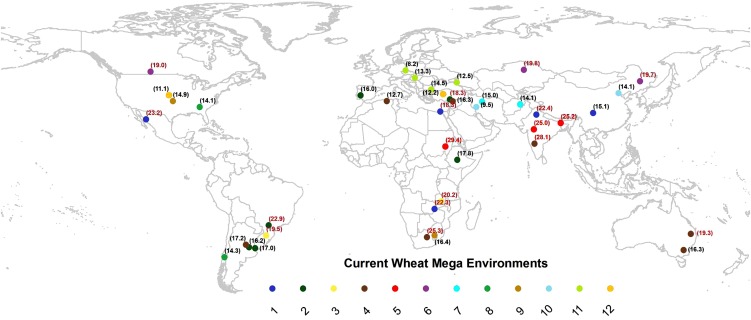
Wheat (Triticum aestivum) represents about 30% of the world’s cereal area, with over 220 million ha cultivated worldwide, often under abiotic stress. Wheat growth can be impaired by heat stress (HS) at any developmental stage, and modeling scenarios predict even warmer temperatures in the future (Easterling and Apps, 2005). The worst impacts of rising temperatures will occur at low latitudes (where approximately 100 million ha of wheat are cultivated, producing approximately 280 million tons of grain), while some benefits at high latitudes are expected. In terms of breeding targets, 12 different wheat mega-environments have been defined worldwide based on cropping system (e.g. rain fed versus irrigated, spring versus winter type) together with biotic and abiotic constraints (Braun et al., 2010). While mega-environment 5 encompasses 7 million ha under continuous HS (e.g. in Sudan and south and central India), over one-half of the total wheat area is prone to periods of HS already, and climate models suggest further increases in average temperatures (Fig. 1; Supplemental Table S1) as well as extreme temperature anomalies, which are already detectable (Hansen et al., 2012). Yield penalties are associated with both chronically high temperatures (mean temperature of the growth cycle being 18°C–25°C, and maximum day temperatures up to 32°C during grain filling) as well as heat shocks, where temperatures greater than 32°C occur during mid or late reproductive wheat stages, including grain filling (Wardlaw and Wrigley, 1994). A recent analysis of extensive international nursery data suggests that spring wheat breeding targeted for abiotic stress delivers better genetic gains in warmer environments (S.M. Gourdji, K.L. Mathews, M.P. Reynolds, J. Crossa, and D.B. Lobell, unpublished data). This Update considers the physiological processes and traits for which there is evidence that genetic improvement could improve wheat adaptation to HS. The issue of biotic threats to wheat is beyond the scope of this review, and readers are referred to other sources (Legreve and Duveiller, 2010).
Figure 1.
Predicted average growing season temperatures for 2050 in each wheat mega-environment (circle color; adapted from Ramirez and Jarvis, 2008). Red numbers show average growing season temperatures above 18°C (indicative of chronically high temperature stress according to Wardlaw and Wrigley, 1994).
IMPROVING GENETIC ADAPTATION OF WHEAT TO HS
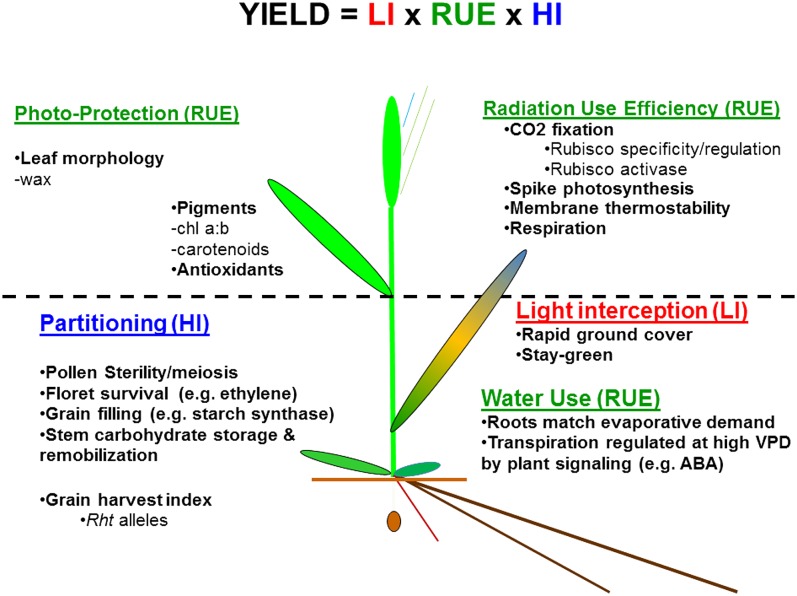
Despite advances in our understanding of genes of major effect conferring disease resistance in wheat (Krattinger et al., 2009), the genetic basis of heat adaptation is poorly understood. Currently, no “heat-tolerance” genes have been cloned. For the time being, physiological traits (PTs) associated with heat adaptation constitute the best available “handle” for genetic improvement of crops, since they represent de facto favorable combinations of alleles. Such alleles are still quite elusive using the quantitative trait locus (QTL) approach, because they show interaction with both environment and genetic background, which typically includes variation in genes of major effect (Reynolds and Tuberosa, 2008; Pinto et al., 2010). While the outcome of combining heat-adaptive PTs in hybridization is, similarly, not always predictable in terms of the net effect on crop productivity, especially over a wide range of environments, crosses made between parents with different but potentially complementary PTs increase the probability of cumulative gene action (Reynolds et al., 2009; Reynolds and Rebetzke, 2011). Both gene discovery and physiological breeding are being facilitated by (1) understanding how adaptive traits enhance yield in heat-stressed environments as well as (2) the development and application of phenotyping platforms to (3) screen experimental populations in order to pinpoint alleles of interest and (4) identify promising new genetic resources. A conceptual model of genetically determined PT to improve heat tolerance in wheat is proposed (Fig. 2), based partly on similar approaches for improving drought adaptation (Reynolds and Tuberosa, 2008), which resulted in the distribution of a new drought-adapted wheat germplasm targeted to mega-environment 4 (Reynolds et al., 2009; http://apps.cimmyt.org/english/wps/obtain_seed/iwin/index.htm). The model is based on the notion that when water and nutrients are not limiting, yield will be a function of three main genetically determined drivers of yield: (1) light interception (LI); (2) radiation use efficiency (RUE); and (3) partitioning of total assimilates.
Figure 2.
Conceptual model of heat-adaptive traits grouped by three main drivers of yield (i.e. yield = LI × RUE × HI) in the absence of water limitations (adapted from Reynolds et al., 2007). ABA, Abscisic acid.
The physiological breeding approach aims to combine traits associated with all three drivers of yield to result in a cumulative genetic effect on yield. Traits for which there is reasonable evidence of being heat adaptive will now be discussed in this framework.
