Elevated CO2 and temperature are altering the interactions between plants and insects with important implications for food security and natural ecosystems. Ecologically, the acceleration of plant phenology by warming is generating mismatches between plants and insect pollinators. Similarly, shifting the rate of plant development relative to insect development can amplify or minimize the consequences of herbivory. Warming also enables some insects to increase the number of generations per year, thus increasing damage to plant communities. The suitability of plant tissues as food for insects also is modulated by global change. Elevated CO2 typically increases the concentration of leaf carbohydrates and in combination with elevated temperature decreases nitrogen (N) content. Together, these changes lower nutritional value, causing certain herbivores to consume more foliage to meet their nutritional needs. Whereas the responses of primary metabolites in plants to global change are reasonably well understood, how elevated CO2 and temperature affect plant defensive compounds (allelochemicals) is considerably less predictable. Recent studies indicate that exposure to elevated CO2 suppresses the plant defense hormone jasmonic acid (JA) while stimulating production of salicylic acid (SA). By differentially affecting defense compounds, these changes in plant hormones potentially increase susceptibility to chewing insects and enhance resistance to pathogens. Exposure to elevated temperature, in contrast, stimulates JA, ethylene (ET), and SA, enhancing defenses. A deeper understanding of how elevated CO2 and temperature, singly and in combination, modulate plant hormones promises to increase our understanding of how these elements of global change will affect the positive and negative interactions between plants and insects.
Chemoautotrophs notwithstanding, plants provide energy in the form of carbohydrates for all nonphotosynthetic organisms, including insects. Not long after the colonization of land by plants 510 million years ago, plants and insects have engaged in an evolutionary arms race that continues today—plants evolve mechanisms to minimize consumption by insects, and insects evolve mechanisms to circumvent these defenses. Rapid changes in Earth’s atmosphere initiated by the human use of fossil fuels is resetting this complex coevolutionary relationship, not only between plants and herbivores but also between plants and their mutualistic partners, including pollinators. Insects have the potential to cause enormous reductions in crop yields and the productivity of natural ecosystems, as well as to provide irreplaceable pollination services that underpin much of the world’s agriculture.
The combustion of fossil fuels during the Industrial Revolution initiated a rapid rise in atmospheric CO2 concentration that is accelerating today; preindustrial levels were approximately 280 μL L−1 and below 300 μL L−1 for the previous 20 million years (Pearson and Palmer, 2000). Today’s atmosphere is approximately 397 μL L−1, and at current rates of fossil fuel combustion, the atmospheric CO2 concentration will double relative to the preindustrial level in the latter half of the 21st century (IPCC, 2007). In addition to directly affecting plant physiology, this increase in CO2, a potent greenhouse gas, is causing an unprecedented rate of planetary warming. Global average temperatures already have increased by 0.8°C, and the last two decades of the 20th century were the warmest in at least four centuries, and possibly in several millennia. At current rates of fossil fuel use, global mean temperatures will rise by 4°C by the end of the 21st century.
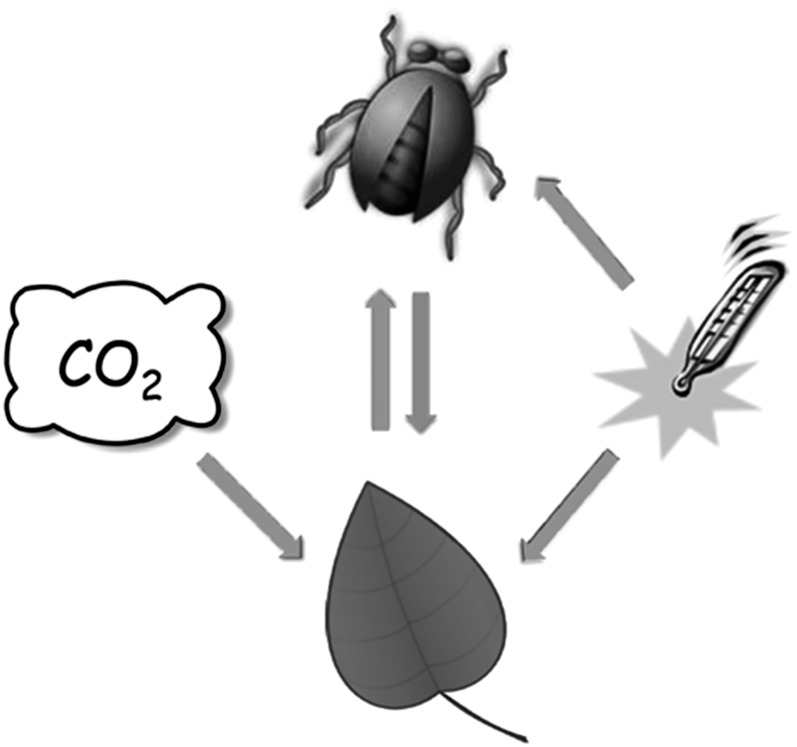
Because of the complex relationships between plants and insects, both favorable and detrimental from the plant’s perspective, how this “one-two punch” of elevated CO2 and elevated temperature will affect plant-insect interactions is difficult to anticipate. Whereas most herbivorous insects do not respond directly to changes in CO2 in the atmosphere (Fig. 1; Guerenstein and Hildebrand, 2008), as exotherms they can be very sensitive to temperature, which affects their life cycle, population size, and geographic distribution (Bale et al., 2002). Populations of many insect herbivores are strongly regulated by invertebrate predators (top down), which also respond to temperature. In this update, we take the “plant’s perspective” and focus on our current understanding and major unresolved questions about how these two major elements of global change, elevated CO2 and temperature, affect insect populations and their relationship with plants. We will elaborate on the concept of ecological mismatches, where global change disrupts the synchrony between plants and insects. Then we will consider how global change affects plants as food for insects, including their chemical defenses, and how changes in leaf chemistry modulate herbivory. We conclude with a discussion of new findings that may reveal the mechanisms governing the interactions between plants, insects, and global change.
Figure 1.
Elevated atmospheric CO2 affects plant-insect interactions through its direct effects on plant metabolism and elevated temperature through its direct effect on plant and insect metabolism. Some insects respond directly to elevated CO2 during oviposition, when foraging for live or dead plant material belowground, or when foraging for nectar, for example (Goyret et al., 2008). At current and projected future levels of atmospheric CO2, the primary effect of elevated CO2 on the suitability of plant tissues as food is mediated largely through changes in plant chemistry and morphology.
ECOLOGICAL MISMATCHES, FOR BETTER OR WORSE
The activities of plants and insects often are coordinated in time—pollinators arrive just when flowers are ready, or feeding larvae enter a resting state (diapause) just as their host plant senesces. The synchrony between plants and insects is mutually constrained by their ecological and evolutionary relationships, yet rising atmospheric CO2 and global temperature challenge the status quo by altering leaf chemistry and advancing phenology. This perturbation increases the selection pressure for asynchrony and, as a result, an increasing number of plant and insect interactions have been characterized as ecologically “mismatched” (Wolkovich et al., 2012).
