CYCLES ALTERED BY HUMAN ACTIVITY: IMPACTS ON THE AVAILABILITY AND STOICHIOMETRY OF NUTRIENTS
The impact on nitrogen (N) and phosphorus (P) cycles of human activity is a growing concern and has several causes and consequences (MacDonald et al., 2011; Peñuelas et al., 2012; Sardans et al., 2012b). Carbon (C) inputs by human CO2 emissions and N inputs from diverse human-driven sources are continuously increasing (Peters et al., 2011; Peñuelas et al., 2012). On the other hand, anthropogenic biospheric inputs of P are increasing much less than emissions of N and P (Peñuelas et al., 2012). These changes impact all ecosystems, including cropland (MacDonald et al., 2011), and seem to lead to shifts in C-N-P ratios and balances (Mackenzie et al., 2002; Peñuelas et al., 2012), with significant impacts on the structures and functions of ecosystems through effects on growth rates and on the competitive abilities of different species (Sterner and Elser, 2002; Peñuelas et al., 2012). Furthermore, increased warming, drought, and concentrations of atmospheric CO2 also change the N and P contents and stoichiometry of plants (Reich et al., 2006; Funk and Vitousek, 2007; Elser et al., 2010; Rivas-Ubach et al., 2012) and, therefore, can indirectly impact soil processes and nutrient availability and stoichiometry. These increases also influence ecosystemic structures and functions and the capacity of Earth to balance its levels of CO2, given the importance of nutrients in the efficiency of plants to take up CO2 (Vicca et al., 2012).
Invasion by plant species, another driver of global change, is also strongly related to the availability and stoichiometry of soil nutrients in most cases (Davis et al., 2000; Chun et al., 2007; Dassonville et al., 2007, 2008; González et al., 2010) and exerts an additional effect on the availability and stoichiometry of N and P in ecosystems and frequently interacts significantly with other drivers of global change, such as N deposition (Huebner et al., 2009; He et al., 2011).
Plants respond to drivers of global change by several metabolic and physiological shifts that frequently alter, among several other functions, a plant’s capacity to take up and reallocate nutrients and, consequently, the elemental composition and stoichiometry of plants. Plants are thus the main factor underlying the links between global change and the status of N and P in ecosystems. This role of plants is critical to Earth’s N and P biogeochemical cycles and to the changes occurring in these cycles. We urgently need to review our knowledge of the role that plant responses to global change plays in changing and/or buffering the availability and stoichiometry of nutrients in ecosystems.
In this Update, we discuss (1) the effects of plants on the availability and stoichiometry of nutrients in ecosystems through their responses to drivers of global change at the individual, population, and community levels, (2) the consequences and feedback mechanisms occurring from changes in the availability and stoichiometry of nutrients in terrestrial ecosystems, and (3) the key aspects we need to investigate to reach a global understanding of the links among drivers of global change, plant responses, and the availability and stoichiometry of nutrients.
INCREASED CONCENTRATIONS OF ATMOSPHERIC CO2
Meta-analyses of herbaria studies and field experiments have observed that elevated concentrations of atmospheric CO2 tend to decrease N (Peñuelas and Matamala, 1990; Sardans et al., 2012b) and P (Peñuelas and Matamala, 1990, 1993; Sardans et al., 2012b) in plant tissues. Foliar C-N and C-P ratios have usually increased as a consequence (Gifford et al., 2000; Tognetti and Peñuelas, 2003; Sardans et al., 2012b). Under high concentrations of atmospheric CO2, the most commonly observed plant response, especially in C3 plants, is an increase in C uptake that frequently leads to an increase in growth (Newton et al., 1995; Reich et al., 2006). Increased concentrations of atmospheric CO2 also lead to reduced transpiration (Niklaus et al., 2001; Del Pozo et al., 2007), because the uptake of CO2 is possible with higher stomatal closure (Samarakoon and Gifford, 1995), which can further limit the capacity to take up N (Berntson, 1994; Pritchard and Rogers, 2000; BassiriRad et al., 2001) and can lead to a progressive limitation of nutrients that can quickly limit the initial increase in plant production under elevated concentrations of atmospheric CO2 (Murray et al., 2000; Zak et al., 2003; Luo et al., 2004; Newton et al., 2010; Norby et al., 2010).
The capacity to enhance plant growth can be further increased by external inputs of nutrients. The current increases in N deposition in several regions of North America and Europe increase the capacity of further plant growth (Finzi et al., 2007). In this case, the increase in plant growth is fundamentally due to an increase in N uptake but not to an improvement in the efficiency of use of N (Finzi et al., 2007). Some experiments of N fertilization have not observed significant increases of plant growth and nutrient cycling under increased atmospheric CO2 concentrations (Lagomarsino et al., 2008).
Plants also tend to take up more N, which can reduce the availability of soil N (Norby et al., 1999; Hovenden et al., 2008; Garten et al., 2011), an effect further enhanced by the frequent increase in N uptake by microbes in response to increased levels of CO2 (Zak et al., 2000; Dijkstra et al., 2010a). The large uptake of N by plants and the decrease of N in soil can prevent losses of N from ecosystems by preventing leaching (Johnson et al., 2004). Plants, however, respond with a series of metabolic and physiological mechanisms for increasing their capacity to take up N in a scenario of high levels of atmospheric CO2. Changes in the structure of mycorrhizal communities and/or increases in mycorrhizal biomass frequently occur (Díaz, 1995; Treseder, 2004; Gamper et al., 2005; Olsrud et al., 2010; Fransson, 2012). Plants also increase their allocation of N to root structures (Norby et al., 1999; Arnone et al., 2000; Norby and Iversen, 2006), including the increase of root exudates that increase the capacity for nutrient chelation (Phillips et al., 2011; Fransson, 2012). An increase in the efficiency of N use is generally observed (Taub and Wang, 2008; Dijkstra et al., 2010a, 2010b) due to these mechanisms, which include an increase in internal plant remobilization (Peñuelas and Estiarte, 1997). An increase in nitrogen fixation is also observed as a short-term response to increases in concentrations of atmospheric CO2 (Reich et al., 2006), but the limitation of other elements such as P and potassium (K) limit the increase in the capacity of ecosystems to fix N2 (Reich et al., 2006; Tobita et al., 2010). The possibility of community invasion by fixers of N2 can increase if levels of atmospheric CO2 rise (Zanetti et al., 1996; Polley et al., 1997).
A follow-on consequence of all these processes is a progressive change in the proportions of individuals of different species of the plant community due to variable capacities of the various species with different ecological strategies, phylogenies, successional stages (Arnone et al., 2000; Handa et al., 2008), or life stages (Shimono and Bunce, 2009). Young plants have a higher capacity to improve uptake than plants in reproductive stages (Shimono and Bunce, 2009). All these mechanisms of increases in nutrient use efficiency and/or uptake in response to higher concentrations of atmospheric CO2 are strongly dependent on soil nutrients and water sources and resources. Nutrient-poor soils may limit the capacity of plants to increase their root production in response to atmospheric concentrations of CO2 and, consequently, limit the capacity to absorb nutrients (Johnson et al., 2006). The capacity to invest to increase nutrient uptake is also limited in arid environments. Clark et al. (2009) observed no changes in mycorrhizal infection in plants growing in the Mojave Desert in response to increased atmospheric CO2 concentration. The capacity of plants to increase their growth by a higher uptake or use efficiency of N is very dependent on the chemical form of the source of N. In general, NH4+ is preferable to NO3− because the allocation of N in plants to nitrate reductase activity must compete with the allocation of other factors (Bauer and Berntson, 2001; Cruz et al., 2003). If most of the N uptake is in the NH4+ form, plants do not need to allocate N to nitrate reductase. A negative feedback can be produced, because increased concentrations of CO2 can increase soil nitrification (Azam et al., 2005). On the other hand, higher levels of soil moisture from reduced transpiration should prevent soil nitrification, as observed in some studies (Cheng et al., 2012), contributing to diminished uptake of NO3− by plants and favoring NH4+ uptake. These processes appear to contribute to a better use of N in the ecosystem.
The decrease in plant transpiration is frequently related to higher soil-water contents (Ebersberger et al., 2003; Staddon et al., 2004). Higher soil-water content, litter production, and root allocation are related to the higher activities of soil enzymes frequently observed under high concentrations of atmospheric CO2 (Ebersberger et al., 2003; Lipson et al., 2006; Jin and Evans, 2007). Higher fungal biomass is also a trait related to higher decomposition rates of soil organic carbon observed in some soils subjected to elevated concentrations of atmospheric CO2 (Lipson et al., 2006). Despite the frequent lower quality of litter resulting from higher C-N ratios and higher concentrations of C-based secondary compounds (Peñuelas et al., 1997; Aerts et al., 2012), these litter changes do not decrease soil organic matter (SOM) or litter decomposition (Zak et al., 2003; Hyvönen et al., 2007; Aerts et al., 2012), which have even been found to increase in several studies (Billings and Ziegler, 2005; Cotrufo et al., 2005; Jin and Evans, 2007). Changes in mineralization under high concentrations of atmospheric CO2 are very dependent on the species of plant (Finzi et al., 2006; Austin et al., 2009).
Thus, increased levels of CO2 concentration lead to high plant production capacity (C3 plants) and lower transpiration. Plants respond by increasing the resource allocation to mechanisms involved in N and P uptake. Nevertheless, plant C-N and C-P ratios tend to increase (Table I). At the end, however, an acclimation effect of plants is observed at medium and long terms, thus diminishing the previous trends. Further research is necessary to reach a more conclusive view of the impacts on N-P ratios of plants and soils.
Table I. Summary of the main results of the studies on plant responses to global change drivers and the flow-on effects on soil fertility and stoichiometry.
The necessary future research to advance in a global overview of this topic is highlighted in the last column.