LI Traits
Temperature modifies developmental and growth rates in plants. Since warmer temperatures are typically associated with a reduction in leaf area index and green area duration, the main targets for improving LI under warm conditions include canopy establishment and architecture and the maintenance of green area.
Rapid Ground Cover and Canopy Structure
Rapid ground cover (RGC) or early vigor characterizes the capacity of genotypes to develop leaf area or aboveground biomass. Genotypic variability in RGC has been associated with differences in seedling emergence rate and/or specific leaf area, grain and embryo size, and tillering capacity (Richards and Lukacs, 2002). These characteristics are relatively heritable, making them easy breeding targets (Rebetzke et al., 2008a); however, their interaction with warm temperatures at crop emergence needs research. Genetic diversity for RGC has been exploited in Mediterranean-type environments (to reduce soil evaporation) and can be readily evaluated in the field using digital imagery or spectral reflectance techniques (Mullan and Reynolds, 2010).
Wheat shows enormous diversity in canopy architecture, and it has long been proposed that optimized light distribution could improve RUE as well as LI (Murchie et al., 2009). In fact, many modern cultivars already present smaller and more erect leaves in the upper canopy, potentially improving RUE and permitting better light penetration to lower leaves. Further modification in canopy architecture may not be easy to address genetically, since the canopy is a highly complex structure; furthermore, light extinction interacts with sun angle and light intensity, making it a constantly moving target. However, modeling studies focused on spatial arrangements of crop rows may provide agronomic strategies that would benefit RUE.
Stay-Green
Leaf senescence is characterized initially by structural changes in the chloroplast, followed by a controlled vacuolar collapse, and a final loss of integrity of plasma membrane and disruption of cellular homeostasis (Lim et al., 2007). Delay in the expression of senescence-related genes permits Stay-Green (SG) genotypes to maintain photosynthesis (Lim et al., 2007). While SG is recognized as an adaptive PT for stress conditions, the optimal pattern of senescence/pigment loss in terms of improving grain yield under HS has not been identified. This is partly because chlorosis is an integral part of programmed senescence, where there are unavoidable tradeoffs between maintaining photosynthetic area and remobilization of nitrogen to the maturing grain (Vijayalakshmi et al., 2010). Nonetheless, relationships between yield and SG have been shown, and QTLs have been identified in mapping populations (Kumar et al., 2010; Vijayalakshmi et al., 2010; Table I). Since chlorosis is expressed heterogeneously on all aboveground organs, even within a single organ such as a leaf, it is not straightforward to parameterize. However, an easy and integrated approach to estimate SG using the spectral reflectance Normalized Difference Vegetation Index showed significant association with yield under HS in two large mapping populations, making it a reliable tool for large-scale screening and gene discovery work (Lopes and Reynolds, 2012). Although the Normalized Difference Vegetation Index, which is a reliable indicator of greenness integrating all chlorophyll, is associated with heat tolerance, studies in other species suggest that chlorophyll a degrades sooner than chlorophyll b (Keskitalo et al., 2005). Therefore, more specific spectral indices related to functional SG would be worth developing as a screening tool for heat tolerance. Transgenic approaches have been used to improve SG. Reduction in levels of wheat No Apical Meristem genes delayed senescence (of spikes and peduncle) 2 weeks but without improvements in grain weight (Uauy et al., 2006), possibly because of the tradeoffs above mentioned.
Table I. Chromosomal location for PTs desirable for hot environments.
| Conceptual Model Factor | Trait | Chromosomal Locations of Genes | Reference |
|---|---|---|---|
| LI | Phenology | 2A, 2B, 2D, 3A, 3B, 5A, 5B, 5D, 6A, 6B, 7A, 7B | Reynolds and Rebetzke (2011) |
| RGC | 2D, 4B, 4D, 5A | Reynolds and Rebetzke (2011) | |
| SG | 1As, 2B, 2D, 3A, 3B, 3BS, 6A, 6B, 7A, 7Ds | Kumar et al. (2010); Vijayalakshmi et al. (2010) | |
| RUE | Stomatal conductance | 1B, 2A, 2B, 2D, 4A, 4B, 4D, 5A, 7A, 7B | Reynolds and Rebetzke (2011) |
| Canopy temperature | 1B, 2B, 3B, 4A | Reynolds and Rebetzke (2011) | |
| Root biomass and seminal roots | 1B, 2AL, 7AL, 7BL | Reynolds and Rebetzke (2011) | |
| Transpiration efficiency (leaf) | 1B, 1D, 2D, 3B, 4A, 4B, 4D, 5A, 7A, 7B | Reynolds and Rebetzke (2011) | |
| Transpiration efficiency (grain) | 1D, 2A, 2D, 4B, 4D, 6D, 7B | Reynolds and Rebetzke (2011) | |
| Photosynthetic capacity | 1B, 1D, 2D, 3B, 4A, 4B, 4D, 5A, 7A, 7B | Reynolds and Rebetzke (2011) | |
| MT | 7A | Ciuca and Petcu (2009) | |
| Glaucousness | 2B, 2D, 3A | Tsunewaki and Ebana (1999); Bennett et al. (2011) | |
| HI | WSC | 1A, 1B, 1D, 2D, 2B, 3D, 4A, 4B, 5B, 6A, 7B, 7D | Rebetzke et al. (2008b) |
| Grain weight, grain-filling rate, and duration | 1A, 1B, 2A, 2D, 3A, 3B, 3D, 4A, 4D, 5A, 5B, 6D, 7D | Wang et al. (2009) | |
| Floret fertility or grain number | 1Ba, 3Bb, 4Aa, 5Ba, 6Ba | Pinto et al. (2010) |
RUE Traits
Assuming that LI is optimized, crop biomass will depend on net RUE, which is the result of gross carbon assimilation minus losses associated with growth, maintenance, and repair (so-called dark respiration), metabolic inefficiencies (mainly photorespiration in C3 species), and a range of photoprotective strategies.
Photosynthesis/Photorespiration
Improving photosynthetic capacity and efficiency are key targets for increasing the yield potential of crops. Interestingly, the main objectives, namely increased affinity of Rubisco for CO2 versus oxygen and a better catalytic rate (Parry et al., 2011), are likely to be particularly beneficial at warmer temperatures. Rubisco’s affinity for CO2 decreases with temperatures (Jordan and Ogren, 1984). Therefore, increasing affinity would simultaneously improve adaptation to warmer conditions, the proof of concept coming from C4 species, in which it is achieved by concentrating CO2 (Sage, 2002).