The rapid advancement of plant phenology by global change brings into question how pollinators and herbivores will keep pace. From the plant’s perspective, rising CO2 increases carbohydrate levels in nectar (Erhardt et al., 2005) and drives greater reproductive investment in many plant species (Jablonski et al., 2002). Sweeter nectar favors some pollinators, such as certain bees and flies (Petanidou, 2005), but not all pollinators (Hoover et al., 2012). The interaction between elevated CO2 and temperature further complicates our understanding of plant interactions with pollinators. For example, elevated CO2 accelerates flowering time for entomophilic C3 species (Springer and Ward, 2007), yet rising temperatures may reduce nectar production by limiting water or by reducing flower longevity (Hoover et al., 2012; Wolkovich et al., 2012). It is important to note that the interactive effects of rising CO2 and temperature as manifested through pollinators remain largely unexplored (but see Hoover et al., 2012). It is likely that pollinator mismatches may be less common among generalists given the long history of adaptation to natural perturbations in climate (e.g. glaciation), but of greater importance for specialist plant-pollinator interactions. Comparisons of historical collections with observations of flight across the last decade indicate many generalist bees avoid mismatches by keeping pace with climate-induced advances in plant phenology (Bartomeus et al., 2011).
For herbivorous insects, mismatches may be more prevalent as traditional host plant resources decrease in availability and nutritional suitability. For example, the comma butterfly (Polygonia c-album) altered its host preference from Humulus lupulus to Ulmus glabra and Urtica dioica, with a corresponding increase in insect performance (Braschler and Hill, 2007). Polyphagous species or those that undergo recent host switching or inclusion events (see also the brown argus butterfly [Aricia agestis]; Pateman et al., 2012) are not constrained by host range and thus are able to expand within their temperature optima faster than the plants on which they feed. This polyphagy may enable insects to track changing climate and move onto new plant resources. How plant resources and defense chemistries have changed, however, remains to be investigated and may resolve mechanisms underlying the host switch. Taken together, these patterns indicate natural selection may go a long way in resolving current mismatches.
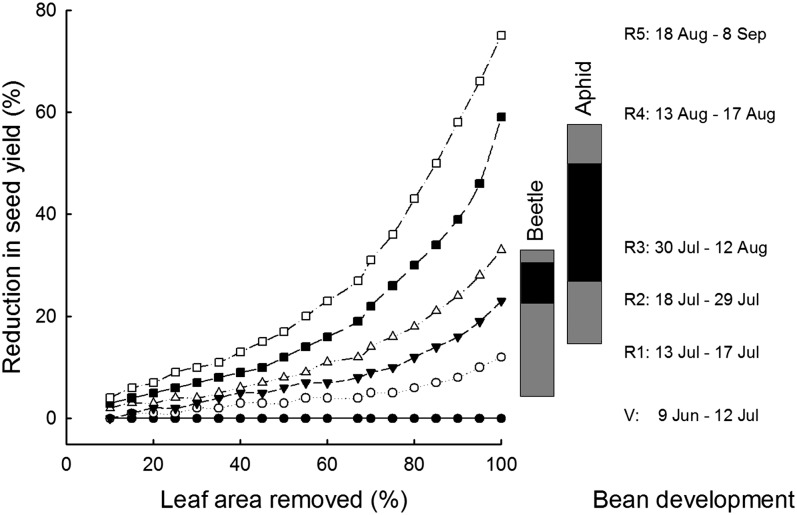
Global change not only modulates the beneficial relationship between plants and their pollinators, it also alters the magnitude of damage caused by herbivores. The largest ecosystem across the United States, the soybean (Glycine max)-corn (Zea mays) rotation, suffers substantial herbivory from the invasive Japanese beetle (Popillia japonica) on soybean. Because soybean is tolerant to herbivory during vegetative development when beetles emerge, damage has little effect on yield (Fig. 2). Earlier emergence of Japanese beetles caused by progressively warmer winters and spring should further reduce the potential for defoliation-induced yield losses by Japanese beetles (Fig. 2). The invasive soybean aphid (Aphis glycines) reduces soybean yield at moderate densities, in part because of its prevalence during reproductive stages when soybean is more vulnerable to herbivory (Fig. 2). As with beetles, if warming causes aphids to move from their alternate host, buckthorn (Rhamnus cathartica) trees, to soybeans earlier when beans are more tolerant of herbivore damage, then warming may reduce potential yield reductions and management costs.
Figure 2.
The consequences of insect damage depend on its timing relative to plant development. In soybean, the sensitivity of seed yield to leaf area removal by herbivory varies strongly among developmental stages (V, vegetative; R1, beginning bloom; R2, full bloom; R3, beginning pod; R4, full pod; R5, beginning seed; from National Crop Insurance Service publication 6302), where a 60% reduction in leaf area during R5 results in greater than 20% yield reduction, but no detectable yield reduction if the same leaf area removal occurs during vegetative growth. The dates (right) for different developmental stages are for soybean in the SoyFACE experiment (Castro et al., 2009). Vertical bars represent the time when Japanese beetles and soybean aphids are abundant (97% of the total annual population), and the black bars represent times of peak abundance (beetles in central Illinois: 26% of total population; Van Timmerman et al., 2000; aphids in southern Wisconsin: approximately 60% of total population; http://www.plantpath.wisc.edu/soyhealth/aglycine.htm). Shifting the timing of insect attack relative to plant development will alter its effect on seed yield.
MULTIPLE INSECT GENERATIONS, MORE HERBIVORY
Herbivorous insect species typically respond to increasing temperatures with an increase in development rates, reproductive potential, overwintering survival, and number of generations within a season (Fig. 1; Ayres and Lombardero, 2000). All of these responses are likely to increase the magnitude of tissue loss, with attendant impacts on plant physiology. Of these responses, global warming has demonstrable effects on herbivore voltinism, the number of life cycles that can be completed in a single season. In many cases, insect life histories are determined facultatively rather than genetically programmed (Solbreck and Sillen-Tullberg, 1981). In temperate-zone species, the initiation of diapause is cued by photoperiod, which, unlike temperature, is invariant and unaffected by human activities. As warm temperatures advance seasonally, insect development can be accelerated such that the sensitive stage (which perceives the cue that triggers diapause initiation) is not exposed to the critical photoperiod, allowing continuous development. Until recently, examples were rare of ostensibly univoltine insects undergoing an additional generation within a season due to unseasonably warm temperatures. Steady increases in global temperatures, however, have increased the frequency of reports of fluctuations in voltinism patterns. Altermatt (2010) examined over 150 years of records of lepidopteran voltinism patterns in central Europe and found that a substantial proportion of the 263 multivoltine species in their dataset exhibited an increased frequency of second and subsequent generations since 1980, with 44 species displaying a stable increase in the number of generations after 1980.
