Abstract
Many auxin responses are dependent on redistribution and/or polar transport of indoleacetic acid. Polar transport of auxin can be inhibited through the application of phytotropins such as 1-naphthylphthalamic acid (NPA). When Arabidopsis thaliana seedlings were grown in the light on medium containing 1.0 μm NPA, hypocotyl and root elongation and gravitropism were strongly inhibited. When grown in darkness, however, NPA disrupted the gravity response but did not affect elongation. The extent of inhibition of hypocotyl elongation by NPA increased in a fluence-rate-dependent manner to a maximum of about 75% inhibition at 50 μmol m−2 s−1 of white light. Plants grown under continuous blue or far-red light showed NPA-induced hypocotyl inhibition similar to that of white-light-grown plants. Plants grown under continuous red light showed less NPA-induced inhibition. Analysis of photoreceptor mutants indicates the involvement of phytochrome and cryptochrome in mediating this NPA response. Hypocotyls of some auxin-resistant mutants had decreased sensitivity to NPA in the light, but etiolated seedlings of these mutants were similar in length to the wild type. These results indicate that light has a significant effect on NPA-induced inhibition in Arabidopsis, and suggest that auxin has a more important role in elongation responses in light-grown than in dark-grown seedlings.
The development of a plant is influenced by a variety of environmental cues. Differences in light quantity and quality can lead to dramatically different growth forms. Light signals are perceived by a number of different photoreceptors, including the phytochromes and the blue light receptors. Developmental processes under the control of the phytochromes include stem elongation, hypocotyl hook unfolding, leaf expansion, seed germination, and flower initiation (for review, see von Arnim and Deng, 1996). Blue light receptors have been shown to be involved in phototropism, regulation of stem elongation, stomatal opening, and the initiation of chloroplast development (for review, see Senger and Schmidt, 1994).
Many of the developmental processes that occur as a result of light signals are dependent, at least in part, on the action of phytohormones. For example, light has been shown to alter the levels of IAA (Bandurski et al., 1977; Jones et al., 1991; Behringer and Davies, 1992), GAs (Ross et al., 1992; Foster and Morgan, 1995), ABA (Weatherwax et al., 1996), cytokinins (Qamuruddin and Tillberg, 1989; Kraepiel et al., 1995), and ethylene (Kathiresan et al., 1996, and refs. therein). Behringer and Davies (1992) proposed that phytochrome regulation of stem elongation is partly the result of changes in IAA levels. Phytochrome-deficient mutants of Arabidopsis thaliana require GAs to express the elongated phenotype of these plants (Peng and Harberd, 1997). Light regulation of BR levels or sensitivity clearly plays a central role in light-regulated development, because Arabidopsis mutants with defects in BR biosynthesis and response are severe dwarfs in both light and dark conditions (Clouse et al., 1996; Kauschmann et al., 1996; Li et al., 1996; Szerkes et al., 1996).
The phytohormone auxin is involved in diverse developmental processes, many of which depend on regulated auxin transport (Went and Thimann, 1937). For example, apical dominance is maintained by auxin produced in the apical meristem and transported basipetally to the target tissues, where it inhibits growth of lateral branches. In the case of tropic responses, lateral redistribution of auxin gives rise to differential growth rates, resulting in curvature of the growing organ. In addition, a gradient in auxin concentration from tip to base is believed to be responsible for differential elongation rates in different regions of shoots (Went and Thimann, 1937; Sanchez-Bravo et al., 1992).
The formation and maintenance of auxin gradients is thought to occur through the action of a specific polar auxin-transport system that requires active efflux of auxin through an auxin-anion uniport (Sabater and Rubery, 1987). Auxin efflux is inhibited by synthetic phytotropins such as NPA (Morgan, 1964; Katekar and Giesler, 1980; Jacobs and Rubery, 1988). The exact nature of this inhibition is not known, but NPA and other auxin-transport inhibitors bind to a single plasma-membrane protein (Lembi et al., 1971; Muday et al., 1993; Bernasconi et al., 1996). The endogenous auxin IAA does not compete with NPA for this binding site (Lomax et al., 1995). In the tir3 mutant of Arabidopsis, reduced NPA binding is associated with defects in auxin-regulated growth processes, suggesting that the NPA-binding site is important for auxin transport (Ruegger et al., 1997).
During the course of genetic studies on auxin transport in Arabidopsis, we noticed that NPA is a potent inhibitor of hypocotyl elongation in light-grown seedlings. In contrast, several recent reports suggest that NPA has little effect on hypocotyl elongation in dark-grown Arabidopsis seedlings (Garbers et al., 1996; Lehman et al., 1996). To further investigate the role of auxin and auxin transport in hypocotyl elongation, we have examined the effects of NPA on hypocotyl elongation under a variety of light conditions and in several different mutants. Our results suggest that auxin is required for hypocotyl elongation during photomorphogenesis, but has a limited role during elongation in the dark.