| Global Change Drivers | Plant Responses | Changes in Soil Nutrient Availability and Stoichiometry | Further Research | |
|---|---|---|---|---|
| Increased atmospheric CO2 concentrations | ↑Plant production capacity at short term (C3 plants), and acclimation capacity at medium and long term | Habitually no observed changes in soil fertility | Effects on N-P stoichiometry | |
| ↓Transpiration | ↑Soil C-N and C-P ratios | |||
| ↑Plant C-N and C-P ratio | ||||
| ↑Plant investment of resources in N and P uptake | ||||
| Warming (cold/wet environments) | ↑Plant production and growth (but the effects can be negative in short, extreme warming events) | ↑Of nutrient cycling, by higher mineralization rates and soil enzyme activity counteracting the negative effect of litter production with high C-N and C-P ratios | Study of the possible different effects on N than on P cycling | |
| ↑Plant C-N and C-P ratios | Study of the effects of increasing frequency of warming events during winter in temperate and cold ecosystems | |||
| ↑Plant investment of resources in N and P uptake | ||||
| Warming (hot/dry environments) and drought | ↑Growth | ↓Soil enzyme activity and nutrient cycling | The role of plant roots in the mechanism of water transport from deep soil layers to upper soil layers | |
| ↑Investment in increasing WUE and in N and P uptake | ↑C-N and C-P ratios | |||
| ↑Plant C-N and C-P ratios by increase in the presence of C-rich structures and compounds linked to water stress avoidance | ↓Decrease of N and P availability | |||
| No significant effects on plant N-P ratios | ↑N and P occuled fractions | |||
| ↑Risk of N and P losses by torrential rainfalls | ||||
| N eutrophication | ↑Aboveground growth and plant competition intensity | ↑P availability, increasing P-limiting role | The impacts of N deposition through its role by increasing plant and soil N-P ratios | |
| ↑Investment in P uptake | ↑N-P ratio | |||
| ↑N-P ratios | ↑Soil phosphatase activity and soil respiration | |||
| ↓Root uptake capacity under high levels of N saturation | ↑Accumulation of recalcitrant C | |||
| ↑N leaching | ||||
| Species invasion | In nutrient-rich soils, high invasive success of species with higher growth rates, low C-N and C-P ratios, fast plasticity in resource acquisition capacity, and high reproductive investment | ↑Of N and P soil concentrations and availability as well as cycling, particularly in rich nutrient soils | The role of N-P ratios (of plant and soil) in invasive success | |
| In nutrient-poor soils, high invasive success related to conservative use of resources, including nutrients | ||||
| Communities dominated by legumes growing in soils with limited availabilities of soil P or water are vulnerable to competition by nonfixing plants with high capacities of P uptake or stomatal control, and low availabilities of P reduce the probability of successful invasion by N2-fixing plants |
WARMING
The Contrast between Cold/Wet and Hot/Dry Ecosystems
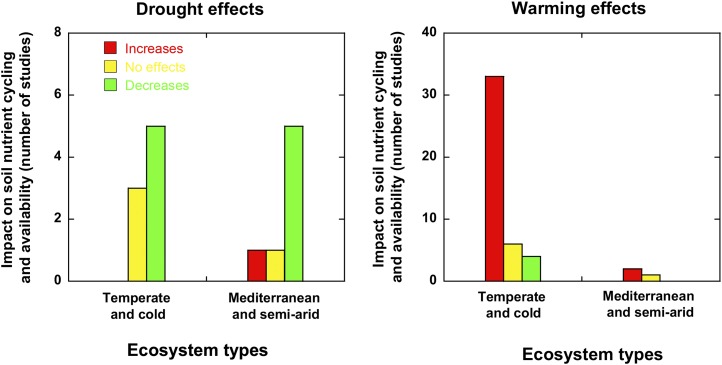
An overview of the information currently available leads to the general conclusion that the responses of plants to warming strongly depend on the indirect effect of warming on soil moisture (Schmidt et al., 2004; Aerts, 2006; Sardans et al., 2012b). In cold and temperate ecosystems, especially in wet conditions, the reduction of soil moisture by warming is insufficient to counteract the enhancement of plant activity in response to rises in temperature. In these conditions, plants increase their biological activity by several mechanisms, such as lengthening the growth and active annual periods (Farnsworth et al., 1995; Peñuelas et al., 2004) and increasing photosynthetic rates (Wookey et al., 1995), vegetative growth (Wookey et al., 1995; Hobbie and Chapin, 1998; Suzuki and Kudo, 2000; Cole et al., 2002; Peñuelas et al., 2004; Day et al., 2008; Olsrud et al., 2010; Melillo et al., 2011; Dreesen et al., 2012), reproductive output (Wookey et al., 1995), and seed reservoirs (Blödner et al., 2007). These increases in photosynthesis activity can be further enhanced under increased atmospheric CO2 concentrations such as observed in some experiments coupling field manipulations of temperature and atmospheric CO2 concentrations (Albert et al., 2011). However, the effects of warming and elevated atmospheric CO2 on other ecosystem processes can be different. For example, Dijkstra et al. (2010a) observed an increase in soil N availability under warming and a decrease in N content (due to the enhancement of N uptake by microbes) that, when acting together, tend to compensate their effects on soil N. On the other hand, nutrient mineralization, cycling rates, and availability frequently increase under warming (Fig. 1). Despite these increases, the enhancement of plant activity and growth involves a dilution of nutrients that leads to frequent decreases in N concentrations in leaves and litter (Suzuki and Kudo, 2000; Shen et al., 2009) and increased C-nutrient ratios in plant tissues and litter (Hyvönen et al., 2007; Sardans et al., 2012b, 2012c). At the individual and community levels, plants respond by increasing nutrient uptake, mycorrhizal symbiosis (Clemmensen et al., 2006; Olsrud et al., 2010), root growth (Volder et al., 2007), and N2 fixation (Sorensen and Michelsen, 2011). These plant responses lead to an increase in nutrient uptake under warming (Wookey et al., 1995; Dijkstra et al., 2010a; Olsrud et al., 2010). The responses of plants to continuous warming generally tend to be limited by the availability of N (Jonasson et al., 2004; Majdi and Öhrvik, 2004) and/or P (Jonasson et al., 2004) in cold and temperate regions (Jarvis and Linder, 2000).
Figure 1.
Impact of drought and warming on rates of soil nutrient cycling and availability. Detailed results and bibliographic references are provided in Supplemental Table S1.
These limitations can be reduced by N deposition, as observed in regions of Europe and North America (Aerts et al., 1992; Majdi and Öhrvik, 2004; Clemmensen et al., 2006). The most direct consequence of the rapid response of plants to warming is the change in competitive relationships among plant species of a community (De Valpine and Harte, 2001). Plant species adapt differently to the new conditions by species-specific differences in metabolism (Schuster and Monson, 1990), phenology (Dreesen et al., 2012), or reproductive strategy (Moulton and Gough, 2011). Reductions in the rates of photorespiration in C3-C4 intermediate-metabolism plants create advantages in photosynthetic capacities at warmer foliar temperatures than in C3 plants, which can only enhance photosynthetic efficiency under warming at substantial costs in water and nutrient use efficiency (Schuster and Monson, 1990). In cold environments, increases in the availability of soil nutrients through enhanced decomposition of SOM and the capacity for plant production favor plants that mainly reproduce sexually (Moulton and Gough, 2011), which frequently leads to a competitive advantage of higher plants over lichens (Moulton and Gough, 2011).
Plants can sometimes respond negatively to warming even in cold and temperate environments. During warming events, an extreme winter snow cover in the Arctic can disappear, and freezing temperatures can reduce a plant’s potential to grow and reproduce the following summer (Bokhorst et al., 2011). In temperate grassland, the large frequency of freeze-thaw cycles has proved to increase the N leaching effect linked to higher water penetration and leaching capacity (Joseph and Henry, 2008). This loss of N can be associated with the increases of plant C-N ratios and growth decreases associated with the increased frequency of freeze-thaw cycles (Kreyling et al., 2010). Moreover, some Alpine plants have roots that grow upward to the snow cover to acquire nitrogen, thereby compensating for the very short season for nutrient uptake (Onipchenko et al., 2009). Plants highly dependent on this strategy can be particularly threatened under a scenario of rapid warming that shortens the period of snow cover. All these sources point at the importance of single events and, by the impacts of extreme warming events, impacts on snow cover and temperature variability in winter.
In contrast, warming in dry environments, such as those of continental, Mediterranean, and dry tropical biomes, can increase soil drought, exacerbating limitations of water and nutrients (Link et al., 2003; Allison and Treseder, 2008). Plants respond by activating mechanisms for water conservation, which can frequently increase C-nutrient ratios in photosynthetic tissues (Sardans et al., 2008b, 2008c, 2012a).
In response to warming, leaves and litter of plants thus tend to increase C-nutrient ratios by the dilution effect and/or by increases in nutrient use efficiency (An et al., 2005) in temperate and cold ecosystems not limited by water and by conservative mechanisms related to the avoidance of water stress in dry areas (Sardans et al., 2012b). Nevertheless, these increased C-nutrient ratios may not be observed in the short and medium terms in cold and temperate sites, because the higher temperatures can increase respiration and enhance soil activity, thus releasing more nutrients, although respiration subsequently tends to acclimate to the new situation (Atkin et al., 2000; Hartley et al., 2006). Anyway, climate-manipulation studies in high-latitude ecosystems report increases (12% on average) in the C-N ratio of litter under warmings ranging from 1°C to 4°C (Cornelissen et al., 2007; Aerts et al., 2012), showing that increases of C-N ratios can be a frequent effect of warming.
Effects on the Availability and Stoichiometry of Soil Nutrients
Along with the responses of plants to warming, soil enzyme activity, SOM decomposition, and rates of nutrient cycling and mineralization tend to change in a direction mainly determined by the impact of warming on soil moisture (Peñuelas et al., 2004; Aerts, 2006; Allison and Treseder, 2008), changes in litter quantity, and the composition and activity of soil microorganisms.
In cold and temperate ecosystems not limited by water, warming also tends to increase nutrient release and availability for plants, despite frequent increases in C-nutrient ratios in litter (Luo, 2007; Fig. 1). Warming frequently increases soil enzyme activities (Bell et al., 2010; Dreesen et al., 2012), soil respiration (Updegraff et al., 2001; Biasi et al., 2008), nutrient mineralization, soil-cycle rates (Fig. 1), and SOM decomposition (Aerts, 2006; Hyvönen et al., 2007; Von Lützow and Kögel-Knabner, 2009). Plants, though, must compete more strongly with microbes for nutrients (Jonasson et al., 2004; Dijkstra et al., 2010b), because the warmer temperatures enhance the activities of the microbes (Von Lützow and Kögel-Knabner, 2009). Nonetheless, plants frequently enhance their uptake of nutrients (Cole et al., 2002). In the long term, when vegetation has a large capacity to take up nutrients, for example in a young forest in its growing stage, this increase in the source of nutrients can imply an increase in leaf concentrations of N (Butler et al., 2012). On the other hand, this increase in the release of soil nutrients can increase leaching to streams (Schmidt et al., 2004). Fewer studies have investigated the effects of warming on soil processes in dry areas than in temperate and cold environments not limited by water. In boreal and dry mountainous and tropical dry areas, the effects of warming are opposite to those in areas not limited by water; soil enzyme activities (Allison and Treseder, 2008), soil respiration (Lellei-Kovacs et al., 2008), and N availability are decreased (Fig. 1).
Some studies have observed that N mineralization is enhanced more rapidly than P mineralization under warming (Rinnan et al., 2007), observing that warming increases more in the N cycle than the P cycle (Sardans et al., 2012b). These results support studies of foliar stoichiometry under natural gradients that have observed positive correlations between temperature and foliar N-P ratios (De Frenne et al., 2011; Kang et al., 2011). In scenarios of rapid rises in temperature, plants in particular and ecosystems in general can respond more quickly and strongly to increases in N availability than to enhanced P availability due to increases in N2 fixation or decreases in denitrification resulting from enhanced evapotranspiration, among other factors. This possibility of increases in soil N-P ratios in the short term can lead to a more persistent rise in N-P ratios if warming continues to increase. Experimentation to specifically study and respond to this question is warranted, because the soil N-P ratio is a key factor in the structures and functions of ecosystems (Sardans et al., 2012c).
Summarizing, in wet/cold ecosystems, warming enhances plant production and growth, increasing the investment in N and P uptake and generally increasing plant C-N and C-P ratios. In spite of the higher litter C-N and C-P ratios, increases in mineralization and nutrient release from soil organic matter are observed as a result of the increases in soil biological activity and, frequently, the higher water availability by increasing defrost. The possible different effects of warming in N and P cycles remain to be clarified. There are some reports suggesting that warming can have an additional impact by the increases of warming periods during winter in cold and temperate ecosystems. All these aspects warrant future research. Contrarily, in hot/dry environments, the effects of warming are determined by the impacts of changing water availability.
DROUGHT
Shifts in Elemental Composition
Prolonged water stress frequently translates into a reduction of aboveground plant growth (Inclán et al., 2005; Xu and Zhou, 2006; Ogaya and Peñuelas, 2007; Prieto et al., 2009; Jyske et al., 2010; Wu et al., 2011b), but plants respond by trying to increase water uptake and enhancing conservative mechanisms to improve water use efficiency (WUE). Single drought events also initiate plant physiological responses that frequently result in changes in soil activity, affecting ecosystem nutrient cycling that allows the survival of the plant community and thereafter the return to initial conditions (Jentsch et al., 2011; Lloret et al., 2012).
Drought increases the production of abscisic acid, the accumulation of water in plant tissues (Radin and Ackerson, 1981; Wittenmayer and Merbach, 2005; Planchet et al., 2011), and mechanisms to protect the PSII enzymatic system based on pigments and antistress enzymes (Munné-Bosch and Lalueza, 2007; Zhang et al., 2011). All these changes tend to reduce photosynthetic efficiency (Munné-Bosch and Lalueza, 2007). Abscisic acid coordinates the protective responses of plants to drought, mainly by increasing stomatal closure, decreasing foliar surface areas, and increasing root-shoot ratios through the allocation of more resources to the root system (Bahrun et al., 2002; Wittenmayer and Merbach, 2005). Increased synthesis of antioxidant enzymes such as superoxide dismutase is characteristic of plants under drought (Ruiz-Lozano et al., 1996). Proteomic and metabolomic analyses have discerned the stepwise changes in N use and allocation in plants responding to drought. Rubisco is hydrolyzed, and N is further allocated to the synthesis of amino acids with osmoprotective functions (Aranjuelo et al., 2011). These “omic” techniques should be used to study shifts in the metabolic use of other nutrients, such as P.