From the plant perspective, multiple generations of herbivores, particularly on long-lived plants, can inflict unprecedented levels of damage over the course of a single season. Such damage may increase the likelihood of mortality and influence subsequent growth and reproductive success. The outbreak of spruce web-spinning sawfly (Cephalcia arvensis) in the Southern Alps between 1985 and 1992 resulted in the death of trees covering hundreds of hectares (Battisti, 2008). Moreover, on a regional scale, warmer temperatures are associated with increased species diversity; in Finland, for every 1°C increase in summer temperature, the species richness of Macrolepidoptera increases in a north-to-south direction by 93 species (Virtanen and Neuvonen 1999).
Long-lived plants are particularly vulnerable to effects of repeated defoliation. Plants have multiple mechanisms for compensating for one-time loss of leaf area; reserves from storage organs can be mobilized and directed to production of replacement foliage, resource allocation to reproduction can be reduced, and photosynthesis can be up-regulated relative to respiration (Anten et al., 2003; Lopez-Toledo et al., 2012). Repeated defoliation, however, can compromise these compensatory mechanisms (Canham et al., 1999; Martinez-Ramos et al., 2009). How rapidly long-lived plants can recover from defoliation depends on size, extent of foliage loss, and resource availability. Much of what is known about impacts of recurrent defoliation events derives from studies of plant species that are harvested for economic purposes (Endress et al., 2004). The existing literature suggests that repeated defoliation induces carbon (C) stress and increases vulnerability to pest and pathogen infestation as well as drought (Anderegg and Callaway, 2012). In trembling aspen (Populus tremuloides), multiple defoliations can cause multiple leaf flushes, incurring drawdown of nonstructural carbohydrate reserves. Kosola et al. (2001) examined effects of experimentally manipulated defoliation by gypsy moth caterpillars (Lymantria dispar) on hybrid poplars (Populus spp.). Defoliation reduced C allocation to starch in trunk, twigs, and fine roots; under the most intense defoliation, uptake of ammonium and nitrate was reduced.
Repeated bouts of herbivory as a consequence of herbivore responses to warming temperatures may be particularly damaging to conifers, which do not typically releaf. Moreover, many species of conifers depend on production of resins to fend off herbivores, defenses that can be circumvented by large numbers of attackers (Trapp and Croteau, 2001). Bark beetles (Scolytidae) overcome the resin defenses of their conifer hosts by way of coordinated mass attacks that overwhelm the resin delivery system and increase the likelihood of tree mortality. The mountain pine beetle (Dendroctonus ponderosae), for example, undergoes periodic outbreaks, during which millions of trees can die. In the Colorado Front Range, increasing air temperatures have resulted in an advance of more than a month in the onset of the flight season, and it has been argued that in some places this species can complete a second generation (Mitton and Ferrenberg, 2012). Similarly, the Eurasian spruce bark beetle (Ips typographus) is an outbreak species that can mass attack and kill spruce trees (Picea abies; Jönsson and Bärring, 2011); higher air temperatures are associated with a second generation in Scandinavia and the potential for a third generation in central Europe.
Increases in the frequency and intensity of insect damage to forests can potentially accelerate global change, as large quantities of C stored in wood decompose and are released to the atmosphere (Hicke et al., 2012). In view of temperature-associated developmental acceleration and changes in voltinism, recurrent herbivore attacks are likely to become more frequent in the future. Under these circumstances, characterizing the physiological impacts of repeated attacks on tree physiology should be a high priority.
PLANTS AS FOOD: CO2 AND TEMPERATURE EFFECTS ON LEAF CHEMISTRY
The quality of foliage as a food source for insects depends on the concentration of primary metabolites, particularly N, which often limits the growth of immature insects, carbohydrates that can act as feeding stimulants, and allelochemicals (secondary metabolites) that can deter feeding. Insects consuming foliage produced under elevated CO2 often find this food source less suitable than foliage developed under current ambient levels. Whereas there is considerable variation among insect taxa and between feeding guilds (e.g. chewers versus sap suckers), on average, growth rates when consuming high CO2 foliage are reduced, resulting in lower pupal and adult weights (Robinson et al., 2012). An unambiguous indicator that elevated CO2 reduces insect growth rates by altering the chemical and physical properties of foliage is that insects consume more high-CO2 foliage, an average of 14% more, to maintain lower growth rates. Increased consumption of low-quality food to meet critical nutrient limitations is referred to as “compensatory feeding” and may portend greater herbivore damage to both managed and natural ecosystems as CO2 continues to increase (Cornelissen, 2011).
How elevated CO2 affects plant primary metabolism and some aspects of leaf chemistry is relatively well understood. For plants using the C3 photosynthetic pathway, Rubisco is the first carboxylating enzyme facilitating the assimilation of gaseous CO2 into carbohydrates. This enzyme operates below its maximum velocity at current CO2 levels, so consequently the rate of photosynthesis and biomass accumulation for C3 plants increases strongly with rising CO2 (Long et al., 2004; Ainsworth and Rogers, 2007). This stimulation of photosynthesis drives a number of changes in primary metabolism, affecting the suitability of leaves to herbivorous insects. Carbohydrates, including starch and soluble sugars, typically increase in foliage grown under elevated CO2, and, either from dilution by increased carbohydrates or reallocation, leaf N content decreases (Stiling and Cornelissen, 2007; Robinson et al., 2012). Increased carbohydrates (23%–50%) and reduced protein levels (10%) drive an average 19% increase in plant C:N ratio (Robinson et al., 2012). Because chewing insects in particular are strongly limited by the N content of their diets, they must consume more high-CO2 foliage to meet their nutritional needs.
Physical characteristics of leaves, including surface waxes, secretory canals, and leaf toughness, also respond to elevated CO2 and affect herbivory, but, with the exception of specific leaf area, less is known about these variables. Specific leaf area (SLA), the ratio of leaf area to leaf mass, is a reasonable proxy for leaf toughness. Typically, SLA decreases (toughness increases) under elevated CO2 (Stiling and Cornelissen, 2007; Robinson et al., 2012), directly reducing the suitability of foliage to insects. The increase in structural mass per unit leaf area associated with a decrease in SLA also dilutes leaf N content.
Because they are ectotherms, elevated temperature directly affects insect metabolism (Fig. 1), influencing their life cycles, ecological interactions, and geographic ranges. The direct effect of elevated temperature can be strong (e.g. Niziolek et al., 2012), often stimulating herbivory (Zvereva and Kozlov, 2006), but relatively little is known about how elevated temperature modulates herbivory indirectly through changes in leaf chemistry (Fig. 1).