While wheat Rubisco, in fact, has among the best affinities for CO2 among crop species, extensive surveys of plant taxa suggest that the kinetic properties of the Rubisco of Limonium gibertii may provide a means to improve its photosynthesis using transgenics. Models where wheat’s substrate specificity factor of Rubisco is replaced from L. gibertii predicted increases of 12% in net assimilation (Parry et al., 2011). However, replacement of wheat Rubisco with that of another species is technically challenging, since it would require context-specific expression, assembly, chloroplast transformation, posttranslational modification, and activity regulation (Bock, 2007; Miyao et al., 2011). The alternative to the transgenic approach is the genetic/phenomic screening of wheat and related species to mine the existing variability in Rubisco kinetic properties, although it is not yet amenable to high-throughput phenotyping (HTP).
Another target for genetic improvement is Rubisco activase (Salvucci and Crafts-Brandner, 2004). Under HS, Rubisco-active sites progressively become inactive either through decarbamylation or catalytic inactivation. Protection of Rubisco activase by preventing its sequestration to the thylakoid membranes from the soluble stroma fraction was associated with higher yields and overexpression of SBPase in transgenic rice (Oryza sativa) plants (Feng et al., 2007). Rubisco activase protection may also occur through the suggested association between Rubisco activase and the chaperone cpn60β (Salvucci, 2008). Natural diversity in the optimum temperature of Rubisco activase exists and may be exploited. Thermotolerant forms of Rubisco activase have also been identified in tropical species (Kurek et al., 2007). Improvements in the thermal stability of Rubisco activase in Arabidopsis (Arabidopsis thaliana) have already been demonstrated by creating a chimeric activase composed of the tobacco (Nicotiana tabacum) activase but containing an Arabidopsis Rubisco recognition domain (Kumar et al., 2009).
Despite significant efforts in identifying mutants with reduced photorespiration and higher photosynthesis and yield, all mutants of photorespiratory enzymes studied to date have shown stunted growth and chlorosis, most probably because 2-phosphoglycolate and other intermediates accumulated in their cells. Nonetheless, photorespiration serves as an energy dissipation mechanism avoiding photoinhibition when CO2 is limited by moisture stress (Peterhansel and Maurino, 2011). Photorespiratory genes have been identified in Arabidopsis and other species, which indicates potential functional overlaps and redundancy, although more genomic information is required to elucidate all repercussions in the photorespiratory pathway (Foyer et al., 2009). The bacterial glycolate pathway and a novel pathway that fully oxidizes glycolate to CO2 in order to improve the efficiency of 2-phosphoglycolate production have been engineered in Arabidopsis chloroplasts. The main advantages compared with the major photorespiratory pathway are (1) CO2 is produced in chloroplasts and potentially enhances the CO2 concentration in the vicinity of Rubisco, (2) both pathways avoid transamination reactions, and (3) in both pathways, additional reducing equivalents are produced in the chloroplast (Peterhansel and Maurino, 2011).
Attempts toward transferring C4 properties to C3 plants are being made by transgenic methods. However, more information is needed, particularly about the establishment and regulation of C4 anatomy and C4-type chloroplast development and their genetic basis (Hibberd and Covshoff, 2010; Parry et al., 2011).
Spike photosynthesis has a number of characteristics that make it a worthwhile target for increasing heat adaptation: (1) spikes intercept up to 40% of incident radiation in wheat canopies and operate at higher temperatures than leaves; (2) their transpiration efficiency is higher than that of leaves; and (3) genetic variation exists in spike senescence. The main difficulties in analyzing spike photosynthesis include the interpretation of results that are potentially confounded by CO2 assimilation/recycling within the spike, the standardization of the units of carbon fixation given the complex geometry of spikes, and the development of HTP; chlorophyll fluorescence represents a good candidate (Parry et al., 2011).
Respiration
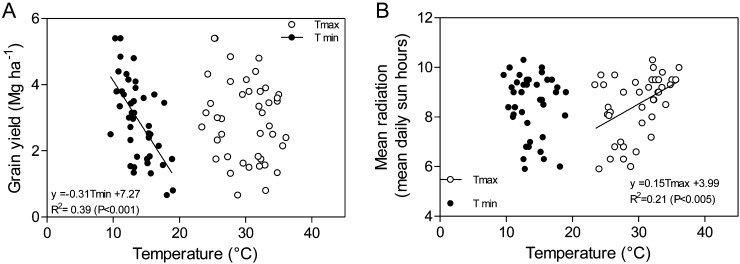
Respiration generally consumes between 30% and 80% of the CO2 taken up by photosynthesis per day (Atkin et al., 2005), increasing with temperature and depending on phenological stage (McCullough and Hunt, 1993). High respiration rates (especially at night) can increase reactive oxygen species (ROS), leading to cell damage and affecting pollen viability (Prasad et al., 1999). Extensive field trials established across some of the hottest wheat-growing environments worldwide (Reynolds et al., 1994) showed that while yield was negatively associated with average daily temperature, night temperature explained most of the variation (which was not a corollary of reduced incident radiation; Fig. 3), suggesting respiration as a strong candidate for genetic improvement. Selection for low respiration was associated with higher biomass in a Lolium spp. breeding program in a temperate environment (Wilson and Jones, 1982), and the development of HTP for respiration in wheat is needed.
Figure 3.
Relationship between grain yield (A) and mean radiation (B) and minimum or maximum temperature observed during the growing season of 16 genotypes from the International Heat Stress Genotype Experiment network of International Maize and Wheat Improvement Center (adapted from Reynolds et al., 1994).
Photoprotective Metabolites
Under abiotic stress, the photosynthetic machinery is especially vulnerable to damage associated with excess light energy and its products like ROS. Scavenging of ROS prior to their diffusion from the site of generation is indispensable for protecting complex molecules. The main photoprotective molecules include carotenoids, flavonoids, glutathione, ascorbate, and tocopherols and are located throughout the cell and its organelles. Depending on specific location, photoprotective molecules (1) avoid photooxidative damage to membranes and proteins caused by excessive light, (2) scavenge ROS like singlet oxygen and dissipate extra energy via xanthophyll-mediated nonphotochemical quenching, (3) interact with the membrane lipids acting like energy receptors and in down-regulation processes, (4) provide protection by direct quenching or chemically scavenging 1O2, O2−, and OH, (5) neutralize free radicals and ROS before they damage the cell under high irradiance levels or HS, or (6) participate in redox signaling (Murchie and Niyogi, 2011). Notwithstanding the relatively extensive knowledge in this area, translational research to crop improvement is scarce. However, spectral radiometry offers a HTP approach to screen for photoprotective molecules in crop canopies (Ollinger, 2011).