Coinciding with these general physiological responses, an increase of mycorrhizal symbiosis under drought is frequently observed in field studies (Ruiz-Lozano et al., 1995; Davies et al., 1996; Al-Karaki and Al-Raddad, 1997; Shi et al., 2002; Roldán et al., 2008). The enhancement of mycorrhizal symbiosis improves the capacity of plants to take up both water and nutrients (Ruiz-Lozano et al., 1995; Alvarez et al., 2009; Wu et al., 2011a). Drought can also increase the allocation of resources to roots (Huang, 2001; Wittenmayer and Merbach, 2005; Suralta, 2010), the acceleration of turnover of fine roots (Meier and Leuschner, 2008), and an increased production of root exudates, contributing to increased chelation and uptake of nutrients (Henry et al., 2007; Kohli et al., 2012). Mycorrhization is positively correlated with an enhanced antioxidant metabolism in plants (Ruiz-Lozano et al., 1996).
In addition to activating mechanisms for improving the capacity to take up water, plants respond to drought by enhancing mechanisms for conserving water and nutrients, such as the retranslocation of nutrients prior to tissue senescence (Heckathorn and DeLucia, 1994, 1996; Correia and Martins-Loução, 1997; Milla et al., 2005) and the internal remobilization of nutrients dependent on the availability of water (Sardans and Peñuelas 2012a). As observed in plants adapted to severe periods of drought, P tends to remobilize from leaves to roots, whereas K has the opposite movement, as observed in Mediterranean trees during summer drought (Milla et al., 2005; Sanz-Pérez et al., 2009; Sardans et al., 2012a). Plants with the genotypic capacity to maintain N, P, and K concentrations and contents under drought have a competitive advantage if drought persists or becomes more severe (Ghandilyan et al., 2009). Plants adapted to drought are able to raise the concentration of N in leaves (Inclán et al., 2005; Weih et al., 2011).
Different metabolic systems of CO2 uptake can provide differences among plants competing under drought. C4 plants maintain higher photosynthetic rates than C3 plants under drought conditions (Taylor et al., 2011). Plants with Crassulacean acid metabolism respond very efficiently to drought even in nutrient-poor soils (Maiquetía et al., 2009).
Drought reduces net photosynthesis and growth as well as the capacity to take up N and P from soil (Chidumayo, 1994), but these effects are ameliorated if N, P, and/or K availability is high, leading to higher root proliferation, water uptake, and WUE (Radin and Ackerson, 1981; DeLucia and Schlesinger, 1991; Borch et al., 2003; Garg et al., 2004; Waraich et al., 2011). Higher concentrations of P in plants improve WUE because they allow a minor suppression of photosynthesis per unit of water transpired (Singh et al., 2000; Jones et al., 2005) and improve the capacity of stomatal control (Waraich et al., 2011). Enzymes of N metabolism play an important role in the acclimation to drought (Xu and Zhou, 2006). Most studies show that decreases in soil moisture also reduce N and/or P uptake by plants (Jupp and Newman, 1987; Sardans et al., 2005; Sardans and Peñuelas, 2007; Cramer et al., 2009; Waraich et al., 2011), suggesting a negative feedback of less water and fewer nutrients on the production capacity and fitness of plants when drought persists or becomes more severe. As a result, plants under drought tend to produce leaves and litter with high C-nutrient ratios (Yarie and Vancleve, 1996; Sardans et al., 2008b; Limousin et al., 2010). The few studies of the specific effects of drought on the N-P ratio in plants, however, have not provided a clear conclusion (Sardans and Peñuelas, 2008; Sardans et al., 2008c, 2012b), in spite of studies in gradients of water availability in Mediterranean regions that have observed a negative correlation between water supply and leaf N-P ratio (Sardans et al., 2011), an effect linked to higher growth capacity in plants with lower N-P ratios (Sardans and Peñuelas, 2012b). Moreover, drought limits the capacity to fix N2 by limiting the metabolic capacity of bacteria and by generating oxidative damage (Streeter, 2003; Naya et al., 2007; Franzini et al., 2010; Aranjuelo et al., 2011), which further decrease the N content of ecosystems. The responses of plants to drought in cold ecosystems, where temperature, light irradiation, and soil nutrients are the main limiting resources, cannot always be detected and may even have effects opposite to those discussed above (Yarie and van Cleve, 1996; Nilsen et al., 1998).
Effects on the Availability and Stoichiometry of Nutrients and on Primary Production and Community Structure
Drought can affect soil activity and fertility as a direct effect of low soil water content and changes in plants. The most common effect of drought is a decrease in soil biological activity, including decreases in soil enzyme activity (Lorenz et al., 2001; Yavitt et al., 2004; Henry et al., 2005; Sardans and Peñuelas, 2005, 2010; Sardans et al., 2006, 2008a, 2008d), mineralization (Fig. 1), and SOM decomposition (Van Meeteren et al., 2008; Sanaullah et al., 2012) that frequently decrease the availability of soil nutrients (Fig. 1). These effects are due most frequently to the decrease in soil moisture (Sardans et al., 2008a; Sardans and Peñuelas, 2010) but also to decreases in food quality and the nutrient contents of leaves and litter (Sardans et al., 2008d; Matías et al., 2011). These effects frequently lead to increases in recalcitrant forms of soil nutrients and decreases in forms available to plants (Sardans and Peñuelas, 2004; Sardans et al., 2006, 2008d, 2008e). As far as we know, only one study has detected increases in soil mineralization under drought (White et al., 2004).
The increase in SOM and nutrient stocks in soils under drought can increase the risk of nutrient losses from the ecosystem, mainly if drought is accompanied by more frequent and intense torrential rainfall, as expected in some areas such as the Mediterranean basin (Sardans and Peñuelas, 2012a; Zaimes et al., 2012). Field studies that experimentally manipulate climate have observed that decreases in soil-water content at a level projected by most climatic models have led to the loss of N from the ecosystem (Ogaya and Peñuelas, 2009). Furthermore, rewetting events in a scenario of increasing drought can further increase the loss of nutrients from the ecosystem; sudden increases of more soluble forms of N in soil have been observed after rewetting events (Ryan et al., 1998). Prolonged drought coupled to less predictable and intense torrential rainfall (Frei et al., 1998) opens a scenario of shifts in the feedbacks and equilibria within the plant-soil system in some semiarid areas such as the Mediterranean region. The most threatening phenomenon for Mediterranean soils, especially in the most xeric areas, is desertification linked to a continual positive feedback: higher frequency and intensity of torrential rainfall and longer, more severe drought periods (both associated with climatic change) with increased soil erosion, which in turn leads to a loss of soil fertility and thus plant cover (Garcia et al., 2002; Moreno-de las Heras et al., 2011; Ruiz-Sinoga et al., 2011, 2012), a phenomenon that finally drives desertification. In this process, the most drought-resistant plants are selected and constitute patches in the middle of bare soil (Ochoa-Hueso et al., 2011; Ruiz-Sinoga et al., 2011). The soil variability of water and nutrient availability are related to the distribution of vegetation patches with respect to bare soil patches, which can be considered as runoff sinks and sources, respectively (Boix-Fayos et al., 1998; Kutiel et al., 1998; Mayor et al., 2009; Ruiz-Sinoga and Martínez Murillo, 2009; Gabarrón-Galeote et al., 2012; Mayor and Bautista, 2012; Merino-Martin et al., 2012). Thus, soil nutrients and water availability are higher in soils with higher infiltration capacity and biological activity that coincides with vegetation patches throughout the slopes (Maestre and Cortina, 2003; Agra and Ne’eman 2012).
In summary, drought reduces net photosynthesis and growth, but these effects are ameliorated if N, P, and/or K availability is high, which allows improving plant WUE by enhancing the capacity of stomatal control (Waraich et al., 2011). Most studies show that decreases in soil moisture also reduce N and/or P uptake by plants; as a result, plants under drought tend to produce leaves and litter with high C-nutrient ratios, with further consequences on litter decomposition by decreasing litter quality. These effects frequently lead to increases in recalcitrant forms of soil nutrients and to decreases of the forms available to plants (Table I). Moreover, deep root systems transfer water from deep to upper soil layers, potentially favoring the mineralization of leaf litter and improving the water status of the community species with superficial root systems (Filella and Peñuelas, 2003), which merits future research.
N EUTROPHICATION
Impacts on Plant Function, Chemical Composition, and Stoichiometry
Human activities have more than doubled the nitrogen inputs in terrestrial ecosystems (Matson et al., 1999; Peñuelas et al., 2012). Individual plants respond to N availability at metabolic and physiological levels. Plant species have an optimal level of N supply for growth and plant function (Baghour et al., 2000; Horchani et al., 2010). Plants also have a notable phenotypic plasticity for adapting to changes in N supply. For example, when the availability of N becomes limiting, high-affinity nitrate and ammonium transport systems are up-regulated and lateral root growth is stimulated (Quaggiotti et al., 2003; Remans et al., 2006; Engineer and Kranz, 2007; Krapp et al., 2011). About 3,000 genes in Medicago truncatula are up-regulated under N starvation (Ruffel et al., 2008), and the main enzymes of the N cycle (nitrite and nitrate reductases, Gln synthetase and dehydrogenase) are very sensitive to N supply (Horchani et al., 2010), indicating the importance that N uptake has for plants. N deficiency reduces foliar area and chlorophyll content, which negatively affect photosynthetic capacity and growth (Zhao et al., 2005).
Increases in N availability due to enhanced N deposition should have a rapid positive effect on plant growth and metabolism. The increases in plant growth under N deposition (Eisenlord and Zak, 2010; Bontemps et al., 2011) enhance the intensity of competition among plants (Friedrich et al., 2012). Ecological responses to increases in N supply, though, are more complex. Several other processes, such as plant competition, plant-herbivore and plant-fungus relationships, and plant-soil feedbacks, among others, are involved in the responses of plants to N deposition in field conditions (Gilliam, 2006). Increased concentrations of N in plants further raise plant respiration (Reich et al., 2008), along with increases in plant activity and the need for resources. Although increases in N supply and uptake result in increases in the uptake of other nutrients such as P, these other nutrients eventually tend to become progressively limiting (Fujita et al., 2010). An increase in phosphate activity in roots is a general plant response to increased P limitation under N deposition (Fujita et al., 2010). The availability and uptake capacity of P is thus a critical factor in the capacities of communities and individual plants to respond to N deposition (Johnson et al., 1999; Phoenix et al., 2003; Menge and Field, 2007; Blanes et al., 2012). Another observed plant response to enhanced P uptake under N deposition is the change of the mycorrhizal community from being specialists in N uptake to being specialists in P uptake (Lilleskov et al., 2002), although some studies have not observed clear changes in plant mycorrhizal infections (Wallenda and Kottke, 1998). In most cases, though, the increases in N availability under N deposition are associated with increases in concentrations of N in plants (Fenn et al., 1998; Baron et al., 2000; Peñuelas and Filella, 2001) and decreases in concentrations of P (Duquesnay et al., 2000; Kowalenko, 2006). Increases in the N-P ratio in plant tissues (Sardans et al., 2012c) and decreases in the C-N ratio (Sardans et al., 2012c) are consequently widely observed in most areas of North America and Europe under prolonged increases in N deposition. This increase in the plant N-P ratio further limits plant growth (Güsewell, 2005; Granath et al., 2012) and photosynthetic rates (Güsewell, 2005). Another frequent effect accompanying N deposition is an increase of root growth (Brunner and Godbold, 2007), but some studies have observed a reduction in root growth (Gundersen et al., 1998; Nadelhoffer 2000) and increases in fine-root turnover (Nadelhoffer 2000) and very frequently a lower root-shoot ratio (Leith et al., 1999).
All these responses are species specific and lead to varying capacities of adaptation to N deposition in the different species. Species with traits that enable better adaptation to P limitation are thus favored (Leith et al., 1999; Fujita et al., 2010; Blanes et al., 2012). The chemical form of the N supply is also important in plant responses. NH4+ generally has a larger effect than NO3− on plant responses (Sas et al., 2003; Cárdenas-Navarro et al., 2006; Horchani et al., 2010). Several species, such as Norway spruce (Picea excelsa), prefer ammonia to nitrate as a source of N (Kronzucker et al., 1997). Increases in the NH4+:NO3− ratio under N deposition have been observed in soils (Stevens et al., 2006; Sparrius et al., 2012). A recent review provides some evidence that the efficiency of carbon use by soil microbes increases with N deposition (Manzoni et al., 2012), suggesting that soil mineralization increases and microbes improve their uptake of nutrients, which, in turn, would increase the competition for nutrients between microbes and plants.