Elevated temperature can influence suitability to insect herbivores directly by modulating leaf carbohydrate concentration through its effect on photosynthesis and respiration and indirectly through its influence on plant development. However, the net effect of temperature on the concentration of soluble sugars depends on whether photosynthesis is operating below or above its thermal optimum, making it difficult to predict how this aspect of leaf suitability will change under field conditions. In a meta-analysis, exposure to elevated temperature in the range predicted in the next 50 to 100 years decreased leaf soluble sugars, starch, and total nonstructural carbohydrates (Zvereva and Kozlov, 2006). Whereas growth under elevated temperature had no statistically resolvable effect on the mechanical properties of leaves (e.g. toughness and SLA), as is the case with growth under elevated CO2, it also causes a reduction in leaf N content. When administered in combination, elevated temperature negated the increase in leaf carbohydrates caused by elevated CO2, but the simultaneous effect of both elements of global change was to amplify decreases in leaf N, causing substantial increases in leaf C:N ratio (Zvereva and Kozlov, 2006). The direct stimulation of insect metabolism by elevated temperature combined with the synergistic reduction of leaf N by the combination of elevated temperature and CO2 suggests that these elements of global change may dramatically increase herbivory in the future.
The discussion here minimizes the enormous variation in plant and insect responses to global change, and although some of this variation can be explained by the behavior of individual taxa, the mechanisms generating other aspects of variation remain elusive. Elevated CO2 causes smaller changes in leaf chemistry in gymnosperms than angiosperms and little or no change in leaf carbohydrates in plants using C4 photosynthesis, as expected from theory. In addition, the reductions in N are considerably smaller in N-fixing than non-N-fixing species (Robinson et al., 2012). Although elucidating the causes of variation in the response of primary plant metabolites to elevated CO2 and temperature is a reachable goal, a full understanding of how these elements of global change affect allelochemicals and, consequently, the full spectrum of responses of herbivorous insects, remains quite challenging.
The coevolutionary arms race with herbivorous insects has produced a dazzling array of secondary defensive compounds (allelochemicals) in plants. Many of these compounds are continuously present in low levels in plant tissues (constitutive) but rise to high concentrations (induced) in response to herbivory. Predicting how global change will affect the production of allelochemicals and their consequent effects on herbivory remains a challenge that is amplified by the absence of comprehensive theory linking primary and secondary metabolism in plants (Hamilton et al., 2001).
Whereas no consistent trend has been identified in the response of allelochemicals, including glucosinolates, tannins, and terpenoids, to global change, and taxon specificity reigns supreme (Bidart-Bouzat and Imeh-Nathaniel, 2008), some generalizations are emerging. On average, growth under elevated CO2 stimulates the production of phenolics, tannins, and flavonoids and suppresses the production of terpenoids (Stiling and Cornelissen, 2007; Robinson et al., 2012). This differential response suggests that elevated CO2 favors the shikimic acid pathway leading to the production of phenolics and suppresses the mevalonic acid and methylerythritol phosphate pathways leading to the production of terpenoids (Lindroth, 2010).
Little is known about how elevated temperature, singly or in combination with elevated CO2, affects allelochemicals. In members of the mustard family (Brassicaceae), including Arabidopsis (Arabidopsis thaliana), elevated temperature increases the production of isothiocyanates, hydrolysis products of some glucosinolates, but decreases nitriles (Bidart-Bouzat and Imeh-Nathaniel, 2008).
Elevated temperature also increases the production of biogenic volatile compounds (BVOCs; Peñuelas and Staudt, 2010). These volatile compounds emitted by plants, including isoprene, monoterpenes, sesquiterpenes, green leaf volatiles, methyl salicylate, and many others, aid in plant defense, pollination, and communication. The emission of BVOCs announces that a plant is under attack and that a meal awaits any predator answering the call (Arimura et al., 2009). Elevated CO2 may directly suppress the synthesis of some BVOCs by its effect on terpenoid synthesis, but the production of larger plants by elevated CO2 and the direct stimulation of metabolism by elevated temperature will likely increase the production of BVOCs in the future.
Given the highly specific responses between some plants and insects and the complex coevolutionary relationships between them, it is not surprising that modulation of allelochemical production under elevated CO2 and temperature is highly complex and often species specific. Much of our understanding of how elevated CO2 in particular affects the production of allelochemicals is observational, and a deeper understanding of the mechanisms regulating these responses may reveal greater generality. It is important to note that most experimental approaches to studying plant and insect responses to global change have not been done in an evolutionary context and thus do not address how organisms will adapt to rapid increases in CO2 and temperature (Shaw and Etterson, 2012). There is considerable variation within populations in responsiveness indicating that elevated CO2 can be a potent selective force (Ward and Kelly, 2004). Similarly, Plutella xylostella caterpillars reared for multiple generations on a high-carbohydrate diet that mimics foliage grown under elevated CO2 developed the ability eat excess carbohydrates without the fitness costs of excess fat storage (Warbrick-Smith et al., 2006). A complete analysis of the response of plants and insects to global change will need to consider potentially important mechanisms of adaptation.
RESETTING PLANT DEFENSE TO HERBIVORES
The induction of a defense response starts with the perception of insect attack in plant tissues. Mechanical damage and insect oral sections, singly or in combination, initiate the response, but our understanding of the precise mechanisms governing the perception of herbivory is incomplete (Koo and Howe, 2009; Wu and Baldwin, 2010). Insect oral secretions are detected by putative receptors and amplify mitogen-activated protein kinases, which control the main molecules involved in signaling JA, as well as ET and SA, hormones that regulate plant development and chemical defenses (Wu and Baldwin, 2010). Phloem-feeding insects, such as aphids (Aphidoidea) and whiteflies (Aleyrodidae), induce SA-dependent responses, whereas wounding caused by chewing insects is associated with rapid accumulation of JA and ET at the wound site (Wu and Baldwin, 2010). However, chemical responses of damaged plants are an integral component of adaptive antiherbivore defense that abiotic factors, such as high temperature or elevated CO2 environment, disrupt by altering plant signaling, that in turn, affects defensive pathways and the plant-insect interaction balance (Zavala et al., 2008; Niziolek et al., 2012).
New studies are bolstering the case that elevated CO2 alters chemical defenses largely by modifying hormone signaling. In tomato (Solanum lycopersicum), elevated CO2 enhances induced defenses derived from the SA-signaling pathway, such as the pathogenesis-related proteins, and reduces JA-signaling and defenses (Sun et al., 2011; Guo et al., 2012). Moreover, whereas enriched CO2 atmosphere increased the susceptibility of soybean to Japanese beetle and western corn rootworm (Diabrotica virgifera virgifera) by down-regulating JA and ET, elevated CO2 increases SA levels in field-grown soybeans and in leaves and rhizomes in two varieties of ginger (Zingiber officinale; Zavala et al., 2008; Ghasemzadeh et al., 2010; Fig. 3).