Wax/Glaucous
The two most important functions of wax (which may be embedded in the polymer matrix [intracuticular wax] or secreted on the tissue surface [epicuticular wax]) are protection against excess radiation and water loss through the reflection of visible and infrared wavelengths (Shepherd and Griffiths, 2006). The reflectivity of radiation (particularly at visible wavelengths) is proportional to the amount of wax, and it is strongly influenced by surface topography. The beginning of wax deposition coincides with meiosis and is greatest when leaf area and crop water use are maximal, suggesting an adaptive advantage under abiotic stress, confirmed by studies with isolines (Richards et al., 1986). Nondestructive methods like confocal laser microscopy were used to analyze the changes in the cuticle structure and thickness of apple (Malus domestica) but have not been applied in wheat breeding (Veraverbeke et al., 2001). Promising recent results show that leaf waxes may reduce leaf temperatures and improve adaptation during HS (Mondal, 2011).
The observation of light bluish-gray or bluish-white color on plant tissues is denominated glaucous and associated with epicuticular wax. Glaucous is generally used to evaluate wax visually and qualitatively in breeding populations. The presence/absence of glaucousness in wheat is controlled by a dominant gene, W1, which acts as an epistatic inhibitor to a glaucousness gene, Iw1 (Tsunewaki and Ebana, 1999). Recently, a new QTL for glaucousness was identified in wheat under warm temperature, explaining around 50% of the genetic variance (Bennett et al., 2011). While epicuticular wax appears to have a relatively simple genetic basis and is easy to select for visually, further research is needed to quantify the cost benefit of different types of wax and its deposition strategies across target environments.
Membrane Thermostability
Heat shock increases cell membrane permeability, thereby inhibiting cellular function, as a result of the denaturation of proteins and increments of unsaturated fatty acids that disrupt water, ion, and organic solute movement across membranes. Thylakoid membranes typically show swelling, increased leakiness, physical separation of the chlorophyll light-harvesting complex II from the PSII core complex, and disruption of PSII-mediated electron transfer (Ristic et al., 2008). ROS accumulation associated with HS also damages membranes (Abdul-Razack and Tarpley, 2009). The lipid composition of membranes is mainly monogalactosyldiacylglycerol (50%) and digalactosyldiacylglycerol (20%) with the rest including phosphatidylglycerol and sulfoquinovosyldiacylglycerol. Thermotolerance is favored by an increased ratio of digalactosyldiacylglycerol to monogalactosyldiacylglycerol and with high levels of saturation of digalactosyldiacylglycerol. Low expression of electrolyte leakage from leaf tissue after an in vitro heat shock, an indication of membrane thermostability (MT), was associated with the performance of 16 wheat genotypes at a range of HS field locations worldwide (Reynolds et al., 1994). Chlorophyll fluorescence will be tested as an HTP surrogate for MT as part of a project to screen wheat genetic resources for heat tolerance. However, more research is needed to explain genetic variation in MT and its relationship with saturation/unsaturation of fatty acids (Essemine et al., 2011). The presence of both additive and dominant types of gene action has been reported for MT in wheat (Dhanda and Munjal, 2012) as well as QTL and associated simple sequence repeat markers (Ciuca and Petcu, 2009; Table I).
Partitioning of Total Assimilate Traits
The adaptation of reproductive growth to HS is obviously crucial to determining grain sink strength, while the ability to synthesize, store, and remobilize starch at high temperature will determine final kernel weight. Recently, it has become apparent that plant growth regulators may also play a crucial role in determining harvest index (HI) under abiotic stress (Ji et al., 2011).
Spike Fertility
Even under relatively optimal conditions, grain set is apparently conservative and sensitive to carbohydrate supply (Fischer, 2011, and refs. therein). Data from hot wheat-growing environments show that grain number is often reduced more than might be expected from reductions in biomass, leading to relatively low HI under HS (Reynolds et al., 2007). Grain abortion has been linked to ethylene levels that induce programmed cell death at high temperature (Hays et al., 2007). When abiotic stress coincides with meiosis, the first phase of gametogenesis fertility may be further impaired (Ji et al., 2010). Wheat plants exposed to 30°C during a 3-d period around anthesis presented abnormal anthers, both structurally and functionally, in 80% of the florets. The chemical composition, metabolism, morphology, and quantity of pollen can be affected by HS in addition to pollen tube growth rate (Hedhly et al., 2009). Genetic diversity for pollen viability under HS has been identified in cotton (Gossypium hirsutum; Burke, 2007), and reduced pollen viability under HS has been linked to carbohydrate metabolism in other cereals like sorghum (Sorghum bicolor; Jain et al., 2007; Prasad and Djanaguiraman, 2011). Although less researched, female reproductive organs are affected by HS. Abnormal ovary development in addition to accelerated stigma and ovule development resulted in reduced pollen tube growth and seed set when HS coincided with meiosis (Barnabás et al., 2008). QTLs for grain number under hot environments have been reported in wheat, and many coincided with yield QTL (Pinto et al., 2010; Table I). These relationships need to be better defined and genetic diversity for spike fertility at high temperature explored in wheat gene pools.
Water-Soluble Carbohydrates
Severe HS occurring after flowering may jeopardize seed filling if adequate stored assimilates are not available for remobilization to the grain. Wheat organs typically accumulate a significant reserve of water-soluble carbohydrates (WSC) when the photosynthesis/respiration ratio is favorable, which can be used for immediate growth or stored and remobilized later in development. The WSC dynamics of stems are relatively well studied, being the largest potential sink, but relatively little is known about other organs. Stem WSC consist of a range fructans (85%), Suc (10%), and other (5%), peaking in concentration 10 to 25 d after anthesis under temperate conditions (Blacklow et al., 1984) and often sooner under stress when the grain-filling period is truncated. Their potential for storage of WSC is determined by stem volume, especially of the peduncle and the penultimate internode (Blum, 1998), and changes in storage capacity were associated also with differences in the speed of translocation and higher concentrations of sugars in the sieve elements (Wardlaw, 1990). Genetic diversity of stem WSC in wheat ranges from 10% to almost 50% of total stem dry weight (Reynolds et al., 2007; Rebetzke et al., 2008b) and has been associated with differential regulation of some carbohydrate metabolic genes at the transcript level (Xue et al., 2008). The Green Revolution Rht1 and Rht2 dwarfing genes are generally associated with a reduction in WSC storage capacity of stems due to shorter peduncles (Borrell et al., 1993). A total of 12 QTLs for stem WSC were mapped in three wheat populations across multiple environments, indicating polygenic control of the trait (Rebetzke et al., 2008b; Table I). Xue et al. (2008) suggested MYB genes (TaMYB13) as positive regulators for controlling the expression of enzymes involved in the WSC synthetic pathway in wheat, while other work has indicated the feasibility of genetic manipulation of regulatory genes for WSC expression (McIntyre et al., 2012).