In sites with N saturation after long-term N deposition, plants can reduce the uptake capacity of roots. Lipson et al. (1996) observed that the Alpine herb Bistorta bistortoides decreases the capacity of its roots to take up N when the N stored in amino acids in rhizomes reaches certain levels. Despite the mechanisms of plants that increase the uptake of other resources or reduce the uptake of N under conditions of N deposition, most studies have observed a decrease in the C-N ratio of leaves and litter under N deposition (Aerts et al., 2012; Sardans et al., 2012b).
The plasticity of plants in reducing their uptake of N under high availabilities of N coupled with enhanced uptake of other resources, such as CO2, P, or K, should be studied. Metabolomic and transcriptomic techniques applied to field experiments are promising tools for advancing our understanding of these responses.
Effects on the Availability and Stoichiometry of Soil Nutrients
As a consequence of the increases in P uptake, the concentration of P in soils tends to decrease (Kowalenko, 2006), and increases in P limitation in plants are observed after long-term N deposition (Bragazza et al., 2004; Güsewell, 2004; Menge and Field, 2007; Lund et al., 2009; Sardans et al., 2012b) and in experiments simulating N deposition (Braun et al., 2010: Sardans et al., 2012b), which can lead to high N-P ratios in soils (Fenn et al., 1998; Manning et al., 2006; Braun et al., 2010). This P limitation can scale up through trophic webs and affect herbivores (Tao and Hunter, 2012). Despite these general trends of increased P limitation when N deposition strongly increases ecosystemic activity, including soil decomposition, ecosystems have large immobilized stocks of P in their soils, which can imply an increase in P availability, as observed in some European heathland (Jones and Power, 2012). Moreover, increases in the activities of soil phosphatases under N deposition have been observed in some studies (Johnson et al., 1998; Turner et al., 2002; Papanikolaou et al., 2010), where an increase in the allocation of P uptake by soil organisms further favors the uptake of P by plants. Increases in the P cycle in soil (Marklein and Houlton, 2012) and the uptake of P by plants, although generally insufficient to prevent the rise of plant and soil N-P ratios, can contribute to the retention of P in the ecosystem (Perring et al., 2008). The effects of N deposition on P limitation in areas with very N-poor soils can remain undetected, as in forests of the northeastern United States (Finzi, 2009; Groffman and Fisk, 2011) and some areas of Europe (Binkley and Högberg, 1997; Stevens et al., 2011). In these situations, an increase in C-N ratios in litter and soil can be observed (Jones et al., 2004; Stevens et al., 2011).
N deposition, in addition to frequently enhancing plant growth and N uptake, also increases the concentration of N in soil (Bobbink et al., 1998; Nissinen and Hari, 1998; Falkegren-Grerup and Diekmann, 2003; Robinson et al., 2004; Manning et al., 2006) and decreases soil C-N ratios, as observed in soil subjected to long-term N deposition (Vourlitis and Zorba, 2007; Vourlitis et al., 2007; Fang et al., 2009; Fahey et al., 2011; Sardans et al., 2012b) or to N fertilization that simulates N deposition (Britton et al., 2008; Esmeijer-Liu et al., 2009; Sardans et al., 2012b). A widely observed feedback effect is the increase in N mineralization in soil observed under N deposition (Carroll et al., 2003; Vourlitis and Zorba, 2007; Vourlitis et al., 2007; Holub and Záhora, 2008; Manning et al., 2008; Duprè et al., 2010), an effect related to the lower C-N ratio in litter under N deposition (Bergkvist and Folkeson, 1992; Tietema, 1998) that leads to an increase in N cycling (Mansson and Falkengren-Grerup, 2003; Michopoulos et al., 2004), nitrification (Nilsson et al., 2006), and N availability of soil (Manning et al., 2008; Duprè et al., 2010; Jones and Power, 2012; Phoenix et al., 2012). This increase in N availability is related to the loss of species diversity observed in some areas of Europe (Duprè et al., 2010) and is probably linked to the homogenization of N availability in soils with the loss of soil diversity in terms of fertility.
A general increase in soil respiration (Tietema, 1998; Allen and Schlesinger, 2004) and SOM decomposition is observed under N deposition (Waldrop et al., 2004a; Dalmonech et al., 2010), but a decrease in the mineralization of the most recalcitrant organic matter, such as lignin and phenolics, is also frequently observed (Magill et al., 1997; Saiya-Cork et al., 2002; Dijkstra et al., 2004; Sinsabaugh et al., 2005), together with a more rapid decomposition of litter (Saiya-Cork et al., 2002). All these effects strongly depend on the type of vegetation; N deposition increases the storage of C in soils when the vegetation’s litter has a natural low nutritional quality and decreases the storage when the nutritional quality is high (Waldrop et al., 2004b). Under N deposition, soil generally tends to accumulate highly recalcitrant forms of soil organic carbon, such as lignin, that are incompatible with high soil activity (Zeglin et al., 2007; Grandi et al., 2008). If the inputs of N are very high, however, soil activities can decay (Waldrop and Zak, 2006; Dalmomech et al., 2010). An increased P limitation can lead to a very high N-P ratio in litter. Güsewell and Freeman (2005) observed that N-P ratios over 22 can limit the decomposition of litter.
By increasing the N-P ratio of plants and soil, N deposition can favor species with slow rates of growth in the long term. Despite a possible initial enhancement of photosynthetic activity, the increasing stoichiometric mismatches, especially high N-P ratios, can lead to slow rates of growth and shifts in species composition (Granath et al., 2012; Sardans et al., 2012b, 2012c).
Some processes can contribute to the buffering of N saturation. Ecosystems saturated with N by N deposition frequently lose N through leaching to streams (Kristensen et al., 2004; Brookshire et al., 2007) and through denitrification (Chen et al., 2012). N deposition also tends to decrease N2 fixation (Compton et al., 2004; Jin et al., 2012), an effect probably associated with the increase in P limitation.
This overview of the current literature thus indicates that long-term N deposition in terrestrial ecosystems, despite general increases of N and P mineralization, leads to larger increases of N in soils than of other elements, to nutrient mismatches, and mainly to increases in N-P ratios in soils and plant tissues that threaten to decrease species diversity by favoring species adapted to P limitation and high N-P ratios (Table I). However, the further possible impacts of N deposition by changing N-P ratios of plants and soil remain mostly ignored.
SPECIES INVASION
The Role of Soil Nutrients in Alien Success
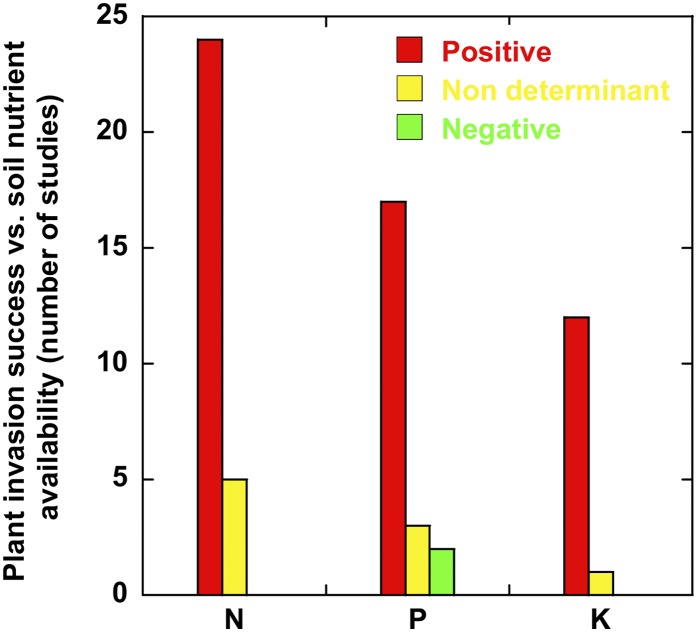
Invasions of alien plant species are currently increasing and are a serious threat to global plant diversity (Vitousek et al., 1987; Vitousek, 1990; Funk and Vitousek, 2007). Most studies that have investigated alien success have identified nutrient availability and the competitive capacities for nutrient uptake and for coping with low levels of nutrients as the key factors in explaining alien success (Fig. 2), factors that can be less important in dry environments as a result of the more limiting role of water (Drenovsky et al., 2012).
Figure 2.
Studies reporting information on the dependence of plant-invasion success on soil nutrient availability. Detailed results and bibliographic references are provided in Supplemental Table S2.
The physiological traits linked to alien success have also been widely studied, and some underlie the changes in nutrient stoichiometries of the plant community and soil (Levine et al., 2002; Daehler, 2003; Leger et al., 2007; González et al., 2010; van Kleunen et al., 2010; Scharfy et al., 2011). Several mechanisms involved in the uptake and use efficiency of nutrients are involved in alien success (Daehler, 2003; González et al., 2010). Low costs of foliar construction and higher phenotypic plasticity in taking up available nutrients frequently contribute to alien success (Daehler, 2003; Sala et al., 2007). The success of the relationship between alien plants and the uptake and use of nutrients varies with the availability of nutrients in the soil. Further increases in nutrient availability generally favor alien success when the soils are rich in nutrients (Fig. 2). Alien invasion in nutrient-rich environments also frequently favors plant species with high rates of photosynthesis and growth (Baruch and Goldstein, 1999; Leishman et al., 2007; Feng et al., 2008; Schumacher et al., 2009; González et al., 2010; Mozdzer and Zieman, 2010; Feng et al., 2011), high reproductive outputs (González et al., 2010), large size (van Kleunen et al., 2010), low C-nutrient ratios in tissues (Monaco et al., 2003; Agrawal et al., 2005; Reed et al., 2005; Packett and Chambers, 2006; Schumacher et al., 2009; González et al., 2010; Peñuelas et al., 2010), low costs of foliar construction (Nagel and Griffin, 2001; Feng et al., 2007; González et al., 2010), large investments of N in photosynthetic production (Ehrenfeld, 2003; Xu et al., 2007; Shen et al., 2011), higher capacities of nutrient uptake (Zabinsky et al., 2002; Harrington et al., 2004; Blank and Sforza, 2007; Feng, 2008; Blank, 2010; Hewins and Hyatt, 2010; Leffler et al., 2011; Peng et al., 2011), and high levels of plasticity in the acquisition of resources as a function of pulses in nutrient availability (Leffler et al., 2011). These factors indicate that nutrient uptake and all foliar traits enabling rapid rates of growth (Zabinsky et al., 2002; Leishman et al., 2007) will help invading species to succeed when resources are not limited (Bray et al., 2003; Shah et al., 2009). Alien plants can increase their symbiotic relationships with fungi by directly increasing their capacity for nutrient uptake (Zabinsky et al., 2002; James et al., 2010) or by inhibiting the germination and growth of native plants (Rudgers and Orr, 2009), but some studies are inconclusive (Shah et al., 2009, 2010). The role of nutrient use efficiency is not clear (Scharfy et al., 2009; Aguilera et al., 2010). The resistance of native plants to alien invasion is linked to their capacity to maintain the lowest possible levels of nutrient availability (Davis et al., 2000; James et al., 2008). Segregating the requirements of nutrient ratios from the invaders can allow native species to persist in invaded communities (James et al., 2008; Peñuelas et al., 2010), a persistence that increases plant diversity (Tilman et al., 1997) and thus helps to reduce competition between native and alien plants. Most studies suggest that increasing the availability of nutrients increases the invasive success of plants (Fig. 2). Current information is insufficient for reaching a clear understanding of whether low N-P ratios in soils favor alien success in nutrient-rich ecosystems, as expected from the growth rate hypothesis, affecting species with higher rates of growth, as is frequently the case for aliens relative to their native competitors (Sterner and Elser, 2002; Sardans et al., 2012c). Despite some studies suggesting the confirmation of this hypothesis, Neves et al. (2010) found lower N-P ratios in alien than in native competitor plants. Therefore, more studies are needed on these two elements in native and alien plants to determine whether some general relationships exist between alien plant success and N-P ratios, at least in nutrient-rich ecosystems.