MATERIALS AND METHODS
Arabidopsis thaliana seeds were surface sterilized for 20 min in 20% (v/v) commercial bleach and 0.1% Triton X-100, rinsed four times with sterile, distilled water, and chilled for 2 d at 4°C. Sterile seeds were placed in square Petri plates on medium containing nutrient salts, 8 g L−1 agar, and 10 g L−1 Suc (Lincoln et al., 1990). NPA was added to the medium after autoclaving from a stock dissolved in ethanol. All plants were ecotype Columbia unless stated otherwise. Petri plates were placed vertically and the seedlings were grown at 22°C in darkness or in continuous light for 7 d. In experiments with the photoreceptor mutants, the seeds were given 16 h of white light to ensure germination before placement in the experimental light conditions. Seeds of the mutants analyzed in this study were obtained from in-house stocks or from the Arabidopsis Biological Resource Center (Ohio State University, Columbus). Seeds of det2 were a gift from J. Chory (Salk Institute, San Diego, CA). Seeds of the transgenic 35S-iaaL plants were obtained from Charles Romano and Harry Klee (University of Florida, Gainesville).
Light Sources
White light at up to 150 μmol m−2 s−1 was obtained with mixed cool-white and warm-fluorescent light bulbs. Blue light up to 30 μmol m−2 s−1 was obtained by filtering light from a bank of cool-white fluorescent bulbs through blue plexiglass (no. 2424, Rohm and Hass, Darmstadt, Ger-many). Red and far-red light up to 30 μmol m−2 s−1 was provided by a solid-state light-emitting diode system (Qbeam 220, Quantum Devices, Barnevel, WI). Neutral-density screens were used to vary the fluence rates. Fluence rates of white, blue, and red light were measured with a quantum photometer (model LI-189, Li-Cor, Lincoln, NE) and fluence rates of far-red light were measured with a spectroradiometer (model LI-1800, Li-Cor).
Growth Measurement
Measurements were made after 7 d of growth. The hypocotyls were straightened with a forceps if necessary and the plates were placed in a photographic enlarger and projected. Magnified images of the hypocotyls were then measured to the nearest 0.1 mm with a ruler. Root lengths were measured to the nearest 0.5 mm by placing the seedlings directly onto a ruler.
To quantify the gravity response, images of undisturbed seedlings on agar plates were captured using a digital imaging system (IS-1000, Innotech Scientific, San Leandro, CA). The orientation angles of the hypocotyls and roots were measured relative to the gravity vector with National Institutes of Health imaging software (see: http://rsb.info.nih.gov/nih-image/), and the sd was calculated as a measure of the randomness of growth.
RESULTS
Light Dependence of the NPA Response
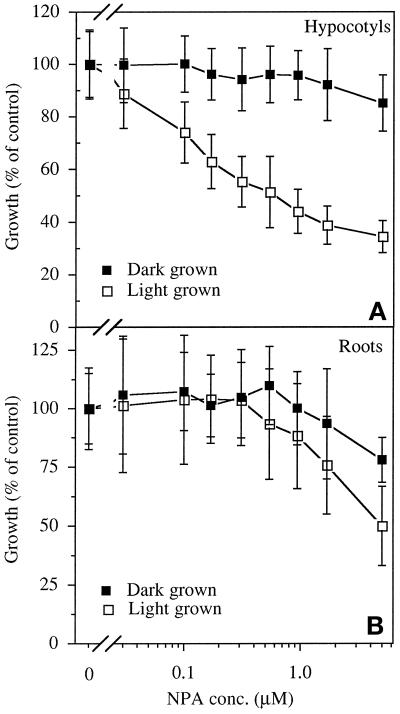
NPA dose-response curves for inhibition of hypocotyl elongation are shown in Figure 1. In dark-grown seedlings there was negligible inhibition of hypocotyl elongation with concentrations up to 5.0 μm NPA (Fig. 1A). In contrast, we observed strong inhibition of elongation in plants grown in the light, with 50% inhibition occurring at approximately 0.5 μm NPA. In roots inhibition of elongation by NPA was not as strong as in the hypocotyl, but the inhibition was greater in light-grown than in dark-grown seedlings (Fig. 1B).
Figure 1.
The effect of light on NPA-induced inhibition of elongation of hypocotyls (A) and roots (B). Growth is normalized to that of seedlings grown without NPA. The fluence rate of the white light was 50 μmol m−2 s−1. Values are the means ± sd from three independent replicates, with at least 10 seedlings per replicate. conc., Concentration.
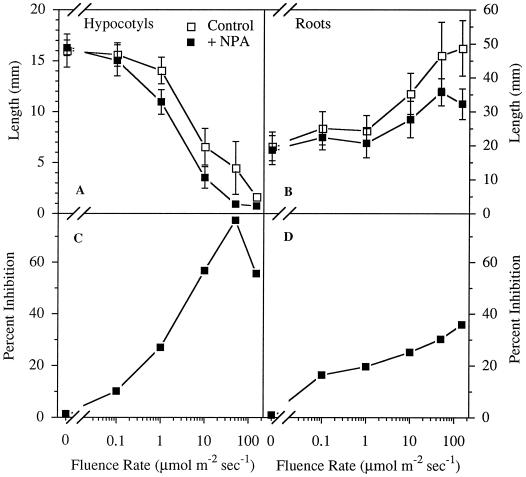
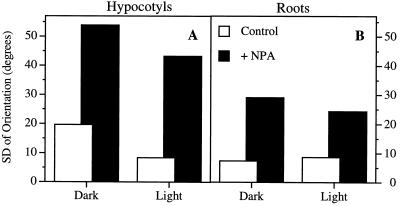
NPA-induced inhibition of hypocotyl elongation was fluence-rate dependent (Fig. 2). In the absence of NPA, seedlings showed characteristic fluence-rate-dependent inhibition of hypocotyl elongation in response to white light (Fig. 2A). When the inhibition caused by 1.0 μm NPA was normalized to the controls at the corresponding light levels, the fluence-rate dependence of the NPA effect was evident and approached a maximum relative inhibition of around 80% (Fig. 2C). The slight decrease in the relative response to NPA at 150 μmol m−2 s−1 was most likely the result of the extreme inhibition caused by the light alone. Although the lengths of hypocotyls from dark-grown plants were unaffected by treatment with 1.0 μm NPA, NPA disrupted the gravity response (Fig. 3A).
