br J Surg. 1970 Oct;57(10):748–752. doi: 10.1002/bjs.1800571013. [DOI] [PubMed] [Google Scholar]
- Mauel J., Buchmüller Y., Behin R. Studies on the mechanisms of macrophage activation. I. Destruction of intracellular Leishmania enriettii in macrophages activated by cocultivation with stimulated lymphocytes. J Exp Med. 1978 Aug 1;148(2):393–407. doi: 10.1084/jem.148.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer M. S. Tumoricidal responses in vitro of peritoneal macrophages from conventionally housed and germ-free nude mice. Cell Immunol. 1976 Mar 1;22(1):176–181. doi: 10.1016/0008-8749(76)90018-6. [DOI] [PubMed] [Google Scholar]
- Newborg M. F., North R. J. On the mechanism of T cell-independent anti-Listeria resistance in nude mice. J Immunol. 1980 Feb;124(2):571–576. [PubMed] [Google Scholar]
- Nickol A. D., Bonventre P. F. Anomalous high native resistance to athymic mice to bacterial pathogens. Infect Immun. 1977 Dec;18(3):636–645. doi: 10.1128/iai.18.3.636-645.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J., Deissler J. F. Nature of "memory" in T-cell mediated antibacterial immunity: cellular parameters that distinguish between the active immune response and a state of "memory". Infect Immun. 1975 Oct;12(4):761–767. doi: 10.1128/iai.12.4.761-767.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J., Kirstein D. P. T-cell-mediated concomitant immunity to syngeneic tumors. I. Activated macrophages as the expressors of nonspecific immunity to unrelated tumors and bacterial parasites. J Exp Med. 1977 Feb 1;145(2):275–292. doi: 10.1084/jem.145.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. Nature of "memory" in T-cell-mediated antibacterial immunity: anamnestic production of mediator T cells. Infect Immun. 1975 Oct;12(4):754–760. doi: 10.1128/iai.12.4.754-760.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. The concept of the activated macrophage. J Immunol. 1978 Sep;121(3):806–809. [PMC free article] [PubMed] [Google Scholar]
- North R. J. The relative importance of blood monocytes and fixed macrophages to the expression of cell-mediated immunity to infection. J Exp Med. 1970 Sep 1;132(3):521–534. doi: 10.1084/jem.132.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLD L. J., BENACERRAF B., CLARKE D. A., CARSWELL E. A., STOCKERT E. The role of the reticuloendothelial system in the host reaction to neoplasia. Cancer Res. 1961 Oct;21:1281–1300. [PubMed] [Google Scholar]
- Otu A. A., Russell R. J., Wilkinson P. C., White R. G. Alterations of mononuclear phagocyte function induced by Lewis lung carcinoma in C57BL mice. Br J Cancer. 1977 Sep;36(3):330–340. doi: 10.1038/bjc.1977.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch M., Manejias R. E., Russo M., Abbey E. E. Increased spreading of macrophages from mice treated with interferon inducers. Cell Immunol. 1977 Mar 1;29(1):86–95. doi: 10.1016/0008-8749(77)90277-5. [DOI] [PubMed] [Google Scholar]
- Rao G. R., Rawls W. E., Perey D. Y., Tompkins W. A. Macrophage activation in congenitally athymic mice raised under conventional or germ-free conditions. J Reticuloendothel Soc. 1977 Jan;21(1):13–20. [PubMed] [Google Scholar]
- Rhodes J. M., Bennedsen J., Larsen S. O., Riisgaard S., Spärck J. V. Correlation between in vivo and in vitro functional tests for activated macrophages. Infect Immun. 1979 Jan;23(1):34–40. doi: 10.1128/iai.23.1.34-40.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocklin R. E., MacDermott R. P., Chess L., Schlossman S. F., David J. R. Studies on mediator production by highly purified human T and B lymphocytes. J Exp Med. 1974 Nov 1;140(5):1303–1316. doi: 10.1084/jem.140.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruskin J., Remington J. S. Immunity and intracellular infection: resistance to bacteria in mice infected with a protozoan. Science. 1968 Apr 5;160(3823):72–74. doi: 10.1126/science.160.3823.72. [DOI] [PubMed] [Google Scholar]
- Salky N. K., Di Luzio N. R., Levin A. G., Goldsmith H. S. Phagocytic activity of the reticuloendothelial system in neoplastic disease. J Lab Clin Med. 1967 Sep;70(3):393–403. [PubMed] [Google Scholar]
- Yoshida T., Sonozaki H., Cohen S. The production of migration inhibition factor by B and T cells of the guinea pig. J Exp Med. 1973 Oct 1;138(4):784–797. doi: 10.1084/jem.138.4.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Blanden R. V., Langman R. E. Early appearance of sensitized lymphocytes in mice infected with Listeria monocytogenes. J Immunol. 1974 Feb;112(2):496–501. [PubMed] [Google Scholar]
- Zinkernagel R. M., Blanden R. V. Macrophage activation in mice lacking thymus-derived (T) cells. Experientia. 1975 May 15;31(5):591–593. doi: 10.1007/BF01932477. [DOI] [PubMed] [Google Scholar]