A higher capacity of N uptake can have further positive consequences for invasive success through the facilitation of the synthesis of N-rich allelopathic compounds that can inhibit the growth of natives (Hewins and Hyatt, 2010). In fact, allelopathic substances in the litter of alien plants can inhibit growth (Hata et al., 2010; Cipollini et al., 2012; Rashid and Reshi, 2012), mycorrhization and the capacities of nutrient uptake (Zhang et al., 2007), and N2 fixation in native plants (Wardle et al., 1994) and change soil nutrient decomposition rates (Chen et al., 2007a), frequently stimulating soil nutrient cycling (Chen et al., 2007b). Allelopathic compounds in the litter of native plants, however, can also inhibit the growth of alien plants (Hou et al., 2012). The “weapon hypothesis” claims that the roots of alien plants exude allelopathic substances that inhibit the growth of native plants, alter soil microbial communities, and confer a competitive advantage to alien plants in the uptake of soil nutrients (Lorenzo et al., 2010; Weidenhamer and Callaway, 2010).
N2-fixing plants can have an advantage in N-poor soils dominated by nonfixing plants (Londsdale et al., 1989; Yelenik et al., 2004; Hughes and Denslow, 2005; Morris et al., 2011). Communities dominated by legumes growing in soils with limited availabilities of soil P or water, however, are vulnerable to competition by nonfixing plants with high capacities of P uptake or stomatal control (Suriyagoda et al., 2011), and low availabilities of P reduce the chances of successful invasion by N2-fixing plants (Haubensak and D’Antonio, 2011). The success of alien nonfixing plants in native communities dominated by N2-fixing plants is reduced when P is added (Brewer and Cralle, 2003). Conversely, in soils poor in N and P, plants able to acquire extra N by a stable symbiosis with N2-fixing bacteria can increase their allocation to P uptake and increase their invasive success (Rout and Chrzanowski, 2009). The more limiting N is, the higher the possibility of alien invasion by N2-fixing plants, especially if P is not so limiting.
Conversely, most studies suggest that the success of invasive plants in nutrient-poor soils depends on more conservative strategies, such as a higher nutrient use efficiency (Funk and Vitousek, 2007; González et al., 2010; Matzek, 2011), especially on short time scales (Funk and Vitousek, 2007), long nutrient residence times (Laungani and Knops, 2009) with high C-nutrient ratios in tissues (González et al., 2010), high nutrient competitive ability enabling resistance to low levels of nutrients (Muth and Pigliucci, 2007; Schumacher et al., 2009; Kueffer, 2010), and high plasticity of stoichiometric ratios (González et al., 2010). In ecosystems poor in resource availability (e.g. with deficits of water and nutrients), stress-tolerant plants with efficient metabolisms, such as C4 plants, can increase their chances of invasive success (Szente et al., 1996). Interestingly, a high capacity to allocate N to photosynthesis aids invasive success in both nutrient-rich and nutrient-poor sites (Feng, 2008; Feng et al., 2008, 2009; Matzek, 2011).
Impacts of Plant Invasion on the Availability and Stoichiometry of Nutrients in Soil
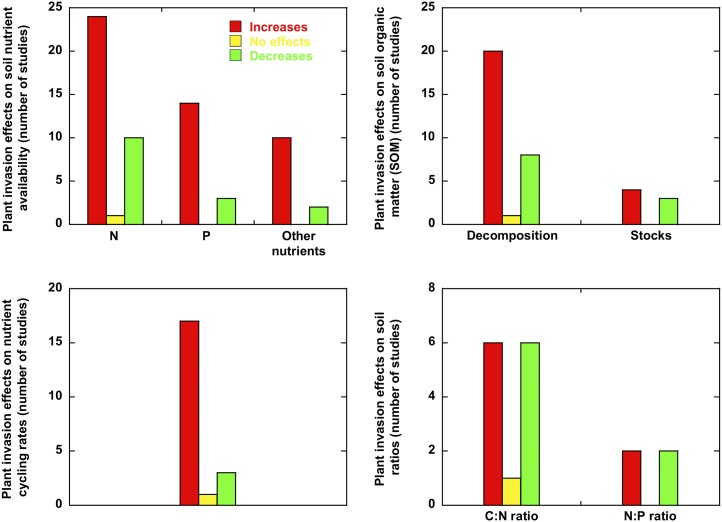
Alien plants frequently alter soil conditions. Several studies report significant impacts on the availability of soil nutrients, the decomposition of organic matter, nutrient cycling, and soil stoichiometry (Fig. 3). Plant invasive success in northern Europe was related to increases in nutrient content in nutrient-poor soils and to decreases in nutrient content in nutrient-rich soils (Dassonville et al., 2008). In a recent review, Pysek et al. (2012) reported that of 436 case studies on the effects of invasive plants on soil nutrient content, 192 found increases, 72 found decreases, and 158 found inconclusive changes. We have reviewed the effect on N and P availability, C-N-P ratios of soils, rates of soil decomposition, and mineralization and nutrient cycling (Fig. 3). Of 65 studies conducted in environments with unclear limitations of nutrients (except some conducted mainly in arid and semiarid areas of the United States), 48 reported increases in the availability of soil nutrients, 14 reported decreases, and three were inconclusive. Most of the 14 studies reporting decreases in soil nutrients were studies with Bromus tectorum, an invader plant of semiarid areas of the United States (six studies) and with N2-fixing invasive plants (three studies), showing that most invasions in nutrient-rich ecosystems tend to increase the availability of soil nutrients. This effect is related to increases in nutrient cycling. The effects on SOM decomposition and mineralization are not as clear, despite a trend of increases in most reports.
Figure 3.
Studies reporting information on plant-invasion effects on soil nutrient availability, soil decomposition rates, soil nutrient cycling, soil organic matter stocks, and soil stoichiometry. Detailed results and bibliographic references are provided in Supplemental Table S3.
Results for C-nutrient and N-P ratios in soil are inconclusive and variable (Fig. 3). Increases in soil nutrient contents are accompanied by higher productions of biomass that lead to more C in the system. In the long term, more C in the soil counteracts higher stocks of soil nutrients. An insufficient number of studies are available for reaching a more solid conclusion of the impact of plant invasion on ecosystemic (plant and soil) N-P ratios. A higher rate of growth in alien species in nutrient-rich soils should be a favorable trait for invasive success, so low N-P ratios in alien species should favor their success. This important question cannot yet be resolved due to the lack of data. N deposition, however, can favor invasion, as has been observed frequently (Tomassen et al., 2003; Bidwell et al., 2006; Dong et al., 2006; Gilliam, 2006; Huebner et al., 2009; He et al., 2011; Miki, 2012). These results thus suggest that possible increases in soil N-P ratios do not decrease alien success, but further studies are warranted to discern the role of plant invasion in ecosystemic stoichiometry. Current data suggest a decrease in C-nutrient ratios of plant communities when the number of alien plants and the availability of soil nutrients increase, but inconclusive results prevent the claim of a clear impact on soil stoichiometry. The role of N-P ratios on the invasive success of plants is thus particularly uncertain in both the invasive phase and the shifts during the invasion.
In summary, plant invasion affects soil conditions, which in turn affect plant growth and competitive relationships by plant-soil feedbacks. Invasion can further interact with other drivers of global change such as N deposition, as discussed above. Moreover, some studies have reported that the effects of plant invasion on soil facilitate further invasion by the initial invasive species or by other species (Allison and Vitousek, 2004; Sharma and Raghubanshi, 2009), although not all studies provide clear results on this topic (Yelenik and Levine, 2011). Thus, alien success in nutrient-rich soils is related to higher growth rates, low C-N and C-P ratios, fast plasticity in resource acquisition capacity and high reproductive investment; in nutrient-poor soils, high invasive success is related to conservative use of resources including nutrients. Apart from the nutrient residence time, the capacity of nutrient uptake and other previously mentioned traits, which can be more or less successful depending on the resource richness of each environment, and the capacity to exploit the resources for production and growth would be useful for alien expansion in all situations. Moreover, the role of N-P ratios (of plant and soil) in invasive success is not well known. These possibilities merit further research.
CONCLUSION AND FINAL REMARKS
Elevated concentrations of atmospheric CO2 tend to increase foliar C-N and C-P ratios, because the initial increase in production can be quickly curtailed by limitations in the availability of N and P, despite the investment of more resources for N and P uptake by plants under high concentrations of atmospheric CO2.
Warming tends to increase C-N and C-P stoichiometry. This effect is related to an increase in growth that progressively gives a more limiting role to nutrients in wet environments and to the enhancement of conservative strategies related to increases in soil-moisture limitation under warming in dry sites. Drought has a negative effect on SOM mineralization and plant growth in currently dry areas, where nutrient cycling slows, contributing to increased accumulations of N and P in the soil, but mainly in recalcitrant forms. All these direct effects decrease soil fertility, increase the risk of nutrient losses caused by torrential rainfall, and have a negative feedback on the capacity of nutrient uptake, plant growth, and WUE. Plants thus frequently present higher C-nutrient ratios under drought in currently semiarid and arid regions. Clear evidence that drought can change N-P ratios in terrestrial ecosystems has not yet been reported.
N deposition can decrease C-N and increase N-P ratios of soils and plant tissues, despite the reaction of ecosystems to increasing rates of P cycling that allow higher P uptakes. The larger increases of soil N than of other elements leads to nutrient mismatches and mainly to increases in N-P ratios that threaten to reduce species diversity by favoring species adapted to P limitation and high N-P ratios. N deposition can have an interactive effect under higher concentrations of atmospheric CO2 and under warming in wetter regions by allowing an increase in growth without C-N stoichiometric changes. However, this can further increase C-P and N-P ratios and the limiting role of P.
Most studies that have investigated the main factors underlying plant invasion have observed that nutrient availability is frequently a key factor in explaining the success of alien plants by the competitive capacity for nutrient uptake in nutrient-rich environments and for coping with low levels of nutrient availability in nutrient-poor sites.
Many unknowns remain in several of these issues, such as the effects of elevated levels of atmospheric CO2 on the N-P ratios in plants, soils, and ecosystems. Although some current reports suggest that warming can lead to increases in N-P ratios in plants, soils, and ecosystems by asymmetrical effects on N and P cycles, more studies are necessary to gain knowledge on this topic. A sufficient number of studies is also lacking to reach a more solid conclusion of the impact of plant invasion on the N-P ratios of ecosystems (plant and soil). The higher rate of growth in alien species in nutrient-rich soils appears to be a favorable trait for invasive success. Low N-P ratios in alien species should thus favor their success being related to rapid growth. This question is very important and warrants extensive and intensive research. Moreover, some global change drivers, such as warming, increased atmospheric CO2 concentrations, and N deposition, can actuate together at continental scales. Thus, more studies on the effects of these drivers one by one and in combination are warranted to reach a better knowledge of the particular effects of each one and the interaction effects among them.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Table S1. Studies reporting information on the relationships between drought and warming with soil-nutrient status (mineralization, content, and availability).
Supplemental Table S2. Studies reporting information on the relationships between plant invasion success and nutrient availability.
Supplemental Table S3. Studies reporting information on plant invasion effects on soil nutrient availability, soil decomposition and mineralization rates, soil nutrient cycling, soil C stocks, and soil stoichiometry.