Figure 3.

Japanese beetles feeding and mating on soybean grown under elevated CO2. Growth under elevated CO2 increases leaf carbohydrates and reduces the concentration of defensive CystPI activity, which together greatly increase the suitability of foliage for beetles. Leaf damage and the number of Japanese beetles, as well as numbers of other folivorous insects, are greater in plots exposed to elevated compared with ambient CO2 (Hamilton et al., 2005; Dermody et al., 2008). Photo credit: B. O’Neill.
The concomitant effects of elevated CO2 on SA and JA/ET in soybean increased the concentration of SA-regulated phenolics, such as flavonoids with antioxidant properties (quercetin, kaempferol, and fisetin), but decreased the concentration of JA-regulated antiherbivore defenses, such as the isoflavonoid genistein and Cys protease inhibitors (CystPI; Zavala et al., 2008; O’Neill et al., 2010). CystPIs are the principal antiherbivore defenses in soybeans against coleopteran herbivores, and the suppression of CystPIs explains greater herbivory under elevated CO2. In addition, elevated CO2 decreased the JA-regulated triterpenoid cardenolides in four different genotypes of milkweed (Asclepias spp.; Vannette and Hunter, 2011). The types of BVOCs emitted by plants also vary with the production of JA or SA. Elevated CO2 decreased the emission of JA-regulated terpene volatile compounds in cabbage (Brassica oleracea) and reduced the host-searching efficiency of the specialist parasitoid Cotesia plutellae (Vuorinen et al., 2004).
Elevated CO2 is not the only global environmental change affecting BVOC emissions; high temperature also can have an additive effect on BVOC emissions in response to insect attack. The combination of high temperature and simulated herbivory in corn resulted in greater JA-regulated BVOC emission than when each stress was applied separately (Gouinguené and Turlings, 2002). In contrast with elevated CO2, high temperature increases JA/ET and SA (Clarke et al., 2009), which also results in high levels of chemical defenses (e.g. trypsin and CystPIs; Mosolov and Valueva, 2011). High temperatures increased allelochemicals regulated by JA/ET, such as trypsin and CystPIs, in many plant species (Mosolov and Valueva, 2011). In addition, high temperature induces the production of antioxidant flavonoids and the pathogen-related response proteins, both regulated by SA (Djanaguiraman et al., 2011). These chemical responses regulated by phytohormones to high temperature explain the higher resistance of plants to both insects and pathogens when grown in a high-temperature environment (Arimura et al., 2009; Maimbo et al., 2007). However, the less explored interactive effects of elevated CO2 with high temperature on plant allelochemical production suggest that the up-regulation of JA/ET by high temperature would eliminate the susceptibility of plants to herbivore attack produced by elevated CO2 alone (Niziolek et al., 2012).
HORMONES AND GLOBAL CHANGE: PUTATIVE MECHANISMS
Emerging data suggest that exposure to either elevated CO2 or high temperatures modulates hormonal signals and, further, that differential responses of plant hormones explain some of the variation in the observed responses of allelochemicals. Whereas the elicitation of the JA pathway may repress SA defense responses, SA-inducing insects and biotrophic pathogens inhibit JA-dependent defenses (Spoel and Loake, 2011). This antagonism is evident under elevated CO2, which down-regulates JA and ET pathways and increases susceptibility to herbivore attack (Casteel et al., 2008; Zavala et al., 2008; Sun et al., 2011). However, SA accumulates in enriched CO2 atmospheres and induces other chemical defense pathways that are not regulated by JA (Ghasemzadeh et al., 2010; Sun et al., 2011). Moreover, the SA-signaling pathway initiates the synthesis of defense compounds against plant pathogens, and this enhanced resistance correlates with a lower incidence of disease in soybeans under field conditions (Eastburn et al., 2010).
Although the mechanisms by which elevated CO2 alters the hormonal response to herbivory are not known, the regulatory protein NPR1 (for NONEXPRESSOR OF PATHOGENESIS-RELATED GENES1) and its response to the redox state of the cell may play a role. NPR1 mediates the antagonistic relationship between SA and JA (Spoel et al., 2003; Yuan et al., 2007). Growth under elevated CO2 alters redox state by affecting the expression of genes coding for thioredoxins and glutathione-S-transferase (Casteel et al., 2008), as well as ascorbic acid concentration and total antioxidant capacity (Gillespie et al., 2011). Recently, Tada et al. (2008) suggested that NPR1 and subsequently the antagonism between SA/JA are activated by redox changes that lead to reduction of the oxidized disulfide bonds in NPR1, a reaction catalyzed by thioredoxins. It may be useful to examine small-molecule redox couples [e.g. NAD(P)H/NAD(P)+, GSH/GSSG, and reduced and oxidized ascorbate] as control points, coordinating the CO2-induced changes in photosynthesis and subsequent electron transport with changes in defense signaling (Spoel and Loake, 2011).
In contrast with the antagonistic interaction between JA/ET and SA under elevated CO2, elevated temperature stimulates the production of JA, ET, and SA, increasing the production of allelochemicals and plant resistance to pathogens and insect herbivores (Maimbo et al., 2007; Arimura et al., 2009; Clarke et al., 2009). Heat shock proteins (HSPs) may be involved in mediating the relationship between plant defensive chemistry and elevated temperature. Exposure to elevated temperatures triggers the production of HSPs, conferring “basal” thermotolerance in plants by protecting proteins against irreversible heat-induced damage (Hong and Vierling, 2000). In addition to thermotolerance, HSPs interact with SA-induced mitogen-activated protein kinase, which acts as a signaling component during plant defense. Silencing HSP70 and HSP90 in Nicotiana benthamiana compromises hypersensitive response and SA-regulated nonhost resistance (Kanzaki et al., 2003).
The stress hormone ET also increases in response to elevated temperature (Djanaguiraman et al., 2011), and through its positive interaction with JA, it may modulate the plant defense response. The antagonistic effect between JA and SA is mediated by ET (Leon-Reyes et al., 2009), and abiotic or biotic factors that induce ET abolished the NPR1 dependency of the SA-JA signal interaction. Therefore, the low endogenous levels of ET in plants grown in enriched CO2 atmospheres enable the antagonistic cross talk between JA and SA pathways, which increases the susceptibility to insect herbivory, particularly chewing insects, and resistance to pathogens (Zavala et al., 2008; Eastburn et al., 2010; Sun et al., 2011). Alternatively, the up-regulation of ET together with JA and SA in plants grown under high temperatures abolishes the antagonistic effect between SA and JA, inducing defenses against herbivores and pathogens (Maimbo et al., 2007; Arimura et al., 2009; Clarke et al., 2009).