Starch Synthesis
Even when adequate assimilates are available, starch accumulation in wheat grains can be reduced by over 30% at temperatures between 30°C and 40°C, with the most critical stage being in early grain filling (Stone and Nicolas, 1995a). Enzymes involved in starch accumulation are a key target for improving adaptation to warmer climates. The main enzymes include ADP-Glc, pyrophosphorylase (AGPase), granule-bound starch synthase, soluble starch synthase (SS), starch-branching enzyme (SBE), starch-debranching enzyme, and plastidial starch phosphorylase. Cereal endosperm uses a unique pathway of starch biosynthesis that requires (1) cytosolic AGPase and (2) ADP-Glc transport (for discussion, see Geigenberger, 2011). Crop transformation with altered expression of enzymes of the starch synthesis pathway allowed systematic investigations into the contributions of each step controlling flux into starch of potato (Solanum tuberosum). However, systematic flux-control studies are lacking for cereal seed endosperm. Hannah and James (2008) suggest a colimitation of AGPase on all starch accumulation. SS activity is maximum between 20°C and 25°C, while at temperatures above 35°C, SS becomes nonfunctional, and 97% of its activity is lost when temperatures rise to 40°C (Keeling et al., 1993). SS and SBE exist as heterocomplexes in wheat. Mechanisms underlying the complex formation are still unresolved, although there is evidence that physical association of these proteins depends on their phosphorylation status (Tetlow et al., 2004). Considerable genetic variability in tolerance to short periods of high temperature during grain filling for both grain yield and quality indicates breeding opportunities (Stone and Nicolas, 1995b). Further research should address the impact of brief heat shock periods and the mechanism behind the genetic variability as well as their biophysical limits. QTLs related to grain-filling rate, maximum grain-filling rate, grain-filling duration, and 1,000 grain weight were reported to be located on 13 different chromosomes (Wang et al., 2009; Table I). Other genetic studies indicate different isoforms of enzymes involved in starch synthesis playing specific roles in determining the complex structure of starch. Transcriptional regulation of gene expression may be the primary mechanism behind the inhibition of starch metabolism under HS.
Plant Signaling
There is evidence that low HI and premature ripening in crops can result from stress-responsive signals whose adaptive significance seems more related to survival than to agronomic improvement. For example, increased ethylene generation was associated with kernel abortion and yield reduction at high temperature in wheat (Hays et al., 2007), and ethylene is also known to accelerate senescence and reduce root and embryo growth (Wilkinson and Davies, 2010). Further evidence that excess ethylene production can be detrimental comes from work with ethylene perception inhibitors like 1-methylcyclopropene, which improved grain set and yield in soybean (Glycine max) and was associated with prolonged photosynthesis and better MT (Djanaguiraman and Prasad, 2010). Root-shoot signaling involving abscisic acid under water deficit has been shown to result in increased transpiration efficiency (Wilkinson and Davies, 2002), which can help optimize water budgets under drought as well as HS conditions, where transpiration efficiency tends to be quite low due to high vapor pressure deficit. However, breeding for favorable abscisic acid expression is challenging, given the likelihood of interactions with other plant signals and the fact that abscisic acid may hasten senescence and reduce floret fertility (Ji et al., 2011). More research in this area is needed to avoid the risk, especially in future more extreme climates, of serious crop losses associated with the switch to conservative adaptive responses.
BREEDING FOR HEAT TOLERANCE
Most of the traits outlined above are good candidates for translational research, while several are already applied in heat tolerance breeding. For example, cooler canopy temperature (CT), which appears to have some common genetic basis under both heat and drought stress (Pinto et al., 2010; Table I), is strongly associated with yield in both environments (Pinto et al., 2010). Recent data show CT to be associated with deeper roots under drought (Lopes and Reynolds, 2010) and HS (M.P. Reynolds, unpublished data). Since cooler canopies are also associated with genetic variation in stomatal conductance under heat (Reynolds et al., 1994, 2007), selection for CT is also likely to improve assimilation capacity per se. More targeted approaches to increase assimilation capacity include screening candidate parents for early vigor and delayed senescence to improve LI as well as MT, photoprotective pigments, and wax to improve RUE. Rubisco and its regulation and spike photosynthesis are longer term targets with potential high payoffs.
As RUE and LI are optimized, yield gains will increasingly depend on the ability of the crop to partition assimilates to grain. Improved understanding of the adaptation of reproductive growth to HS, including interactions with plant growth regulators (Hays et al., 2007), will require significant investment if genetic gains in assimilation capacity are to translate into agronomic yield through stable expression of HI. In this context, capacity for WSC storage and remobilization are traits that can help stabilize HI by buffering assimilate supply against highly variable weather.
Adaptation to warm night temperatures will be a high priority in more humid wheat environments (e.g. Bangladesh, Brazil, Kenya), and low respiratory costs and heat-stable starch synthesis remain key targets to improve RUE and HI, respectively, in spite of the difficulties of screening.
While cumulative gene action can be expected through strategic crossing of complementary traits (Reynolds et al., 2009), there will inevitably be tradeoffs, as observed between WSC concentration and investment in root mass at depth under drought, for example (Lopes and Reynolds, 2010). When the true variability of crop-growing environments is better characterized, and the precise genetic bases of heat-adaptive traits are established, such tradeoffs will be able to be effectively modeled and targeted (Chapman, 2008).
In the meantime, HTP platforms can accelerate both physiological breeding and gene discovery. CT is an ideal HTP field screen in many ways, showing a good genetic association with yield (Saint Pierre et al., 2010), being highly accessible as indicated by economic analysis (Brennan et al., 2007), and given that it allows rapid estimation of difficult-to-phenotype traits such as transpirational flux and root depth (Lopes and Reynolds, 2010). In addition, infrared and other remote spectral sensing platforms are being continually refined, allowing CT, pigment composition, hydration status, and ground cover to be measured on a breeding scale (Ollinger, 2011). More direct procedures for HTP of root structure are also under investigation, including ground-penetrating radar, x-ray, γ-ray, thermal, neutron, and magnetic-resonance tomography (Tracy et al., 2010). Integrated use of spectral reflectance spectroscopy and other remote-sensing HTP methods with simulation models, incorporating field and environmental data, will make extensive screening of genetic resources more feasible as well as contribute to the detection of molecular markers for their future allele mining. There are almost 500,000 wheat accessions available worldwide in germplasm banks, encompassing landraces, nondomesticated species, and advanced and obsolete cultivars (Ortiz et al., 2008). Screening and crossing with exotic material will be prerequisites to maintaining genetic gains as the limitations of conventional gene pools under HS are revealed.
As target traits are more clearly defined by precision phenotyping, and the costs of molecular tools continue to decline, transcriptomic and genomic analysis are expected to provide definite genetic bases of heat-adaptive traits, allowing more routine use of diagnostic molecular markers in breeding and genetic resource exploration.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Table S1. Main expected changes for each International Maize and Wheat Improvement Center mega-environment due to climate change (adapted from Braun et al., 2010).