Glossary
- N
nitrogen
- P
phosphorus
- C
carbon
- K
potassium
- SOM
soil organic matter
- WUE
water use efficiency
References
- Aerts R. (2006) The freezer defrosting: global warming and litter decomposition rates in cold biomes. J Ecol 94: 713–724 [Google Scholar]
- Aerts R, van Bodegom PM, Cornelissen JHC. (2012) Litter stoichiometric traits of plant species of high-latitude ecosystems show high responsiveness to global change without causing strong variation in litter decomposition. New Phytol 196: 181–188 [DOI] [PubMed] [Google Scholar]
- Aerts R, Wallén B, Malmer N. (1992) Growth-limiting nutrients in Sphagnum-dominated bogs subject to low and high atmospheric nitrogen supply. J Ecol 80: 131–140 [Google Scholar]
- Agra HE, Ne’eman G. (2012) Composition and diversity of herbaceous patches in woody vegetation: the effects of grazing, soil seed bank, patch spatial properties and scale. Flora 207: 310–317 [Google Scholar]
- Agrawal AA, Kotanen PM, Mitchell CE, Power AG, Godsoe W, Klironomos J. (2005) Enemy release? An experiment with congeneric plant pairs and diverse above- and belowground enemies. Ecology 86: 2979–2988 [Google Scholar]
- Aguilera AG, Alpert P, Dukes JS, Harrington R. (2010) Impacts of the invasive plant Fallopia japonica (Houtt.) on plant communities and ecosystem processes. Biol Invasions 12: 1243–1252 [Google Scholar]
- Albert KR, Ro-Poulsen H, Mikkelsen TN, Michelsen A, van der Linden L, Beier C. (2011) Interactive effects of elevated CO2, warming, and drought on photosynthesis of Deschampsia flexuosa in a temperate heath ecosystem. J Exp Bot 62: 4253–4266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Karaki GN, Al-Raddad A. (1997) Effects of arbuscular mycorrhizal fungi and drought stress on growth and nutrient uptake of two wheat genotypes differing in drought resistance. Mycorrhiza 7: 81–88 [Google Scholar]
- Allen AS, Schlesinger WH. (2004) Nutrient limitations to soil microbial biomass and activity in loblolly pine forest. Soil Biol Biochem 36: 581–589 [Google Scholar]
- Allison SD, Treseder KK. (2008) Warming and drying suppress microbial activity and carbon cycling in boreal forest soils. Glob Change Biol 14: 2898–2909 [Google Scholar]
- Allison SD, Vitousek PM. (2004) Rapid nutrient cycling in leaf litter from invasive plants in Hawai’i. Oecologia 141: 612–619 [DOI] [PubMed] [Google Scholar]
- Alvarez M, Huygens D, Olivares E, Saavedra I, Alberdi M, Valenzuela E. (2009) Ectomycorrhizal fungi enhance nitrogen and phosphorus nutrition of Nothofagus dombeyi under drought conditions by regulating assimilative enzyme activities. Physiol Plant 136: 426–436 [DOI] [PubMed] [Google Scholar]
- An Y, Wan S, Zhou X, Subedar AA, Wallace LL, Luo Y. (2005) Plant nitrogen concentration, use efficiency, and contents in a tallgrass prairie ecosystem under experimental warming. Glob Change Biol 11: 1733–1744 [Google Scholar]
- Aranjuelo I, Molero G, Erice G, Avice JC, Nogués S. (2011) Plant physiology and proteomics reveals the leaf response to drought in alfalfa (Medicago sativa L.). J Exp Bot 62: 111–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone JA, III, Zaller JG, Spehn EM, Niklaus PA, Wells CE, Körner C. (2000) Dynamics of root systems in native grasslands: effects of elevated atmospheric CO2. New Phytol 147: 73–85 [Google Scholar]
- Atkin OK, Edwards EJ, Loveys BR. (2000) Response of root respiration to changes in temperature and its relevance to global warming. New Phytol 147: 141–154 [Google Scholar]
- Austin EE, Castro HF, Sides KE, Schadt CW, Classen AT. (2009) Assessment of 10 years of CO2 fumigation on soil microbial communities and function in a sweetgum plantation. Soil Biol Biochem 41: 514–520 [Google Scholar]
- Azam F, Gill S, Farooq S. (2005) Availability of CO2 as a factor affecting the rate of nitrification in soil. Soil Biol Biochem 37: 2141–2144 [Google Scholar]
- Baghour M, Ruiz JM, Romero L. (2000) Metabolism and efficiency in nitrogen utilization during senescence in pepper plants: response to nitrogenous fertilization. J Plant Nutr 23: 91–101 [Google Scholar]
- Bahrun A, Jensen CR, Asch F, Mogensen VO. (2002) Drought-induced changes in xylem pH, ionic composition, and ABA concentration act as early signals in field-grown maize (Zea mays L.). J Exp Bot 53: 251–263 [DOI] [PubMed] [Google Scholar]
- Baron JS, Rueth HM, Wolje AM, Nydick KR, Allstott EJ, Minear JT, Moraska B. (2000) Ecosystem responses to nitrogen deposition in the Colorado front range. Ecosystems (N Y) 3: 352–368 [Google Scholar]
- Baruch Z, Goldstein G. (1999) Leaf construction cost, nutrient concentration, and net CO2 assimilation of native and invasive species in Hawaii. Oecologia 121: 183–192 [DOI] [PubMed] [Google Scholar]
- BassiriRad H, Gutschick VP, Lussenhop J. (2001) Root system adjustments: regulation of plant nutrient uptake and growth responses to elevated CO2. Oecologia 126: 305–320 [DOI] [PubMed] [Google Scholar]
- Bauer GA, Berntson GM. (2001) Ammonium and nitrate acquisition by plants in response to elevated CO2 concentration: the roles of root physiology and architecture. Tree Physiol 21: 137–144 [DOI] [PubMed] [Google Scholar]
- Bell TH, Klironomos JN, Henry HAL. (2010) Seasonal responses of extracellular enzyme activity and microbial biomass to warming and nitrogen addition. Soc Soil Sci Am J 74: 820–828 [Google Scholar]
- Bergkvist B, Folkeson L. (1992) Soil acidification and element fluxes of a Fagus sylvatica forest influenced by simulated nitrogen deposition. Water Air Soil Pollut 65: 111–133 [Google Scholar]
- Berntson GM. (1994) Modelling root architecture: are there tradeoffs between efficiency and potential of resource acquisition? New Phytol 127: 483–493 [Google Scholar]
- Biasi C, Meyer H, Rusalimova O, Hämmerie R, Kaiser C, Baranyi C, Daims H, Lashchinsky N, Barsukov P, Richter A. (2008) Initial effects of experimental warming on carbon exchange rates, plant growth and microbial dynamics of a lichen-rich dwarf shrub tundra in Siberia. Plant Soil 307: 191–205 [Google Scholar]
- Bidwell S, Attiwill PM, Adams MA. (2006) Nitrogen availability and weed invasion in a remnant native woodland in urban Melbourne. Austral Ecol 31: 262–270 [Google Scholar]
- Billings SA, Ziegler SE. (2005) Linking microbial activity and soil organic matter transformations in forest soils under elevated CO2. Glob Change Biol 11: 203–212 [Google Scholar]
- Binkley D, Högberg P. (1997) Does atmospheric deposition of nitrogen threaten Swedish forest? For Ecol Manag 92: 119–152
- Blanes MC, Emmett BA, Viñegla B, Carreira JA. (2012) Alleviation of P limitation makes tree roots competitive for N against microbes in a N-saturated conifer forest: a test through P fertilization and 15N labeling. Soil Biol Biochem 48: 51–59 [Google Scholar]
- Blank RR. (2010) Intraspecific and interspecific pair-wise seedling competition between exotic annual grasses and native perennials: plant-soil relationships. Plant Soil 326: 331–343 [Google Scholar]
- Blank RR, Sforza R. (2007) Plant-soil relationships of the invasive annual grass Taeniatherum caput-medusae: a reciprocal transplant experiment. Plant Soil 298: 7–19 [Google Scholar]
- Blödner C, Goebel C, Feussner I, Gatz C, Polle A. (2007) Warm and cold parental reproductive environments affect seed properties, fitness, and cold responsiveness in Arabidopsis thaliana progenies. Plant Cell Environ 30: 165–175 [DOI] [PubMed] [Google Scholar]
- Bobbink R, Horning M, Roelofs JM. (1998) The effects of air-borne nitrogen pollutants on species diversity in natural and semi-natural European vegetation. J Ecol 86: 717–738 [Google Scholar]
- Boix-Fayos C, Calvo-Cases A, Imeson AC, Soriano-Soto MD, Tiemessen IR. (1998) Spatial and short-term temporal variations in runoff, soil aggregation and other soil properties along a Mediterranean climatological gradient. Catena 33: 123–128 [Google Scholar]
- Bokhorst S, Bjerke JW, Street LE, Callaghan TV, Phoenix GK. (2011) Impacts of multiple extreme winter warming events on sub-Artic heathland: phenology, reproduction, growth and CO2 flux responses. Glob Change Biol 17: 2817–2830 [Google Scholar]
- Bontemps JD, Hervé JC, Leban JM, Dhôte JF. (2011) Nitrogen footprint in a long-term observation of forest growth over the twentieth century. Trees 25: 237–251 [Google Scholar]
- Borch K, Miller C, Brown KM, Lynch JP. (2003) Improved drought tolerance in marigold by manipulation of root growth with buffered-phosphorus nutrition. Hortic Sci 38: 212–216 [Google Scholar]
- Bragazza L, Tahvanainen T, Kutmar L, Rydin H, Limpens J, Hájek M, Grosvernier P, Hájek T, Hajkova P, Hansen I, et al. (2004) Nutritional constraints in ombrotrophic Sphagnum plants under increasing atmospheric nitrogen deposition in Europe. New Phytol 163: 609–616 [DOI] [PubMed] [Google Scholar]
- Braun S, Thomas VFD, Quiring R, Flückiger W. (2010) Does nitrogen deposition increase forest production? The role of phosphorus. Environ Pollut 158: 2043–2052 [DOI] [PubMed] [Google Scholar]
- Bray SR, Kitayima K, Sylvia DM. (2003) Mycorrhizae differentially alter growth physiology, and competitive ability of an invasive shrub. Ecol Appl 13: 565–574 [Google Scholar]
- Brewer JS, Cralle SP. (2003) Phosphorus addition reduces invasion of a longleaf pine savanna (Southeastern USA) by a non-indigenous grass (Imperata cylindrica). Plant Ecol 167: 237–245 [Google Scholar]
- Britton AJ, Helliwell RC, Fisher JM, Gibbs S. (2008) Interactive effects of nitrogen deposition and fire on plant and soil chemistry in an alpine heathland. Environ Pollut 156: 409–416 [DOI] [PubMed] [Google Scholar]
- Brookshire ENJ, Valett HM, Thomas SA, Webster JR. (2007) Atmospheric N deposition increases organic N loss from temperate forest. Ecosystems (New York) 10: 252–262 [Google Scholar]
- Brunner I, Godbold DL. (2007) Tree roots in a changing world. J For Res 12: 78–82 [Google Scholar]
- Butler SM, Melillo JM, Johnson JE, Mohan J, Steudler PA, Lux H, Burrows E, Smith RM, Vario CL, Scott L, et al. (2012) Soil warming alters nitrogen cycling in a New England forest: implications for ecosystem function and structure. Oecologia 168: 819–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cárdenas-Navarro R, López-Pérez L, Lobit P, Ruiz-Corro R, Castellanos-Morales VC. (2006) Effects of nitrogen source on growth and development of strawberry plants. J Plant Nutr 29: 1699–1707 [Google Scholar]
- Carroll JA, Caporn SJM, Johnson D, Morecroft MD, Lee JA. (2003) The interactions between plant growth, vegetation structure and soil processes in semi-natural acidic and calcareous grasslands receiving long-term inputs of simulated pollutant nitrogen deposition. Environ Pollut 121: 363–376 [DOI] [PubMed] [Google Scholar]
- Chen BM, Ni GY, Ren WT, Peng SL. (2007a) Effects of aqueous extracts of Mikania micrantha on litter decomposition of native plants in south China. Allelophatic J 20: 307–314 [Google Scholar]
- Chen H, Li B, Fang C, Chen J, Wu J. (2007b) Exotic plant influences soil nematode communities through litter input. Soil Biol Biochem 39: 1782–1793 [Google Scholar]