Increasing our understanding of how elevated CO2 and temperature, singly and in combination, affect the complex interactions between JA, ET, and SA will greatly enhance our ability to predict changes in allelochemistry and the relationship between plants and herbivorous insects. Given their centrality in plant defense against biotic attack, it may be worth noting that the receptors for SA (Fu et al., 2012) and JA (Sheard et al., 2010) recently have been identified.
CONCLUSION AND FUTURE RESEARCH
The intimate relationship between plants and insects that in some cases results in devastating crop losses and, in other cases, facilitates agricultural production through pollination is altered by global climate change. Although some of these changes are well understood, others remain elusive. How elevated CO2 and temperature independently affect the primary chemistry of leaves, including leaf carbohydrate contents and leaf N, is relatively well understood, as is how these changes affect the performance of many herbivorous insects. Atmospheric CO2 and temperature, however, are increasing in parallel, and even for primary plant chemistry, our knowledge of the interactive effects of these two elements of global change remains shallow.
Considerably less is know about how these elements of global change affect plant allelochemicals and the subsequent responses of herbivorous insects to these changes. The diverse array of defensive chemicals produced by plants and the differential responses of generalist and specialist herbivores to these chemicals challenge simple predictions of cause and effect. Recent studies of how elevated CO2 and temperature modulate hormonal signaling and subsequently plant defense show great promise in advancing our ability to predict how global change will affect plant-insect interactions.
Understanding how global change will affect the ecological and evolutionary relationships between plants and insects remains challenging as these interactions integrate all behavioral, physiological, and molecular interactions at lower organizational scales. Whereas many or most generalist herbivores may be preadapted via host switching to changes in plant phenology and hence resource availability, many or most specialists may face local extinction due to ecological mismatching. Few studies have examined the interactive effects of elevated CO2 and temperature on plant-insect interactions at any level of organization. Understanding the trajectory of natural ecosystems and ensuring food security will require a deeper understanding of these processes.
Acknowledgments
We thank Scott Woolbright for his thoughtful comments on an earlier draft of this manuscript, and we are deeply indebted to our dear friend Art Zangerl, who passed away recently, for his insights into the world of plants and insects.
Glossary
- N
nitrogen
- C
carbon
- SLA
specific leaf area
- BVOC
biogenic volatile compound
- JA
jasmonic acid
- ET
ethylene
- SA
salicylic acid
- CystPI
Cys protease inhibitors
- HSP
heat shock protein
References
- Ainsworth EA, Rogers A. (2007) The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant Cell Environ 30: 258–270 [DOI] [PubMed] [Google Scholar]
- Altermatt F. (2010) Climatic warming increases voltinism in European butterflies and moths. Proc Biol Sci 277: 1281–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderegg WRL, Callaway ES. (2012) Infestation and hydraulic consequences of induced carbon starvation. Plant Physiol 159: 1866–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anten NPR, Martínez-Ramos M, Ackerly DD. (2003) Defoliation and growth in an understory palm: quantifying the contributions of compensatory responses. Ecology 84: 2905–2918 [Google Scholar]
- Arimura G-I, Matsui K, Takabayashi J. (2009) Chemical and molecular ecology of herbivore-induced plant volatiles: proximate factors and their ultimate functions. Plant Cell Physiol 50: 911–923 [DOI] [PubMed] [Google Scholar]
- Ayres MP, Lombardero MJ. (2000) Assessing the consequences of global change for forest disturbance from herbivores and pathogens. Sci Total Environ 262: 263–286 [DOI] [PubMed] [Google Scholar]
- Bale JS, Masters GJ, Hodkinson ID, Awmack C, Bezemer TM, Brown VK, Butterfield J, Buse A, Coulson JC, Farrar J, et al. (2002) Herbivory in global climate change research: direct effects of rising temperature on insect herbivores. Glob Change Biol 8: 1–16 [Google Scholar]
- Bartomeus I, Ascher JS, Wagner D, Danforth BN, Colla S, Kornbluth S, Winfree R. (2011) Climate-associated phenological advances in bee pollinators and bee-pollinated plants. Proc Natl Acad Sci USA 108: 20645–20649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battisti A. (2008) Forests and climate change—lessons from insects. iForest 1: 1–5 [Google Scholar]
- Braschler B, Hill JK. (2007) Role of larval host plants in the climate-driven range expansion of the butterfly Polygonia c-album. J Anim Ecol 76: 415–423 [DOI] [PubMed] [Google Scholar]
- Bidart-Bouzat MG, Imeh-Nathaniel A. (2008) Global change effects on plant chemical defenses against insect herbivores. J Integr Plant Biol 50: 1339–1354 [DOI] [PubMed] [Google Scholar]
- Canham CD, Kobe RK, Latty EF, Chazdon RL. (1999) Interspecific and intraspecific variation in tree seedling survival: effects of allocation to roots versus carbohydrate reserves. Oecologia 121: 1–11 [DOI] [PubMed] [Google Scholar]
- Casteel CL, O’Neill BF, Zavala JA, Bilgin DD, Berenbaum MR, Delucia EH. (2008) Transcriptional profiling reveals elevated CO2 and elevated O3 alter resistance of soybean (Glycine max) to Japanese beetles (Popillia japonica). Plant Cell Environ 31: 419–434 [DOI] [PubMed] [Google Scholar]
- Castro JC, Dohleman FG, Bernacchi CJ, Long SP. (2009) Elevated CO2 significantly delays reproductive development of soybean under Free-Air Concentration Enrichment (FACE). J Exp Bot 60: 2945–2951 [DOI] [PubMed] [Google Scholar]
- Clarke SM, Cristescu SM, Miersch O, Harren FJM, Wasternack C, Mur LA. (2009) Jasmonates act with salicylic acid to confer basal thermotolerance in Arabidopsis thaliana. New Phytol 182: 175–187 [DOI] [PubMed] [Google Scholar]
- Cornelissen T. (2011) Climate change and its effects on terrestrial insects and herbivory patterns. Neotrop Entomol 40: 155–163 [DOI] [PubMed] [Google Scholar]