Acknowledgments
We thank H.-J. Braun for useful discussions and suggestions about the manuscript, K. Sonder for support in Geographic Information Systems, and J. Pietragalla for advice during the preparation of the manuscript.
Glossary
- HS
heat stress
- PT
physiological trait
- QTL
quantitative trait locus
- LI
light interception
- RUE
radiation use efficiency
- RGC
rapid ground cover
- SG
Stay-Green
- HTP
high-throughput phenotyping
- ROS
reactive oxygen species
- MT
membrane thermostability
- HI
harvest index
- WSC
water-soluble carbohydrates
- CT
canopy temperature
References
- Abdul-Razack M, Tarpley L. (2009) Impact of high nighttime temperature on respiration, membrane stability, antioxidant capacity, and yield of rice plants. Crop Sci 49: 313–322 [Google Scholar]
- Atkin OK, Bruhn D, Hurry VM, Tjoelker MG. (2005) Evans Review No. 2. The hot and the cold: unravelling the variable response of plant respiration to temperature. Funct Plant Biol 32: 87–105 [DOI] [PubMed] [Google Scholar]
- Barnabás B, Jäger K, Fehér A. (2008) The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ 31: 11–38 [DOI] [PubMed] [Google Scholar]
- Bennett D, Izanloo A, Edwards J, Kuchel H, Chalmers K, Tester M, Reynolds MP, Schnurbusch T, Langridge P. (2011) Identification of novel quantitative trait loci for days to ear emergence and flag leaf glaucousness in a bread wheat (Triticum aestivum L.) population adapted to southern Australian conditions. Theor Appl Genet 124: 1–15 [DOI] [PubMed] [Google Scholar]
- Blacklow WM, Darbyshire B, Pheloung P. (1984) Fructan polymerized and depolymerized in the internodes of winter wheat as grain filling progressed. Plant Sci Lett 36: 213–218 [Google Scholar]
- Blum A. (1998) Improving wheat grain filling under stress by stem reserve mobilisation. Euphytica 100: 77–83 [Google Scholar]
- Bock R. (2007) Plastid biotechnology: prospects for herbicide and insect resistance, metabolic engineering and molecular farming. Curr Opin Biotechnol 18: 100–106 [DOI] [PubMed] [Google Scholar]
- Borrell AK, Incoll LD, Dalling MJ. (1993) The influence of the Rht1 and Rht2 alleles on the deposition and use of stem reserves in wheat. Ann Bot (Lond) 71: 317–326 [Google Scholar]
- Braun H-J, Atlin G, Payne T. (2010) Multi-location testing as a tool to identify plant response to global climate change. In MP Reynolds, ed, Climate Change and Crop Production. CABI, Oxfordshire, UK, pp 115–138
- Brennan JP, Condon AG, van Ginkel M, Reynolds MP. (2007) An economic assessment of the use of physiological selection for stomatal aperture-related traits in CIMMYT’s wheat breeding program. J Agric Sci 145: 187–194 [Google Scholar]
- Burke JJ. (2007) Genetic diversity in pollen abiotic stress tolerance (no. 1509). In World Cotton Research Conference-4. September 10–14, Lubbock, TX
- Chapman S. (2008) Use of crop models to understand genotype by environment interactions for drought in real-world and simulated plant breeding trials. Euphytica 161: 195–208 [Google Scholar]
- Ciuca M, Petcu E. (2009) SSR markers associated with membrane stability in wheat (Triticum aestivum L.). Romanian Agricultural Research 26: 21–24 [Google Scholar]
- Dhanda SS, Munjal R. (2012) Heat tolerance in relation to acquired thermotolerance for membrane lipids in bread wheat. Field Crops Res 135: 30–37 [Google Scholar]
- Djanaguiraman M, Prasad PVV. (2010) Ethylene production under high temperature stress causes premature leaf senescence in soybean. Funct Plant Biol 37: 1071–1084 [Google Scholar]
- Easterling DR, Apps M. (2005) Assessing the consequences of climate change for food and forest resources: a view from the IPCC. Clim Change 70: 165–189 [Google Scholar]
- Essemine J, Govindachary S, Ammar S, Bouzid S, Carpentier R. (2011) Functional aspects of the photosynthetic light reactions in heat stressed Arabidopsis deficient in digalactosyl-diacylglycerol. J Plant Physiol 168: 1526–1533 [DOI] [PubMed] [Google Scholar]
- Feng L, Wang KJ, Li Y, Tan Y, Kong J, Li H, Li Y, Zhu Y. (2007) Overexpression of SBPase enhances photosynthesis against high temperature stress in transgenic rice plants. Plant Cell Rep 26: 1635–1646 [DOI] [PubMed] [Google Scholar]
- Fischer RA. (2011) Wheat physiology: a review of recent developments. Crop Pasture Sci 62: 95–114 [Google Scholar]
- Foyer CH, Bloom AJ, Queval G, Noctor G. (2009) Photorespiratory metabolism: genes, mutants, energetics, and redox signaling. Annu Rev Plant Biol 60: 455–484 [DOI] [PubMed] [Google Scholar]
- Geigenberger P. (2011) Regulation of starch biosynthesis in response to a fluctuating environment. Plant Physiol 155: 1566–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannah LC, James M. (2008) The complexities of starch biosynthesis in cereal endosperms. Curr Opin Biotechnol 19: 160–165 [DOI] [PubMed] [Google Scholar]
- Hansen J, Sato M, Ruedy R. (2012) Perception of climate change. Proc Natl Acad Sci USA (in press)
- Hays DB, Do JH, Mason RE, Morgan G, Finlayson SA. (2007) Heat stress induced ethylene production in developing wheat grains induces kernel abortion and increased maturation in a susceptible cultivar. Plant Sci 172: 1113–1123 [Google Scholar]
- Hedhly A, Hormaza JI, Herrero M. (2009) Global warming and sexual plant reproduction. Trends Plant Sci 14: 30–36 [DOI] [PubMed] [Google Scholar]
- Hibberd JM, Covshoff S. (2010) The regulation of gene expression required for C4 photosynthesis. Annu Rev Plant Biol 61: 181–207 [DOI] [PubMed] [Google Scholar]
- Jain M, Prasad PV, Boote KJ, Hartwell AL, Jr, Chourey PS. (2007) Effects of season-long high temperature growth conditions on sugar-to-starch metabolism in developing microspores of grain sorghum (Sorghum bicolor L. Moench). Planta 227: 67–79 [DOI] [PubMed] [Google Scholar]
- Ji X, Shiran B, Wan J, Lewis DC, Jenkins CLD, Condon AG, Richards RA, Dolferus R. (2010) Importance of pre-anthesis anther sink strength for maintenance of grain number during reproductive stage water stress in wheat. Plant Cell Environ 33: 926–942 [DOI] [PubMed] [Google Scholar]