Figure 2.
The effect of fluence rate on inhibition of elongation by 1.0 μm NPA. A, Comparison of hypocotyl lengths in 1.0 μm NPA-treated and untreated seedlings. B, Comparison of root lengths in 1.0 μm NPA-treated and untreated seedlings. C, Percentage inhibition of hypocotyl elongation calculated as growth on 1.0 μm NPA compared with untreated seedlings at the corresponding fluence rate. D, Percentage inhibition of root elongation calculated as growth on 1.0 μm NPA compared with untreated seedlings (see C). Values are the means ± sd from three independent replicates, with at least 10 seedlings per replicate.
Figure 3.
The effect of 1.0 μm NPA on the gravity response of hypocotyls (A) and roots (B) of light- and dark-grown seedlings. Data are presented as the sd of the orientation angles of the hypocotyls and roots as a measure of the randomness of orientation.
Roots also exhibited a light-dependent response to NPA (Fig. 2, B and D). Untreated roots increased in length in seedlings grown with increasing fluence rates, but 1.0 μm NPA prevented the roots from elongating to control lengths. As with the hypocotyls, the percentage inhibition of elongation caused by NPA increased with increased fluence rates (Fig. 2D). However, the maximum response of the roots was less than that of the hypocotyls (40% inhibition in roots compared with 80% in hypocotyls). Again, although root elongation in dark-grown plants was unaffected by NPA, the gravity response was still disrupted (Fig. 3B).
Effect of Light Quality
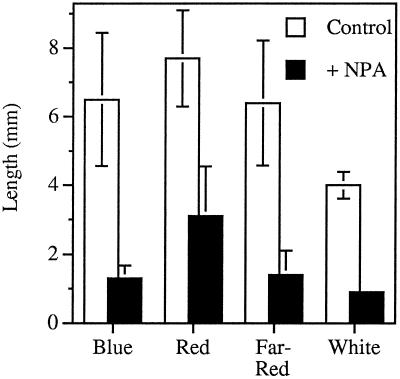
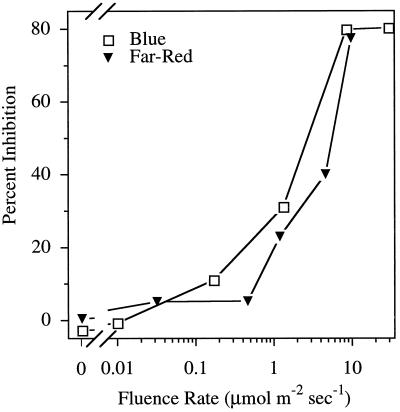
Because growth in white light had a strong effect on NPA-induced inhibition of hypocoytl elongation, we examined the response in seedlings grown under various colors of light (Fig. 4). In continuous blue, red, or far-red light, NPA inhibited hypocotyl elongation. The response to NPA under blue and far-red light was similar in magnitude to that seen under white light, but there was less effect under red light. Blue and far-red fluence-rate dependence of the response to 1.0 μm NPA (Fig. 5) was very similar to that seen for white light (Fig. 2A). The fluence-rate dependence for red light was not determined because red light was not as effective at inhibiting hypocotyl elongation. The inhibition of root elongation under the different colors of light was negligible compared with that observed with white light, although a slight inhibition was observed with blue light (data not shown).
Figure 4.
The effect of light quality on the inhibition of hypocotyl elongation by 1.0 μm NPA. Seedlings were grown under continuous white light (80 μmol m−2 s−1), blue light (30 μmol m−2 s−1), far-red light (10 μmol m−2 s−1), or red light (25 μmol m−2 s−1) for 7 d. Values are the means ± sd from three independent replicates, with at least 10 seedlings per replicate.
Figure 5.
The effect of blue and far-red light fluence rate on inhibition of hypocotyl elongation by 1.0 μm NPA. The percentage inhibition of elongation is calculated as growth on 1.0 μm NPA compared with untreated seedlings at the corresponding fluence rate. Values are the means ± sd from three independent replicates, with at least 10 seedlings per replicate.