- Dermody O, O’Neill BF, Zangerl AR, Berenbaum MR, DeLucia EH. (2008) Effects of elevated CO2 and O3 on leaf damage and insect abundance in a soybean agroecosystem. Arthropod-Plant Interact 2: 125–135 [Google Scholar]
- Djanaguiraman M, Prasad PVV, Al-Khatib K. (2011) Ethylene perception inhibitor 1-MCP decreases oxidative damage of leaves through enhanced antioxidant defense mechanisms in soybean plants grown under high temperature stress. Environ Exp Bot 71: 215–223 [Google Scholar]
- Eastburn DM, Gegennaro MM, DeLucia EH, Dermody O, McElrone AJ. (2010) Elevated atmospheric carbon dioxide and ozone alter soybean diseases at SoyFACE. Glob Change Biol 16: 320–330 [Google Scholar]
- Endress BA, Gorchov DL, Noble RB. (2004) Non-timber forest product extraction: effects of harvest and browsing on an understory palm. Ecol Appl 14: 1139–1153 [Google Scholar]
- Erhardt A, Rusterholz HP, Stöcklin J. (2005) Elevated carbon dioxide increases nectar production in Epilobium angustifolium L. Oecologia 146: 311–317 [DOI] [PubMed] [Google Scholar]
- Fu ZQ, Yan SP, Saleh A, Wang W, Ruble J, Oka N, Mohan R, Spoel SH, Tada Y, Zheng N, et al. (2012) NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants. Nature 486: 228–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemzadeh A, Jaafar HZ, Rahmat A. (2010) Elevated carbon dioxide increases contents of flavonoids and phenolic compounds, and antioxidant activities in Malaysian young ginger (Zingiber officinale Roscoe.) varieties. Molecules 15: 7907–7922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie KM, Rogers A, Ainsworth EA. (2011) Growth at elevated ozone or elevated carbon dioxide concentration alters antioxidant capacity and response to acute oxidative stress in soybean (Glycine max). J Exp Bot 62: 2667–2678 [DOI] [PubMed] [Google Scholar]
- Gouinguené SP, Turlings TCJ. (2002) The effects of abiotic factors on induced volatile emissions in corn plants. Plant Physiol 129: 1296–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyret J, Markwell PM, Raguso RA. (2008) Context- and scale-dependent effects of floral CO2 on nectar foraging by Manduca sexta. Proc Natl Acad Sci USA 105: 4565–4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerenstein PG, Hildebrand JG. (2008) Roles and effects of environmental carbon dioxide in insect life. Annu Rev Entomol 53: 161–178 [DOI] [PubMed] [Google Scholar]
- Guo H, Sun Y, Ren Q, Zhu-Salzman K, Kang L, Wang C, Li C, Ge F. (2012) Elevated CO2 reduces the resistance and tolerance of tomato plants to Helicoverpa armigera by suppressing the JA signaling pathway. PLoS ONE 7: e41426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JG, Dermody OC, Aldea M, Zangerl AR, Rogers A, Berenbaum MR, DeLucia EH. (2005) Anthropogenic changes in tropospheric composition increase susceptibility of soybean to insect herbivory. Environ Entomol 34: 479–485 [Google Scholar]
- Hamilton JG, Zangerl AR, DeLucia EH, Berenbaum MR. (2001) The carbon-nutrient balance hypothesis: its rise and fall. Ecol Lett 4: 86–95 [Google Scholar]
- Hicke JA, Allen CD, Desai AR, Dietze MC, Hall RJ, Hogg EH, Kashian DM, Moore D, Raffa KF, Sturrock RN, et al. (2012) Effects of biotic disturbances on forest carbon cycling in the United States and Canada. Glob Change Biol 18: 7–34 [Google Scholar]
- Hong SW, Vierling E. (2000) Mutants of Arabidopsis thaliana defective in the acquisition of tolerance to high temperature stress. Proc Natl Acad Sci USA 97: 4392–4397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover SER, Ladley JJ, Shchepetkina AA, Tisch M, Gieseg SP, Tylianakis JM. (2012) Warming, CO2, and nitrogen deposition interactively affect a plant-pollinator mutualism. Ecol Lett 15: 227–234 [DOI] [PubMed] [Google Scholar]
- IPCC (2007) Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. In S Solomon, D Qin, M Manning, Z Chen, M Marquis, KB Averyt, M Tignor, HL Miller, eds, Climate Change 2007: The Physical Science Basis. Cambridge University Press, Cambridge, UK
- Jablonski LM, Wang XZ, Curtis PS. (2002) Plant reproduction under elevated CO2 conditions: a meta-analysis of reports on 79 crop and wild species. New Phytol 156: 9–26 [Google Scholar]
- Jönsson AM, Bärring L. (2011) Future climate impact on spruce bark beetle life cycle in relation to uncertainties in regional climate model data ensembles. Tellus 63: 158–173 [Google Scholar]
- Kanzaki H, Saitoh H, Ito A, Fujisawa S, Kamoun S, Katou S, Yoshioka H, Terauchi R. (2003) Cytosolic HSP90 and HSP70 are essential components of INF1-mediated hypersensitive response and non-host resistance to Pseudomonas cichorii in Nicotiana benthamiana. Mol Plant Pathol 4: 383–391 [DOI] [PubMed] [Google Scholar]
- Koo AJK, Howe GA. (2009) The wound hormone jasmonate. Phytochemistry 70: 1571–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosola KR, Dickmann DI, Paul EA, Parry D. (2001) Repeated insect defoliation effects on growth, nitrogen acquisition, carbohydrates, and root demography of poplars. Oecologia 129: 65–74 [DOI] [PubMed] [Google Scholar]
- Leon-Reyes A, Spoel SH, De Lange ES, Abe H, Kobayashi M, Tsuda S, Millenaar FF, Welschen RAM, Ritsema T, Pieterse CMJ. (2009) Ethylene modulates the role of NONEXPRESSOR OF PATHOGENESIS-RELATED GENES1 in cross talk between salicylate and jasmonate signaling. Plant Physiol 149: 1797–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindroth RL. (2010) Impacts of elevated atmospheric CO2 and O3 on forests: phytochemistry, trophic interactions, and ecosystem dynamics. J Chem Ecol 36: 2–21 [DOI] [PubMed] [Google Scholar]
- Long SP, Ainsworth EA, Rogers A, Ort DR. (2004) Rising atmospheric carbon dioxide: plants FACE the future. Annu Rev Plant Biol 55: 591–628 [DOI] [PubMed] [Google Scholar]
- Lopez-Toledo L, Anten NPR, Endress BA, Ackerly DD, Martinez-Ramos M. (2012) Resilience to chronic defoliation in a dioecious understory tropical rain forest palm. J Ecol : 1245–1256100 [Google Scholar]
- Maimbo M, Ohnishi K, Hikichi Y, Yoshioka H, Kiba A. (2007) Induction of a small heat shock protein and its functional roles in Nicotiana plants in the defense response against Ralstonia solanacearum. Plant Physiol 145: 1588–1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Ramos M, Anten NPR, Ackerly DD. (2009) Defoliation and ENSO effects on vital rates of an understory tropical rain forest palm. J Ecol 97: 1050–1061 [Google Scholar]