- Chen J, Wu FH, Liu TW, Chen L, Xiao Q, Dong XJ, He JX, Pei ZM, Zheng HL. (2012) Emissions of nitric oxide from 79 plant species in response to simulated nitrogen deposition. Environ Pollut 160: 192–200
- Cheng Y, Cai ZC, Zhang JB, Lang M, Mary B, Chang SX. (2012) Soil moisture effects on gross nitrification differ between adjacent grassland and forested soils in central Alberta, Canada. Plant Soil 352: 289–301 [Google Scholar]
- Chidumayo EN. (1994) Phenology and nutrition of miombo woodland trees in Zambia. Trees (Berl) 9: 67–72 [Google Scholar]
- Chun YJ, Collyer ML, Moloney KA, Nason JD. (2007) Phenotypic plasticity of native vs. invasive purple loosestrife: a two-state multivariate approach. Ecology 88: 1499–1512 [DOI] [PubMed] [Google Scholar]
- Cipollini K, Titus K, Wagner C. (2012) Allelophatic effects of invasive species (Alliaria petiolata, Lonicera maackii, Ranunculus ficaria) in the midwestern United States. Allelopathy J 29: 63–75 [Google Scholar]
- Clark NM, Rillig MC, Nowak RS. (2009) Arbuscular mycorrhizal fungal abundance in the Mojave Desert: seasonal dynamics and impacts of elevated CO2. J Arid Environ 73: 834–843 [Google Scholar]
- Clemmensen KE, Michelsen A, Jonasson S, Shaver GR. (2006) Increased ectomycorrhizal fungal abundance after long-term fertilization and warming of two arctic tundra ecosystems. New Phytol 171: 391–404 [DOI] [PubMed] [Google Scholar]
- Cole L, Bardgett RD, Ineson P, Hobbs PJ. (2002) Enchytraeid worm (Oligochaeta) influences on microbial community structure, nutrient dynamics and plant growth in blanket peat subject to warming. Soil Biol Biochem 34: 83–92 [Google Scholar]
- Compton JE, Watrud LS, Porteous LA, DeGrood S. (2004) Response of soil microbial biomass and nitrogen additions at Harvard forest. For Ecol Manag 196: 143–158
- Cornelissen JHC, van Bodegom PM, Aerts R, Callaghan TV, van Logtestijn RSP, Alatalo J, Chapin FS, Gerdol R, Gudmundsson J, Gwynn-Jones D, et al. (2007) Global negative vegetation feedback to climate warming responses of leaf litter decomposition rates in cold biomes. Ecol Lett 10: 619–627 [DOI] [PubMed] [Google Scholar]
- Correia PJ, Martins-Loução MA. (1997) Leaf nutrient variation in mature carob (Ceratonia siliqua) trees in response to irrigation and fertilization. Tree Physiol 17: 813–819 [DOI] [PubMed] [Google Scholar]
- Cotrufo MF, De Angelis P, Polle A. (2005) Leaf litter production and decomposition in a poplar short-rotation coppice exposed to free air CO2 enrichment (POPFACE). Glob Change Biol 11: 971–982 [Google Scholar]
- Cramer MD, Hawkins HJ, Verboom GA. (2009) The importance of nutritional regulation of plant water flux. Oecologia 161: 15–24 [DOI] [PubMed] [Google Scholar]
- Cruz C, Lips H, Martins-Loução MA. (2003) Nitrogen use efficiency by a slow-growing species as affected by CO2 levels, root temperature, N source and availability. J Plant Physiol 160: 1421–1428 [DOI] [PubMed] [Google Scholar]
- Daehler CC. (2003) Performance comparisons of co-occuring native and alien invasive plants: implications for conservation and restoration. Annu Rev Ecol Syst 34: 183–211 [Google Scholar]
- Dalmonech D, Lagomarsino A, Moscatelli MC, Chiti T, Valentini R. (2010) Microbial performance under increasing nitrogen availability in a Mediterranean forest soil. Soil Biol Biochem 42: 1596–1606 [Google Scholar]
- Dassonville N, Vanderhoeven S, Gruber W, Maerts P. (2007) Invasion by Fallopia japonica increases topsoil mineral nutrient concentrations. Ecoscience 14: 230–240 [Google Scholar]
- Dassonville N, Vanderhoeven S, Vanparys V, Hayez M, Gruber W, Meerts P. (2008) Impacts of alien invasive plants on soil nutrients are correlated with initial site conditions in NW Europe. Oecologia 157: 131–140 [DOI] [PubMed] [Google Scholar]
- Davies FT, Jr, Svenson SE, Cole JC, Phavaphutanon L, Duray SA, Olalde-Portugal V, Meier CE, Bo SH. (1996) Non-nutritional stress acclimation of mycorrhizal woody plants exposed to drought. Tree Physiol 16: 985–993 [DOI] [PubMed] [Google Scholar]
- Davis MA, Grime JP, Thompson K. (2000) Fluctuating resources in plant communities: a general theory of invasibility. J Ecol 88: 528–534 [Google Scholar]
- Day TA, Ruhland C, Xiong FS. (2008) Warming increases aboveground plant biomass and C stocks in vascular-plant-dominated Antarctic tundra. Glob Change Biol 14: 1827–1843 [Google Scholar]
- De Frenne P, Kolb A, Graae BJ, Decocq G, Baltora S, De Schrijver A, Brunet J, Chabrerie O, Cousins SAO, Dhondt R, et al. (2011) A latitudinal gradient in seed nutrients of the forest herb Anemone nemorosa. Plant Biol (Stuttg) 13: 493–501 [DOI] [PubMed] [Google Scholar]
- Del Pozo A, Pérez P, Gutiérrez D, Alonso A, Moscuende R, Martínez-Carrasco R. (2007) Gas exchange acclimation to elevated CO2 in upper-sunlit and lower-shaded canopy leaves in relation to nitrogen acquisition and partitioning in wheat grown in field chambers. Environ Exp Bot 59: 371–380 [Google Scholar]
- DeLucia EH, Schlesinger WH. (1991) Resource-use efficiency and drought tolerance in adjacent great basin and sierran plants. Ecology 72: 51–58 [Google Scholar]
- De Valpine P, Harte J. (2001) Plant responses to experimental warming in a montane meadow. Ecology 82: 637–648 [Google Scholar]
- Díaz S. (1995) Effects of elevated [CO2] at the community level mediated by root symbionts. Plant Soil 187: 309–320 [Google Scholar]
- Dijkstra FA, Blumenthal D, Morgan JA, LeCain DR, Follett RF. (2010b) Elevated CO2 effects on semi-arid grassland plants in relation to water availability and competition. Funct Ecol 24: 1152–1161 [Google Scholar]
- Dijkstra FA, Blumenthal D, Morgan JA, Pendall E, Carrillo Y, Follett RF. (2010a) Contrasting effects of elevated CO2 and warming on nitrogen cycling in a semiarid grassland. New Phytol 187: 426–437 [DOI] [PubMed] [Google Scholar]
- Dijkstra FA, Hobbie SE, Knops JMH, Reich PB. (2004) Nitrogen deposition and plant species interact to influence soil carbon stabilization. Ecol Lett 7: 1192–1198 [Google Scholar]
- Dong M, Lu JZ, Zhang WJ, Chen JK, Li B. (2006) Canada goldenrod (Solidago canadensis): an invasive alien weed rapidly spreading in China. Acta Phytotaxon Sin 44: 72–85 [Google Scholar]
- Dreesen PE, De Boeck HJ, Janssens IA, Nijs I. (2012) Summer heat and drought extremes trigger unexpected changes in productivity of a temperate annual/biannual plant community. Environ Exp Bot 79: 21–30 [Google Scholar]
- Drenovsky RE, Khasanova A, James JJ. (2012) Trait convergence and plasticity among native and invasive species in resource-poor environments. Am J Bot 99: 629–639 [DOI] [PubMed] [Google Scholar]
- Duprè C, Stevens CJ, Ranke T, Bleekers A, Peppler-Lisbach C, Cowing DJG, Dise NB, Dorland E, Bobbink R, Diekmann M. (2010) Changes in species richness and composition in European acidic grasslands over the past 70 years: the contribution of cumulative atmospheric nitrogen deposition. Glob Change Biol 16: 344–357 [Google Scholar]
- Duquesnay A, Dupouey JL, Clement A, Ulrich E, Le Tacon F. (2000) Spatial and temporal variability of foliar mineral concentration in beech (Fagus sylvatica) stands in northeastern France. Tree Physiol 20: 13–22 [DOI] [PubMed] [Google Scholar]
- Ebersberger D, Niklaus PA, Kandeler E. (2003) Long term CO2 enrichment stimulates N-mineralization and enzyme activities in calcareous grassland. Soil Biol Biochem 35: 965–972 [Google Scholar]
- Ehrenfeld JG. (2003) Effects of exotic plant invasion on soil nutrient cycling processes. Ecosystems (N Y) 6: 503–523 [Google Scholar]
- Eisenlord SD, Zak DR. (2010) Simulated atmospheric nitrogen deposition alters actinobacterial community composition in forest soils. Soil Sci Soc Am J 74: 1157–1166 [Google Scholar]
- Elser JJ, Peace AL, Kyle M, Wojewodzic M, McCrackin ML, Andersen T, Hessen DO. (2010) Atmospheric nitrogen deposition is associated with elevated phosphorus limitation of lake zooplankton. Ecol Lett 13: 1256–1261 [DOI] [PubMed] [Google Scholar]
- Engineer CB, Kranz RG. (2007) Reciprocal leaf and root expression of AtAmt1.1 and root architectural changes in response to nitrogen starvation. Plant Physiol 143: 236–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmeijer-Liu A, Aerts R, Kürschner WM, Bobbink R, Lotter AF, Verhoeven JTA. (2009) Nitrogen enrichment lowers Betula pendula green and yellow leaf stoichiometry irrespective of effects of elevated carbon dioxide. Plant Soil 316: 311–322 [Google Scholar]
- Fahey TJ, Yavitt JB, Sherman RE, Groffman PM, Fisk MC, Maerz JC. (2011) Transport of carbon and nitrogen between litter and soil organic matter in a northern hardwood forest. Ecosystems (N Y) 14: 326–340 [Google Scholar]
- Falkengren-Grerup U, Diekmann M. (2003) Use of a gradient of N-deposition to calculate effect-related soil and vegetation measures in deciduous forest. For Ecol Manage 180: 113–124 [Google Scholar]
- Fang HJ, Yu GR, Cheng SL, Mo JM, Yan JH, Li S. (2009) 13C abundance, water-soluble and microbial biomass carbon as potential indicators of soil organic carbon dynamics in subtropical forest at different successional stages and subject to different nitrogen loads. Plant Soil 320: 243–254 [Google Scholar]
- Feng YL. (2008) Photosynthesis, nitrogen allocation and specific leaf area in invasive Eupatorium adenophorum and native Eupatorium japonicum grown at different irradiances. Physiol Plant 133: 318–326 [DOI] [PubMed] [Google Scholar]
- Feng YL, Auge H, Ebeling SK. (2007) Invasive Buddleja davidii allocates more nitrogen to its photosynthetic machinery than five native woody species. Oecologia 153: 501–510 [DOI] [PubMed] [Google Scholar]
- Feng YL, Fu GL, Zheng YL. (2008) Specific leaf area relates to the differences in leaf construction cost, photosynthesis, nitrogen allocation, and use efficiencies between invasive and noninvasive alien congeners. Planta 228: 383–390 [DOI] [PubMed] [Google Scholar]
- Feng YL, Lei YB, Wang RF, Callaway RM, Valiente-Banuet A, Inderjit, Li YP, Zheng YL. (2009) Evolutionary tradeoffs for nitrogen allocation to photosynthesis versus cell walls in an invasive plant. Proc Natl Acad Sci USA 106: 1853–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng YL, Li YP, Wang RF, Callaway RM, Valiente-Banuet A, Inderjit (2011) A quicker return energy-use strategy by populations of a subtropical invader in the non-native range: a potential mechanism for the evolution of increased competitive ability. J Ecol 99: 1116–1123 [Google Scholar]
- Fenn ME, Poth MA, Aber JD, Baron JS, Bormann BT, Johnson DW, Lemly AD, McNully SG, Ryan DE, Stottlemeyer R. (1998) Nitrogen excess in North American ecosystems: predisposing factors, ecosystem responses, and management strategies. Ecol Appl 8: 706–733 [Google Scholar]
- Filella I, Peñuelas J. (2003) Indications of hydraulic lift by Pinus halepensis and its effects on the water relations of neighbour species. Biol Plant 47: 209–214 [Google Scholar]
- Finzi AC. (2009) Decades of atmospheric deposition have not resulted in widespread phosphorus limitation or saturation of tree demand for nitrogen in southern New England. Biogeochemistry 92: 217–229 [Google Scholar]