Mutant Analysis
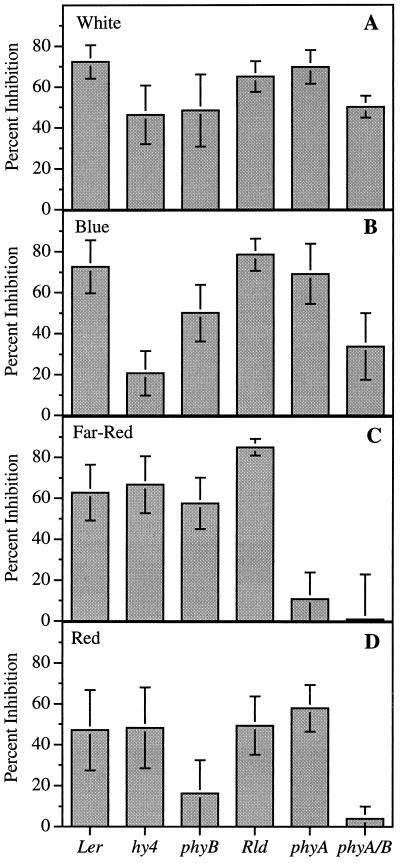
The observation that growth in blue, red, and far-red light resulted in NPA-induced inhibition of hypocotyl elongation suggested that multiple photoreceptors were functioning in this response. To examine this further, several photoreceptor mutants were tested, including hy4, which is defective in CRY1-dependent blue-light responses; phyA, which is defective in PHYA-dependent responses; phyB, which is defective in PHYB-dependent responses; and a phyA-phyB double mutant designated phyA/B. When the mutants were grown in darkness, they behaved like the wild type and no appreciable effect of NPA was observed in the hypocotyls (data not shown). When plants were grown under white light, 1.0 μm NPA inhibited hypocotyl elongation in all of the mutants (Fig. 6A). However, with the exception of phyA, the inhibition was not as great as that observed for the wild type of the corresponding ecotype.
Figure 6.
The effect of 1.0 μm NPA on the hypocotyl elongation of photoreceptor mutants and wild types (Ler and Rld). Values are the means ± sd from three independent replicates, with at least 10 seedlings per replicate. The percentage-inhibition values were calculated by determining the percentage inhibition for each NPA-treated seedling using the average length of the appropriate control seedlings. The values for each seedling were then averaged to generate the data points ± sd. Seedlings were grown as described in Figure 4.
Under blue light, hy4 and the phyA/B double mutant showed only slight inhibition in the presence of NPA (Fig. 6B). The phyA and phyB mutants showed greater inhibition than hy4 and phyA/B, but less than that of the corresponding wild-type seedlings. In far-red light the phyA and the phyA/B double mutants showed no effects of NPA on elongation, whereas phyB, hy4, and the wild type were strongly inhibited (Fig. 6C). In all of the genotypes, less inhibition was observed under red light compared with the other light treatments. However, the NPA response in red light was similar in hy4, phyA, and the wild type, whereas phyB showed only slight inhibition and phyA/B showed none (Fig. 6D). All genotypes in all light treatments showed a perturbation of the gravity response in the presence of NPA regardless of the effect on elongation (data not shown).
In an attempt to gain some insight into the mechanism of the light effect, we examined the NPA response in several hormone mutants, including axr1-12 and axr1-3, which are allelic mutants resistant to auxin; det2, which is defective in BR biosynthesis and has a constitutive light phenotype; eto1-1, which is an ethylene overproducer; and the ethylene-resistant mutant etr1 (Table I). In addition, we examined transgenic 35S-iaaL plants, which express an enzyme that conjugates IAA to Lys, thereby causing a reduction in the levels of free IAA compared with the wild type (C. Romano, M. Ruegger, P.J. Jensen, G. Sandberg, and M.A. Estelle, unpublished data). The phenotype of these transgenic plants is consistent with a reduction in free IAA, including a reduction in overall plant size, leaf size, apical dominance, and fertility. Tobacco plants containing the same transgene have 5 times less free IAA (Romano et al., 1991).
Table I.
Effect of 1.0 μm NPA on hypocotyl length ± sd of various hormone mutants grown for 7 d in darkness or white light (80 μmol m−2 s−1)
| Genotype | Light
|
Dark
|
||||
|---|---|---|---|---|---|---|
| −NPA | +NPA | Inhibition | −NPA | +NPA | Inhibition | |
| mm | % | mm | % | |||
| Wild type | 4.1 ± 1.1 | 1.1 ± 0.2 | 74 | 13.9 ± 1.8 | 14.1 ± 2.3 | −1 |
| iaaL | 2.0 ± 0.6 | 1.1 ± 0.3 | 45 | 16.9 ± 2.3 | 14.7 ± 2.1 | 13 |
| axr1-3 | 1.7 ± 0.3 | 1.0 ± 0.1 | 37 | 15.6 ± 2.0 | 15.7 ± 2.1 | −1 |
| axr1-12 | 1.4 ± 0.3 | 1.1 ± 0.2 | 22 | 7.7 ± 2.1 | 3.4 ± 1.2 | 56 |
| det2 | 0.8 ± 0.2 | 0.5 ± 0.1 | 40 | 2.9 ± 0.9 | 2.9 ± 0.6 | −3 |
| eto1-1 | 4.3 ± 1.0 | 1.3 ± 0.3 | 70 | 7.4 ± 1.3 | 5.7 ± 1.2 | 22 |
| etr1 | 4.4 ± 0.8 | 1.3 ± 0.3 | 72 | 19.0 ± 2.5 | 14.7 ± 3.2 | 23 |
In white light (50 μmol m−2 s−1) the 35S-iaaL, axr1-12, and axr1-3 plants were less sensitive to NPA than the wild type, showing 45, 22, and 37% inhibition of hypocoytl elongation, respectively. The response of det2 was similar to that of 35S-iaaL and axr1-3 plants. When grown in darkness there was only minor inhibition of hypocotyl elongation in NPA-treated plants, with the exception of axr1-12, which was inhibited by 56%. In white light both eto1-1 and etr1 had NPA-induced hypocotyl inhibition responses similar to the wild type, but in darkness both of these mutants showed slight inhibition. Wild-type roots were inhibited about 20% by NPA in light-grown seedlings but there was no inhibition in darkness. The roots of all of the hormone mutants responded similarly to the wild type (data not shown), except eto1-1, which was inhibited by 42 and 32% in the light and dark, respectively.