- Ji XM, Dong B, Shiran B, Talbot MJ, Edlington JE, Hughes T, White RG, Gubler F, Dolferus R. (2011) Control of abscisic acid catabolism and abscisic acid homeostasis is important for reproductive stage stress tolerance in cereals. Plant Physiol 156: 647–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan DB, Ogren WL. (1984) The CO2/O2 specificity of ribulose 1,5-bisphosphate carboxylase/oxygenase: dependence on ribulosebisphosphate concentration, pH and temperature. Planta 161: 308–331 [DOI] [PubMed] [Google Scholar]
- Keeling PL, Bacon PJ, Holt DC. (1993) Elevated temperature reduces starch deposition in wheat endosperm by reducing the activity of soluble starch synthase. Planta 191: 342–348 [Google Scholar]
- Keskitalo J, Bergquist G, Gardeström P, Jansson S. (2005) A cellular timetable of autumn senescence. Plant Physiol 139: 1635–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krattinger SG, Lagudah ES, Spielmeyer W, Singh RP, Huerta-Espino J, McFadden H, Bossolini E, Selter LL, Keller B. (2009) A putative ABC transporter confers durable resistance to multiple fungal pathogens in wheat. Science 323: 1360–1363 [DOI] [PubMed] [Google Scholar]
- Kumar A, Li C, Portis AR., Jr (2009) Arabidopsis thaliana expressing a thermostable chimeric Rubisco activase exhibits enhanced growth and higher rates of photosynthesis at moderately high temperatures. Photosynth Res 100: 143–153 [DOI] [PubMed] [Google Scholar]
- Kumar U, Joshi A, Kumari M, Paliwal R, Kumar S, Röder M. (2010) Identification of QTLs for stay green trait in wheat (Triticum aestivum L.) in the ‘Chirya 3’ × ‘Sonalika’ population. Euphytica 174: 437–445 [Google Scholar]
- Kurek I, Chang TK, Bertain SM, Madrigal A, Liu L, Lassner MW, Zhu GH. (2007) Enhanced thermostability of Arabidopsis Rubisco activase improves photosynthesis and growth rates under moderate heat stress. Plant Cell 19: 3230–3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legreve A, Duveiller E. (2010) Preventing potential disease and pest epidemics under a climate change. In MP Reynolds, ed, Climate Change and Crop Production. CABI, Oxfordshire, UK, pp 50–70
- Lim PO, Kim HJ, Nam HG. (2007) Leaf senescence. Annu Rev Plant Biol 58: 115–136 [DOI] [PubMed] [Google Scholar]
- Lopes MS, Reynolds MP. (2010) Partitioning of assimilates to deeper roots is associated with cooler canopies and increased yield under drought in wheat. Funct Plant Biol 37: 147–156 [Google Scholar]
- Lopes MS, Reynolds MP. (2012) Stay-green in spring wheat can be determined by spectral reflectance measurements (normalized difference vegetation index) independently from phenology. J Exp Bot 63: 3789–3798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough DE, Hunt LA. (1993) Mature tissue and crop canopy respiratory characteristics of rye, triticale and wheat. Ann Bot (Lond) 72: 269–282 [Google Scholar]
- McIntyre CL, Seung D, Casu RE, Rebetzke GJ, Shorter RA, Xue GP. (2012) Genotypic variation in the accumulation of water soluble carbohydrates in wheat. Funct Plant Biol 39: 560–568 [DOI] [PubMed] [Google Scholar]
- Miyao M, Masumoto C, Miyazawa S-I, Fukayama H. (2011) Lessons from engineering a single-cell C(4) photosynthetic pathway into rice. J Exp Bot 62: 3021–3029 [DOI] [PubMed] [Google Scholar]
- Mondal S. (2011) Defining the molecular and physiological role of leaf cuticular waxes in reproductive stage heat tolerance in wheat. PhD thesis. Texas A&M University, College Station
- Mullan DJ, Reynolds MP. (2010) Quantifying genetic effects of ground cover on soil water evaporation using digital imaging. Funct Plant Biol 37: 703–712 [Google Scholar]
- Murchie EH, Niyogi KK. (2011) Manipulation of photoprotection to improve plant photosynthesis. Plant Physiol 155: 86–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchie EH, Pinto M, Horton P. (2009) Agriculture and the new challenges for photosynthesis research. New Phytol 181: 532–552 [DOI] [PubMed] [Google Scholar]
- Ollinger SV. (2011) Sources of variability in canopy reflectance and the convergent properties of plants. New Phytol 189: 375–394 [DOI] [PubMed] [Google Scholar]
- Ortiz R, Braun H-J, Crossa J, Crouch J, Davenport G, Dixon J, Dreisigacker S, Duveiller E, He Z, Huerta J, et al. (2008) Wheat genetic resources enhancement by the International Maize and Wheat Improvement Center (CIMMYT). Genet Resour Crop Evol 55: 1095–1140 [Google Scholar]
- Parry MAJ, Reynolds M, Salvucci ME, Raines C, Andralojc PJ, Zhu X-G, Price GD, Condon AG, Furbank RT. (2011) Raising yield potential of wheat. II. Increasing photosynthetic capacity and efficiency. J Exp Bot 62: 453–467 [DOI] [PubMed] [Google Scholar]
- Peterhansel C, Maurino VG. (2011) Photorespiration redesigned. Plant Physiol 155: 49–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto RS, Reynolds MP, Mathews KL, McIntyre CL, Olivares-Villegas J-J, Chapman SC. (2010) Heat and drought adaptive QTL in a wheat population designed to minimize confounding agronomic effects. Theor Appl Genet 121: 1001–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad PVV, Craufurd PQ, Summerfield RJ. (1999) Fruit number in relation to pollen production and viability in groundnut exposed to short episodes of heat stress. Ann Bot (Lond) 84: 381–386 [Google Scholar]
- Prasad PVV, Djanaguiraman M. (2011) High night temperature decreases leaf photosynthesis and pollen function in grain sorghum. Funct Plant Biol 38: 993–1003 [DOI] [PubMed] [Google Scholar]
- Ramirez J, Jarvis A. (2008) High resolution statistically downscaled future climate surfaces. Center for Tropical Agriculture, CGIAR Research Program on Climate Change, Agriculture, and Food Security. http://www.ccafs-climate.org/data/ (August 2012)
- Rebetzke GJ, López-Castañeda C, Botwright Acuña TL, Condon AG, Richards RA. (2008a) Inheritance of coleoptile tiller appearance and size in wheat. Aust J Agric Res 59: 863–873 [Google Scholar]