- Mitton JB, Ferrenberg SM. (2012) Mountain pine beetle develops an unprecedented summer generation in response to climate warming. Am Nat 179: E163–E171 [DOI] [PubMed] [Google Scholar]
- Mosolov VV, Valueva TA. (2011) Inhibitors of proteolytic enzymes under abiotic stresses in plants. Appl Biochem Microbiol 47: 453–459 [PubMed] [Google Scholar]
- Niziolek OK, Berenbaum MR, DeLucia EH. (2012) Impact of elevated CO2 and temperature on Japanese beetle herbivory. Insect Sci (in press) [DOI] [PubMed] [Google Scholar]
- O’Neill BF, Zangerl AR, Dermody O, Bilgin DD, Casteel CL, Zavala JA, DeLucia EH, Berenbaum MR. (2010) Impact of elevated levels of atmospheric CO2 and herbivory on flavonoids of soybean (Glycine max Linnaeus). J Chem Ecol 36: 35–45 [DOI] [PubMed] [Google Scholar]
- Pateman RM, Hill JK, Roy DB, Fox R, Thomas CD. (2012) Temperature-dependent alterations in host use drive rapid range expansion in a butterfly. Science 336: 1028–1030 [DOI] [PubMed] [Google Scholar]
- Pearson PN, Palmer MR. (2000) Atmospheric carbon dioxide concentrations over the past 60 million years. Nature 406: 695–699 [DOI] [PubMed] [Google Scholar]
- Peñuelas J, Staudt M. (2010) BVOCs and global change. Trends Plant Sci 15: 133–144 [DOI] [PubMed] [Google Scholar]
- Petanidou T. (2005) Sugars in Mediterranean floral nectars: an ecological and evolutionary approach. J Chem Ecol 31: 1065–1088 [DOI] [PubMed] [Google Scholar]
- Robinson EA, Ryan GD, Newman JA. (2012) A meta-analytical review of the effects of elevated CO2 on plant-arthropod interactions highlights the importance of interacting environmental and biological variables. New Phytol 194: 321–336 [DOI] [PubMed] [Google Scholar]
- Shaw RG, Etterson JR. (2012) Rapid climate change and the rate of adaptation: insight from experimental quantitative genetics. New Phytol 195: 752–765 [DOI] [PubMed] [Google Scholar]
- Sheard LB, Tan X, Mao HB, Withers J, Ben-Nissan G, Hinds TR, Kobayashi Y, Hsu FF, Sharon M, Browse J, et al. (2010) Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. Nature 468: 400–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solbreck C, Sillen-Tullberg B. (1981) Control of diapause in a “monovoltine” insect, Lygaeus equestris (Heteroptera). Oikos 36: 68–74 [Google Scholar]
- Spoel SH, Koornneef A, Claessens SM, Korzelius JP, van Pelt JA, Mueller MJ, Buchala AJ, Métraux JP, Brown R, Kazan K, et al. (2003) NPR1 modulates cross-talk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. Plant Cell 15: 760–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoel SH, Loake GJ. (2011) Redox-based protein modifications: the missing link in plant immune signalling. Curr Opin Plant Biol 14: 358–364 [DOI] [PubMed] [Google Scholar]
- Springer CJ, Ward JK. (2007) Flowering time and elevated atmospheric CO2. New Phytol 176: 243–255 [DOI] [PubMed] [Google Scholar]
- Stiling P, Cornelissen T. (2007) How does elevated carbon dioxide (CO2) affect plant-herbivore interactions? A field experiment and meta-analysis of CO2-mediated changes on plant chemistry and herbivore performance. Glob Change Biol 13: 1823–1842 [Google Scholar]
- Sun Y, Yin J, Cao H, Li C, Kang L, Ge F. (2011) Elevated CO2 influences nematode-induced defense responses of tomato genotypes differing in the JA pathway. PLoS ONE 6: e19751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada Y, Spoel SH, Pajerowska-Mukhtar K, Mou Z, Song J, Wang C, Zuo J, Dong X. (2008) Plant immunity requires conformational changes [corrected] of NPR1 via S-nitrosylation and thioredoxins. Science 321: 952–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp S, Croteau R. (2001) Defensive resin biosynthesis in conifers. Annu Rev Plant Physiol Plant Mol Biol 52: 689–724 [DOI] [PubMed] [Google Scholar]
- Van Timmerman SJ, Switzer PV, Kruse KC. (2000) Emergence and reproductive patterns in the Japanese beetle Popillia japonica (Coleoptera: Scarabaeidae). J Kans Entomol Soc 74: 17–27 [Google Scholar]
- Vannette RL, Hunter MD. (2011) Genetic variation in expression of defense phenotype may mediate evolutionary adaptation of Asclepias syriaca to elevated CO2. Glob Change Biol 17: 1277–1288 [Google Scholar]
- Virtanen T, Neuvonen S. (1999) Climate change and macrolepidopteran biodiversity in Finland. Chemosphere, Glob Chang Sci 1: 439–448 [Google Scholar]
- Vuorinen T, Nerg AM, Ibrahim MA, Reddy GVP, Holopainen JK. (2004) Emission of Plutella xylostella-induced compounds from cabbages grown at elevated CO2 and orientation behavior of the natural enemies. Plant Physiol 135: 1984–1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warbrick-Smith J, Behmer ST, Lee KP, Raubenheimer D, Simpson SJ. (2006) Evolving resistance to obesity in an insect. Proc Natl Acad Sci USA 103: 14045–14049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JK, Kelly JK. (2004) Scaling up evolutionary responses to elevated CO2; lessons from Arabidopsis. Eco Letters 7: 427–440 [Google Scholar]
- Wolkovich EM, Cook BI, Allen JM, Crimmins TM, Betancourt JL, Travers SE, Pau S, Regetz J, Davies TJ, Kraft NJB, et al. (2012) Warming experiments underpredict plant phenological responses to climate change. Nature 485: 494–497 [DOI] [PubMed] [Google Scholar]
- Wu J, Baldwin IT. (2010) New insights into plant responses to the attack from insect herbivores. Annu Rev Genet 44: 1–24 [DOI] [PubMed] [Google Scholar]
- Yuan Y, Zhong S, Li Q, Zhu Z, Lou Y, Wang L, Wang J, Wang M, Li Q, Yang D, et al. (2007) Functional analysis of rice NPR1-like genes reveals that OsNPR1/NH1 is the rice orthologue conferring disease resistance with enhanced herbivore susceptibility. Plant Biotechnol J 5: 313–324 [DOI] [PubMed] [Google Scholar]
- Zavala JA, Casteel CL, Delucia EH, Berenbaum MR. (2008) Anthropogenic increase in carbon dioxide compromises plant defense against invasive insects. Proc Natl Acad Sci USA 105: 5129–5133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvereva EL, Kozlov MV. (2006) Consequences of simultaneous elevation of carbon dioxide and temperature for plant-herbivore interactions: a metaanalysis. Glob Change Biol 12: 27–41 [Google Scholar]