Lateral Root Inhibition
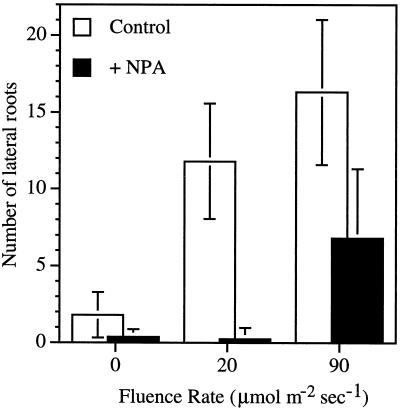
The effect of NPA on lateral root formation was tested under three different light conditions. In untreated plants lateral root production increased with increasing fluence rates (Fig. 7). At a concentration of 0.5 μm, NPA almost abolished lateral root formation in dark and in low-light conditions (Fig. 7). However, plants grown at higher fluence rates were able to overcome partially the inhibitory affect of NPA on lateral root formation.
Figure 7.
Inhibition of lateral root formation by 0.5 μm NPA at various fluence rates. Values are the means ± sd from three independent replicates, with at least 10 seedlings per replicate.
DISCUSSION
Light Quantity and the Response to NPA
Plant growth and development are controlled by the coordinated activities of numerous external and endogenous factors. Light is one of the most important external factors controlling plant development, and plant hormones are among the major endogenous regulators. Among the plant hormones, auxin has important roles in developmental processes including cell division, cell elongation, apical dominance, vascular development, and tropisms. Moreover, the function of auxin is strongly dependent on its controlled transport.
The NPA dose-response curves for seedlings grown in light and dark conditions show that there was a light-dependent difference in response to NPA. When grown in white light, 50% inhibition of hypocotyl elongation was obtained with only 0.5 μm NPA. In dark-grown seedlings there was no NPA-induced inhibition of elongation up to 5.0 μm NPA, above which there was only slight inhibition. Others have also noted a lack of response to NPA in dark-grown plants. For example, when a ring of lanolin containing NPA was applied just below the hook of dark-grown sunflower and mallow seedlings, there was no effect on hypocotyl elongation during a 5-h experimental period (Tamimi and Firn, 1985). It was suggested that in the short term, elongation was not auxin limited. In their characterization of the rcn1 Arabidopsis mutant, Garbers et al. (1996) reported no inhibition of elongation in dark-grown wild type (ecotype Wassilewskija) until a concentration of 100 μm NPA was reached. Based on our findings we conclude that light is necessary for NPA to inhibit hypocotyl elongation in Arabidopsis. It is unclear at this time whether this NPA response is directly mediated by light or is a consequence of photomorphogenesis.
Light Quality and the Response to NPA
Our experiments with different colors of light and different photoreceptor mutants indicate that PHYA, PHYB, and CRY1 are involved in the light-dependent NPA effect on hypocotyl elongation (Figs. 4–6). For example, under far-red light, NPA did not cause hypocotyl inhibition in a phyA mutant, whereas hy4, phyB, and the wild type strongly responded to NPA. Similarly, NPA did not cause hypocotyl inhibition in the phyB mutant grown under red light and the hy4 mutant grown under blue light, whereas the wild type and other photoreceptor mutants were affected by NPA under red and blue light. Therefore, it seems that multiple photoreceptors are able to induce the changes that result in the light-dependent inhibition of hypocotyl elongation caused by NPA. It appears that the response to blue light is dependent on both CRY1 and phytochrome, because the hy4 mutant and the phyA/B double mutant are not responsive to NPA in blue light. Indeed, the phyA/B double mutant behaves in many respects like the CRY1-deficient hy4 mutant.
This apparent co-action of phytochrome and cryptochrome has recently been described for blue-light-dependent inhibition of hypocotyl elongation and anthocyanin production (Ahmad and Cashmore, 1997). Thus, our data indicate that the light-induced inhibitory effect of NPA on hypocotyl elongation is controlled by downstream events that are shared among the different photoreceptor signal transduction systems. Moreover, the data indicate that the NPA response described here reflects a change in auxin physiology that is a fundamental part of photomorphogenesis. Others have also reported light-dependent changes in auxin physiology, including changes in auxin levels (Iino, 1982; Jones et al., 1991; Behringer and Davies, 1992) and in the amount of auxin-binding protein (Walton and Ray, 1981; Jones et al., 1991). In addition, an examination of tobacco phytochrome mutants showed that they have higher levels of free IAA than the wild type (Kraepiel et al., 1995).