- Rebetzke GJ, van Herwaarden AF, Jenkins C, Weiss M, Lewis D, Ruuska S, Tabe L, Fettell NA, Richards RA. (2008b) Quantitative trait loci for water-soluble carbohydrates and associations with agronomic traits in wheat. Aust J Agric Res 59: 891–905 [Google Scholar]
- Reynolds M, Balota M, Delgado M, Amani I, Fischer R. (1994) Physiological and morphological traits associated with spring wheat yield under hot, irrigated conditions. Funct Plant Biol 21: 717–730 [Google Scholar]
- Reynolds MP, Manes Y, Izanloo A, Langridge P. (2009) Phenotyping approaches for physiological breeding and gene discovery in wheat. Ann Appl Biol 155: 309–320 [Google Scholar]
- Reynolds MP, Pierre CS, Saad ASI, Vargas M, Condon AG. (2007) Evaluating potential genetic gains in wheat associated with stress-adaptive trait expression in elite genetic resources under drought and heat stress. Crop Sci 47: S-172–S-189 [Google Scholar]
- Reynolds MP, Rebetzke G. (2011) Application of plant physiology in wheat breeding. In AP Bonjean, WJ Angus, M Van Ginkel, eds, The World Wheat Book: A History of Wheat Breeding, Vol 2. Editions TEC, Paris, pp 877\x{2013}906
- Reynolds MP, Tuberosa R. (2008) Translational research impacting on crop productivity in drought-prone environments. Curr Opin Plant Biol 11: 171–179 [DOI] [PubMed] [Google Scholar]
- Richards RA, Lukacs Z. (2002) Seedling vigour in wheat: sources of variation for genetic and agronomic improvement. Aust J Agric Res 53: 41–50 [Google Scholar]
- Richards RA, Rawson HM, Johnson DA. (1986) Glaucousness in wheat: its development and effect on water-use efficiency, gas exchange and photosynthetic tissue temperatures. Funct Plant Biol 13: 465–473 [Google Scholar]
- Ristic Z, Bukovnik U, Vara Prasad PV, West M. (2008) A model for prediction of heat stability of photosynthetic membranes. Crop Sci 48: 1513–1522 [Google Scholar]
- Sage RF. (2002) Variation in the k(cat) of Rubisco in C(3) and C(4) plants and some implications for photosynthetic performance at high and low temperature. J Exp Bot 53: 609–620 [DOI] [PubMed] [Google Scholar]
- Saint Pierre C, Crossa J, Manes Y, Reynolds MP. (2010) Gene action of canopy temperature in bread wheat under diverse environments. Theor Appl Genet 120: 1107–1117 [DOI] [PubMed] [Google Scholar]
- Salvucci ME. (2008) Association of Rubisco activase with chaperonin-60beta: a possible mechanism for protecting photosynthesis during heat stress. J Exp Bot 59: 1923–1933 [DOI] [PubMed] [Google Scholar]
- Salvucci ME, Crafts-Brandner SJ. (2004) Inhibition of photosynthesis by heat stress: the activation state of Rubisco as a limiting factor in photosynthesis. Physiol Plant 120: 179–186 [DOI] [PubMed] [Google Scholar]
- Shepherd T, Griffiths DW. (2006) The effects of stress on plant cuticular waxes. New Phytol 171: 469–499 [DOI] [PubMed] [Google Scholar]
- Stone P, Nicolas M. (1995a) Effect of timing of heat stress during grain filling on two wheat varieties differing in heat tolerance. I. Grain growth. Funct Plant Biol 22: 927–934 [Google Scholar]
- Stone P, Nicolas M. (1995b) A survey of the effects of high temperature during grain filling on yield and quality of 75 wheat cultivars. Aust J Agric Res 46: 475–492 [Google Scholar]
- Tetlow IJ, Wait R, Lu Z, Akkasaeng R, Bowsher CG, Esposito S, Kosar-Hashemi B, Morell MK, Emes MJ. (2004) Protein phosphorylation in amyloplasts regulates starch branching enzyme activity and protein-protein interactions. Plant Cell 16: 694–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy SR, Roberts JA, Black CR, McNeill A, Davidson R, Mooney SJ. (2010) The X-factor: visualizing undisturbed root architecture in soils using X-ray computed tomography. J Exp Bot 61: 311–313 [DOI] [PubMed] [Google Scholar]
- Tsunewaki K, Ebana K. (1999) Production of near-isogenic lines of common wheat for glaucousness and genetic basis of this trait clarified by their use. Genes Genet Syst 74: 33–41 [Google Scholar]
- Uauy C, Distelfeld A, Fahima T, Blechl A, Dubcovsky J. (2006) A NAC gene regulating senescence improves grain protein, zinc, and iron content in wheat. Science 314: 1298–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veraverbeke EA, Van Bruaene N, Van Oostveldt P, Nicolaï BM. (2001) Non destructive analysis of the wax layer of apple (Malus domestica Borkh.) by means of confocal laser scanning microscopy. Planta 213: 525–533 [DOI] [PubMed] [Google Scholar]
- Vijayalakshmi K, Fritz A, Paulsen G, Bai G, Pandravada S, Gill B. (2010) Modeling and mapping QTL for senescence-related traits in winter wheat under high temperature. Mol Breed 26: 163–175 [Google Scholar]
- Wang RX, Hai L, Zhang XY, You GX, Yan CS, Xiao SH. (2009) QTL mapping for grain filling rate and yield-related traits in RILs of the Chinese winter wheat population Heshangmai × Yu8679. Theor Appl Genet 118: 313–325 [DOI] [PubMed] [Google Scholar]
- Wardlaw IF. (1990) Tansley Review No. 27 The control of carbon partitioning in plants. New Phytol 116: 341–381 [DOI] [PubMed] [Google Scholar]
- Wardlaw IF, Wrigley CW. (1994) Heat tolerance in temperate cereals: an overview. Aust J Plant Physiol 21: 695–703 [Google Scholar]
- Wilkinson S, Davies WJ. (2002) ABA-based chemical signalling: the co-ordination of responses to stress in plants. Plant Cell Environ 25: 195–210 [DOI] [PubMed] [Google Scholar]
- Wilkinson S, Davies WJ. (2010) Drought, ozone, ABA and ethylene: new insights from cell to plant to community. Plant Cell Environ 33: 510–525 [DOI] [PubMed] [Google Scholar]
- Wilson D, Jones JG. (1982) Effect of selection for dark respiration rate of mature leaves on crop yields of Lolium perenne cv. S23. Ann Bot (Lond) 49: 313–320 [Google Scholar]
- Xue G-P, McIntyre CL, Jenkins CLD, Glassop D, van Herwaarden AF, Shorter R. (2008) Molecular dissection of variation in carbohydrate metabolism related to water-soluble carbohydrate accumulation in stems of wheat. Plant Physiol 146: 441–454 [DOI] [PMC free article] [PubMed] [Google Scholar]