Role of Auxin and Auxin Transport in Light- versus Dark-Mediated Elongation
Because NPA is believed to act by inhibiting auxin transport, our results indicate that basipetal auxin transport is not important for hypocotyl elongation in dark-grown seedlings. This implies either that auxin is synthesized in elongating tissue and does not need to be transported, or that auxin is not important for elongation during skotomorphogenesis. The phenotypes of the transgenic auxin overproducers and underproducers are more consistent with the latter possibility. The 35S-iaaL plants have decreased auxin levels, and the 19S-iaaM plants have 4 times greater free IAA than the wild type, yet all three genotypes have hypocotyls of similar length when grown in the dark (Table I) (Romano et al., 1995). In contrast, when grown in the light, hypocotyls of axr1-12 and axr1-3 mutants and 35S-iaaL seedlings are shorter than those of the wild type (Table I). Light-grown seedlings of axr1-3 and 35S-iaaL also have a reduced sensitivity to NPA, most likely because of their respective auxin defects. In addition, light-grown seedlings of the 19S-iaaM auxin overproducer have longer hypocotyls (Romano et al., 1995). These results suggest that auxin is not as important during dark elongation as was previously believed, and highlights its importance in light-grown plants, at least in Arabidopsis. It is not possible from currently available data to determine if the light-dependent effects of NPA are caused by changes in auxin levels, auxin sensitivity, or both.
Light increases root production. Auxin from the shoot is a strong inducer of lateral root formation (Wightman and Thimann, 1980), as is exogenous auxin. Because auxin is thought to be produced in young, expanding leaves and light induces leaf growth, light should result in an increased supply of auxin to roots. This is consistent with the observation that increased fluence rates were able to restore partially the lateral root formation in NPA-treated seedlings (Fig. 7), and a reduction in IAA sensitivity causes a decrease in lateral root formation (Hobbie and Estelle, 1995).
Although hypocotyl elongation was not inhibited by NPA in dark-grown plants, the gravity response was still blocked (Fig. 3A). Thus, NPA was still active under these experimental conditions. Gravitropism is generally thought to involve lateral movement of auxin across an organ, whereas stem elongation is considered to be regulated by the transport of auxin from the sites of production in the shoot apex. Our results are consistent with the idea that there are two distinct transport systems, as was previously suggested by Firn and Tamimi (1986). According to this model, the cells involved in lateral IAA movement are distinct from the cells involved in basipetal transport. Thus, even under conditions in which NPA-induced inhibition of hypocotyl elongation varies dramatically, such as light versus dark growth, the effect of NPA on the gravity response is relatively constant.
Several recent studies demonstrated that BRs are necessary for elongation of hypocotyls during skotomorphogenesis. Three Arabidopsis mutants have been identified with defects in BR biosynthesis (Kauschmann et al., 1996; Li et al., 1996; Szerkes et al., 1996), as well as one that is insensitive to BRs (Clouse et al., 1996). All of these mutants have short hypocotyls when grown in darkness. A variety of experiments with these mutants show that BRs play a major role in hypocotyl elongation in the light and dark. Although the hypocotyls of the det2 mutant, which is defective in BR biosynthesis, were very short, the addition of NPA caused further length reduction in light-grown plants (Table I). However, the percentage inhibition was less than that observed in wild type.
As with the other mutants tested, NPA had no effect on elongation in dark-grown det2 plants. In a variety of bioassay systems, BR and IAA were found to interact synergistically (Arteca, 1995). The effects of NPA in the det2 mutant, the auxin mutants, and the transgenic plants suggest that the roles of auxin and BR in hypocotyl elongation function independently, at least to some extent. In addition to BR-auxin interactions, a role for ethylene in regulating auxin transport has been proposed (Suttle, 1988). Our results on the NPA response in etr1 and eto1-1, the ethylene-resistant and the ethylene-overproducing mutants, respectively, failed to show any interaction in light-grown plants.
Our findings show that basipetal auxin transport is important in hypocotyl elongation during light-dependent but not dark-dependent development. This is consistent with the recent demonstration that hypocotyl elongation in light-grown Arabidopsis seedlings proceeds by a different developmental program than in seedlings grown in darkness (Gendreau et al., 1997). Although our results indicate that basipetal auxin transport does not play a significant role in regulating hypocotyl elongation during etiolated development, our results are consistent with auxin transport as being important in the gravity response of dark-grown seedlings. At this time it is unclear if the difference in the light-dependent response to NPA is the result of light-induced changes in auxin levels, changes in auxin sensitivity, or both.
Hypocotyl elongation is a complex developmental process regulated by many factors. The findings presented here, along with those from other studies of the roles of auxin, GAs, ethylene, BRs, and various photoreceptors in hypocotyl elongation, attest to this fact. It is evident that the actions of the various plant hormones are part of a complex set of overlapping processes that are ultimately regulated through the activity of multiple photoreceptors and other environmental sensory systems.
Abbreviations:
- BR
brassinosteroid
- NPA
1-naphthylphthalamic acid
Footnotes
This work was supported by National Science Foundation grants to M.E. (no. IBN-9604398) and R.P.H. (no. IBN-9596186).
LITERATURE CITED
- Ahmad M, Cashmore AR. Plant J. 1997;11:421–427. doi: 10.1046/j.1365-313x.1997.11030421.x. [DOI] [PubMed] [Google Scholar]
- Arteca RN. Brassinosteroids. In: Davies PJ, editor. Plant Hormones. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 206–213. [Google Scholar]
- Bandurski RS, Schulze A, Cohen JD. Photoregulation of the ratio of ester to free indole-3-acetic acid. Biochem Biophys Res Commun. 1977;79:1219–1223. doi: 10.1016/0006-291x(77)91136-6. [DOI] [PubMed] [Google Scholar]
- Behringer FJ, Davies PJ. Indole-3-acetic acid levels after phytochrome-mediated changes in the stem elongation rate of dark- and light-grown Pisum seedlings. Planta. 1992;188:85–92. doi: 10.1007/BF00198943. [DOI] [PubMed] [Google Scholar]
- Bernasconi P, Patel BC, Regan JD, Subramanian MV. The N-1-naphthylphthalamic acid-binding protein is an integral membrane protein. Plant Physiol. 1996;111:427–432. doi: 10.1104/pp.111.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouse SD, Langford M, McMorris TC. A brassinosteroid mutant in Arabidopsis thaliana exhibits multiple defects in growth and development. Plant Physiol. 1996;111:671–678. doi: 10.1104/pp.111.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firn RD, Tamimi S. Auxin transport and shoot tropisms: the need for precise models. In: Bopp M, editor. Plant Growth Substances 1985. Berlin: Springer-Verlag; 1986. pp. 236–240. [Google Scholar]
- Foster KR, Morgan PW. Genetic regulation of development in Sorghum bicolor. IX. The ma3R allele disrupts diurnal control of gibberellin biosynthesis. Plant Physiol. 1995;108:337–343. doi: 10.1104/pp.108.1.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbers C, DeLong A, Deruére J, Bernasconi P, Soll D. A mutation in protein phosphatase 2A regulatory subunit A affects auxin transport in Arabidopsis. EMBO J. 1996;15:2115–2124. [PMC free article] [PubMed] [Google Scholar]
- Gendreau E, Traas J, Desnos T, Grandjean O, Cabache M, Hofte H. Plant Physiol. 1997;114:295–305. doi: 10.1104/pp.114.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie L, Estelle M. The axr4 auxin resistant mutants of Arabidopsis thaliana define a gene important for root gravitropism and lateral root initiation. Plant J. 1995;7:211–220. doi: 10.1046/j.1365-313x.1995.7020211.x. [DOI] [PubMed] [Google Scholar]
- Iino M. Inhibitory action of red light on growth of the maize mesocotyl: evaluation of the auxin hypothesis. Planta. 1982;156:388–395. doi: 10.1007/BF00393308. [DOI] [PubMed] [Google Scholar]
- Jacobs M, Rubery PH. Naturally occurring auxin transport regulators. Science. 1988;241:346–349. doi: 10.1126/science.241.4863.346. [DOI] [PubMed] [Google Scholar]
- Jones AM, Cochran DS, Lamerson PM, Evans ML, Cohen JD. Red light-regulated growth. I. Changes in the abundance of indoleacetic acid and a 22-kilodalton auxin-binding protein in the maize mesocotyl. Plant Physiol. 1991;97:352–358. doi: 10.1104/pp.97.1.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katekar GF, Giesler AE. Auxin transport inhibitors. IV. Evidence of a common mode of action for a proposed class of auxin transport inhibitors, the phytotropins. Plant Physiol. 1980;66:1190–1195. doi: 10.1104/pp.66.6.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathiresan A, Reid DM, Chinnappa CC. Light- and temperature-entrained circadian regulation of activity and mRNA accumulation of 1-aminocyclopropane-1-carboxylic acid oxidase in Stellaria longipes. Planta. 1996;199:329–335. doi: 10.1007/BF00195723. [DOI] [PubMed] [Google Scholar]
- Kauschmann A, Jessop A, Koncz C, Szerkes M, Willmitzer L, Altmann T. Genetic evidence for an essential role of brassinosteroids in plant development. Plant J. 1996;9:710–713. [Google Scholar]
- Kraepiel Y, Marrec K, Sotta B, Caboche M, Miginiac E. In vitro morphogenic characteristics of phytochrome mutants in Nicotiana plumbaginifolia are modified and correlated to high indole-3-acetic acid levels. Planta. 1995;197:142–146. [Google Scholar]
- Lehman A, Black R, Ecker JR. HOOKLESS1, an ethylene response gene, is required for differential cell elongation in the Arabidopsis hypocotyl. Cell. 1996;85:183–194. doi: 10.1016/s0092-8674(00)81095-8. [DOI] [PubMed] [Google Scholar]
- Lembi CA, Morré DJ, St.-Thomson K, Hertel R. N-1-Naphthylphthalamic-acid-binding activity of a plasma membrane rich fraction from maize coleoptiles. Planta. 1971;99:37–45. doi: 10.1007/BF00392118. [DOI] [PubMed] [Google Scholar]
- Li J, Nagpal P, Vitart V, McMorris TC, Chory J. A role for brassinosteroids in light-dependent development of Arabidopsis. Science. 1996;272:398–401. doi: 10.1126/science.272.5260.398. [DOI] [PubMed] [Google Scholar]
- Lincoln C, Britton JH, Estelle M. Growth and development of the axr1 mutants of Arabidopsis. Plant Cell. 1990;2:1071–1080. doi: 10.1105/tpc.2.11.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax TL, Muday GK, Rubery PH. Auxin transport. In: Davies PJ, editor. Plant Hormones. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 509–530. [Google Scholar]
- Morgan DG. Influence of α-naphthylphthalamic acid on the movement of indolyl-3-acetic acid in plants. Nature. 1964;201:476–477. doi: 10.1038/201476a0. [DOI] [PubMed] [Google Scholar]
- Muday GK, Brunn SA, Haworth P, Subramanian M. Evidence for a single naphthylphthalamic acid binding site on the zucchini plasma membrane. Plant Physiol. 1993;103:449–456. doi: 10.1104/pp.103.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muday GK, Haworth P. Tomato root growth, gravitropism, and lateral development: correlation with auxin transport. Plant Physiol Biochem. 1994;32:193–203. [PubMed] [Google Scholar]
- Peng J, Harberd NP. Gibberellin deficiency and response mutations suppress the stem elongation phenotype of phytochrome-deficient mutants of Arabidopsis. Plant Physiol. 1997;113:1051–1058. doi: 10.1104/pp.113.4.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qamuruddin M, Tillberg E. Rapid effects of red light on the isopentenyladenosine content in Scots pine seeds. Plant Physiol. 1989;91:5–8. doi: 10.1104/pp.91.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano C, Hein M, Klee H. Inactivation of auxin in tobacco transformed with the indoleacetic acid-lysine synthetase gene of Pseudomonas savastonoi. Genes Dev. 1991;5:438–446. doi: 10.1101/gad.5.3.438. [DOI] [PubMed] [Google Scholar]
- Romano CP, Robson PRH, Smith H, Estelle M, Klee H. Transgene-mediated auxin overproduction in Arabidopsis: hypocotyl elongation phenotype and interactions with the hy6-1 hypocotyl elongation and axr1 auxin resistant mutants. Plant Mol Biol. 1995;27:1071–1083. doi: 10.1007/BF00020881. [DOI] [PubMed] [Google Scholar]
- Ross JJ, Willis CL, Gaskin P, Reid JB. Shoot elongation in Lathyrus elongatus L.: gibberellin levels in light and dark-grown tall and dwarf seedlings. Planta. 1992;187:10–13. doi: 10.1007/BF00201618. [DOI] [PubMed] [Google Scholar]
- Ruegger M, Dewey E, Hobbie L, Brown D, Bernasconi P, Turner J, Muday G, Estelle M. Reduced naphthylphthalamic acid binding in the tir3 mutant of Arabidopsis is associated with a reduction in polar auxin transport and diverse morphological defects. Plant Cell. 1997;9:745–757. doi: 10.1105/tpc.9.5.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabater M, Rubery PH. Auxin carriers in Cucurbita vesicles. Planta. 1987;171:507–513. doi: 10.1007/BF00392299. [DOI] [PubMed] [Google Scholar]
- Sánchez-Bravo J, Ortuño AM, Botía JM, Acosta M, Sabater F. The decrease in auxin polar transport down the lupin hypocotyl could produce the indole-3-acetic acid distribution response responsible for the elongation growth pattern. Plant Physiol. 1992;99:108–114. doi: 10.1104/pp.100.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senger H, Schmidt W. Diversity of photoreceptors. In: Kendrick RE, Kronenberg GHM, editors. Photomorphogenesis in Plants, Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 301–322. [Google Scholar]
- Suttle JC. Effect of ethylene treatment on polar IAA transport, net IAA uptake and specific binding of N-1-naphthyl-phthalamic acid in tissues and microsomes isolated from etiolated pea epicotyls. Plant Physiol. 1988;88:795–799. doi: 10.1104/pp.88.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szerkes M, Nemeth K, Koncz-Kálmán Z, Mathur J, Kauschmann A, Altmann T, Rédei GP, Nagy F, Schell H, Koncz C. Brassinosteroids rescue the deficiency of CYP90, a cytochrome P450, controlling cell elongation and de-etiolation in Arabidopsis. Cell. 1996;85:171–182. doi: 10.1016/s0092-8674(00)81094-6. [DOI] [PubMed] [Google Scholar]
- Tamimi S, Firn RD. The basipetal auxin transport system and the control of cell elongation in hypocotyls. J Exp Bot. 1985;36:955–962. [Google Scholar]
- von Arnim A, Deng X-W. Light control of seedling development. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:215–243. doi: 10.1146/annurev.arplant.47.1.215. [DOI] [PubMed] [Google Scholar]
- Walton JD, Ray PM. Evidence for receptor function of auxin binding sites in maize. Red light inhibition of mesocotyl elongation and auxin binding. Plant Physiol. 1981;68:1334–1338. doi: 10.1104/pp.68.6.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherwax SC, Ong MS, Degenhardt J, Bray EA, Tobin EM. The interaction of light and abscisic acid in the regulation of plant gene expression. Plant Physiol. 1996;111:363–370. doi: 10.1104/pp.111.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Went F, Thimann K (1937) Phytohormones. MacMillan, New York
- Wightman F, Thimann KV. Hormonal factors controlling the initiation and development of lateral roots. I. Sources of primordia-inducing substances in the primary root of pea seedlings. Physiol Plant. 1980;49:13–20. [Google Scholar]