- Finzi AC, Moore DJP, DeLucia EH, Lichter J, Hofmockel KS, Jackson RB, Kim HS, Matamala R, McCarthy HR, Oren R, et al. (2006) Progressive nitrogen limitation of ecosystem processes under elevated CO2 in a warm-temperate forest. Ecology 87: 15–25 [DOI] [PubMed] [Google Scholar]
- Finzi AC, Norby RJ, Calfapietra C, Gallet-Budynek A, Gielen B, Holmes WE, Hoosbeek MR, Iversen CM, Jackson RB, Kubiske ME, et al. (2007) Increases in nitrogen uptake rather than nitrogen-use efficiency support higher rates of temperate forest productivity under elevated CO2. Proc Natl Acad Sci USA 104: 14014–14019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. (2012) Elevated CO2 impacts ectomycorrhiza-mediated forest soil carbon flow: fungal biomass production, respiration and exudation. Fungal Ecol 5: 85–98 [Google Scholar]
- Farnsworth EJ, Núñez-Farfán J, Careaga SA, Bazzaz FA. (1995) Phenology and growth of three temperate forest life forms in response to artificial soil warming. J Ecol 83: 967–977 [Google Scholar]
- Franzini VI, Azcón R, Mendes FL, Aroca R. (2010) Interactions between Glomus species and Rhizobium strains affect the nutritional physiology of drought-stressed legume hosts. J Plant Physiol 167: 614–619 [DOI] [PubMed] [Google Scholar]
- Frei C, Schär C, Lüthi D, Davies HC. (1998) Heavy precipitation processes in a warmer climate. Geophys Res Lett 25: 1431–1434 [Google Scholar]
- Friedrich U, von Oheimb G, Kriebitzsch WU, Schleßelmann K, Weber MS, Härdtle W. (2012) Nitrogen deposition increases susceptibility to drought: experimental evidence with the perennial grass Molinia caerulea (L.) Moench. Plant Soil 353: 59–71 [Google Scholar]
- Fujita Y, Robroek BJM, de Ruiter PC, Heil GW, Wassen MJ. (2010) Increased N affects P uptake of eight grassland species: the role of root surface phosphatase activity. Oikos 119: 1665–1673 [Google Scholar]
- Funk JL, Vitousek PM. (2007) Resource-use efficiency and plant invasion in low-resource systems. Nature 446: 1079–1081 [DOI] [PubMed] [Google Scholar]
- Gabarrón-Galeote MA, Martínez-Murillo JF, Ruiz-Sinoga JD. (2012) Relevant effects of vegetal cover and litter on the soil hydrological response of two contrasting Mediterranean hillslopes at the end of the dry season (south of Spain). Hydrol Processes 26: 1729–1738 [Google Scholar]
- Gamper H, Hartwig UA, Leuchtmann A. (2005) Mycorrhizas improve nitrogen nutrition of Trifolium repens after 8 yr of selection under elevated atmospheric CO2 partial pressure. New Phytol 167: 531–542 [DOI] [PubMed] [Google Scholar]
- Garcia C, Hernandez T, Roldan A, Martin A. (2002) Effect of plant cover decline on chemical and microbiological parameters under Mediterranean climate. Soil Biol Biochem 34: 635–642 [Google Scholar]
- Garg BK, Burman U, Kathju S. (2004) The influence of phosphorus nutrition on the physiological response of moth bean genotypes to drought. J Plant Nutr Soil Sci 167: 503–508 [Google Scholar]
- Garten CT, Jr, Iversen CM, Norby RJ. (2011) Litterfall 15N abundance indicates declining soil nitrogen availability in a free-air CO2 enrichment experiment. Ecology 92: 133–139 [DOI] [PubMed] [Google Scholar]
- Ghandilyan A, Barboza L, Tisné S, Granier C, Reymond M, Koornneef M, Schat H, Aarts MGM. (2009) Genetic analysis identifies quantitative trait loci controlling rosette mineral concentrations in Arabidopsis thaliana under drought. New Phytol 184: 180–192 [DOI] [PubMed] [Google Scholar]
- Gifford RM, Barrett DJ, Lutze JL. (2000) The effects of elevated [CO2] on the C:N and C:P mass ratios of plant tissues. Plant Soil 224: 1–14 [Google Scholar]
- Gilliam FS. (2006) Response of the herbaceous layer of forest ecosystems to excess nitrogen deposition. J Ecol 94: 1176–1191 [Google Scholar]
- González AL, Kominoski JS, Danger M, Ishida S, Iwai N, Rubach A. (2010) Can ecological stoichiometry help explain patterns of biological invasion? Oikos 119: 779–790 [Google Scholar]
- Granath G, Strengbom J, Rydin H. (2012) Direct physiological effects of nitrogen on Sphagnum: a greenhouse experiment. Funct Ecol 26: 353–364 [Google Scholar]
- Grandi AS, Sinsabaugh RL, Neff JC, Stursova M, Zak DR. (2008) Nitrogen deposition effects on soil organic matter chemistry are linked to variation in enzymes, ecosystems and size fractions. Biogeochemistry 91: 37–49 [Google Scholar]
- Groffman PM, Fisk M. (2011) Phosphate additions have no effects on microbial biomass and activity in a northern hardwood forest. Soil Biol Biochem 43: 2441–2449 [Google Scholar]
- Gundersen P, Emmett BA, Kjonaas OJ, Koopmans CJ, Tietema A. (1998) Impact of nitrogen deposition on nitrogen cycling in forest: a synthesis of NITREX data. For Ecol Manag 101: 37–55
- Güsewell S. (2004) N:P ratios in terrestrial plants: variation and functional significance. New Phytol 164: 243–266 [DOI] [PubMed] [Google Scholar]
- Güsewell S. (2005) High nitrogen:phosphorus ratios reduce nutrient retention and second-year growth of wetland sedges. New Phytol 166: 537–550 [DOI] [PubMed] [Google Scholar]
- Güsewell S, Freeman C. (2005) Nutrient limitation and enzyme activities during litter decomposition of nine wetland species in relation to litter N:P ratios. Funct Ecol 19: 582–593 [Google Scholar]
- Handa IT, Hagedorn F, Hättenschwiler S. (2008) No stimulation in root production in response to 4 years of in situ CO2 enrichment at the Swiss treeline. Funct Ecol 22: 348–358 [Google Scholar]
- Harrington RA, Fownes JH, Cassidy TM. (2004) Japanese barberry (Berberis thunbergii) in forest understory: leaf and whole plant responses to nitrogen availability. Am Midl Nat 151: 206–216 [Google Scholar]
- Hartley IP, Amstrong AF, Murthy R, Barron-Gafford G, Ineson P, Atkin OK. (2006) The dependence of respiration on photosynthetic substrate supply and temperature: integrating leaf, soil and ecosystem measurements. Glob Change Biol 12: 1954–1968 [Google Scholar]
- Hata K, Kato H, Kachi N. (2010) Litter of an alien tree, Casuarina equisetifolia, inhibits seed germination and initial growth of a native tree on the Ogasawara Islands (subtropical oceanic islands). J For Res 15: 384–390 [Google Scholar]
- Haubensak KA, D’Antonio CM. (2011) The importance of nitrogen-fixation for an invader of a coastal California grassland. Biol Invasions 13: 1275–1282 [Google Scholar]
- He WM, Yu GL, Sun ZK. (2011) Nitrogen deposition enhances Bromus tectorum invasion: biogeographic differences in growth and competitive ability between China and North America. Ecography 34: 1059–1066 [Google Scholar]
- Heckathorn SA, DeLucia EH. (1994) Drought-induced nitrogen retranslocation in perennial C4 grasses of tallgrass praire. Ecology 75: 1877–1886 [Google Scholar]
- Heckathorn SA, DeLucia EH. (1996) Retranslocation of shoot nitrogen to rhizomes and roots in praire grasses may limit loss of N to grazing and fire during drought. Funct Ecol 10: 396–400 [Google Scholar]
- Henry A, Doucette W, Norton J, Bugbee B. (2007) Changes in crested wheatgrass root exudation caused by flood, drought, and nutrient stress. J Environ Qual 36: 904–912 [DOI] [PubMed] [Google Scholar]
- Henry HA, Juarez JD, Field CB, Vitousek PM. (2005) Interactive effects of elevated CO2, N deposition and climate change on extracellular enzyme activity and soil density fractionation in a California annual grassland. Glob Change Biol 11: 1808–1815 [Google Scholar]
- Hewins DB, Hyatt LA. (2010) Flexible N uptake and assimilation mechanisms may assist biological invasion by Alliaria petiolata. Biol Invasions 12: 2639–2647 [Google Scholar]
- Hobbie SE, Chapin FS., III (1998) The response of tundra plant biomass, aboveground production, nitrogen, and CO2 flux to experimental warming. Ecology 79: 1526–1544 [Google Scholar]
- Holub P, Záhora J. (2008) Effects of nitrogen addition on nitrogen mineralization and nutrient content of expanding Calamagrostis epigejos in the Podyji National Park, Czech Republic. J Plant Nutr Soil Sci 171: 795–803 [Google Scholar]
- Horchani F, Hajri R, Aschi-Smiti S. (2010) Effect of ammonium or nitrate nutrition on photosynthesis, growth and nitrogen assimilation in tomato plants. J Plant Nutr Soil Sci 173: 610–617 [Google Scholar]
- Hou YP, Peng SL, Ni GY, Chen LY. (2012) Inhibition of invasive species Mikania micrantha HBK by native dominant trees in China. Allelopathy J 29: 307–314 [Google Scholar]
- Hovenden MJ, Newton PCD, Carran RA, Theobald P, Wills KE, Vander Schoor JK, Williams AL, Osanai Y. (2008) Warming prevents the elevated CO2-induced reduction in available soil nitrogen in a temperate, perennial grassland. Glob Change Biol 14: 1018–1024 [Google Scholar]
- Huang B. (2001) Nutrient accumulation and associated root characteristics in response to drought stress in tall fescue cultivars. Hortic Sci 36: 148–152 [Google Scholar]
- Huebner CD, Morin RS, Zurbriggen A, Whitre RL, Moore A, Twardus D. (2009) Patterns of exotic plant invasions in Pennsylvania’s Allegheny National Forest using intensive forest inventory and analysis plots. For Ecol Manag 257: 258–270
- Hughes RF, Denslow JS. (2005) Invasion by a N2-fixing tree alters function and structure in wet lowland forest of Hawaii. Ecol Appl 15: 1615–1628 [Google Scholar]
- Hyvönen R, Agren GI, Linder S, Persson T, Cotrufo MF, Ekblad A, Freeman M, Grelle A, Janssens IA, Jarvis PG, et al. (2007) The likely impact of elevated [CO2], nitrogen deposition, increased temperature and management on carbon sequestration in temperate and boreal forest ecosystems: a literature review. New Phytol 173: 463–480 [DOI] [PubMed] [Google Scholar]
- Inclán R, Gimeno BS, Dizengremel P, Sanchez M. (2005) Compensation processes of Aleppo pine (Pinus halepensis Mill.) to ozone exposure and drought stress. Environ Pollut 137: 517–524 [DOI] [PubMed] [Google Scholar]
- James JJ, Davies KW, Sheley RL, Aanderud ZT. (2008) Linking nitrogen partitioning and species abundance to invasion resistance in the Great Basin. Oecologia 156: 637–648 [DOI] [PubMed] [Google Scholar]
- James JJ, Ziegenhagen L, Aanderud ZT. (2010) Exploitation of nutrient-rich soil patches by invasive annual and native grasses. Invasive Plant Sci Manag 3: 169–177 [Google Scholar]
- Jarvis P, Linder S. (2000) Constraints to growth of boreal forests. Nature 405: 904–905 [DOI] [PubMed] [Google Scholar]
- Jentsch A, Kreyling J, Elmer M, Gellesch E, Glaser B, Grant K, Hein R, Lara M, Mirzae H, Nadler E, et al. (2011) Climate extremes initiate ecosystem-regulating functions while maintaining productivity. J Ecol 99: 689–702 [Google Scholar]
- Jin J, Watt M, Mathesius U. (2012) The autoregulation gene SUNN mediates changes in root organ formation in response to nitrogen through alteration of shoot-to-root auxin transport. Plant Physiol 159: 489–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin VL, Evans RD. (2007) Elevated CO2 increases microbial carbon substrate use and nitrogen cycling in Mojave Desert soils. Glob Change Biol 13: 452–465 [Google Scholar]
- Johnson D, Leake JR, Lee JA. (1999) The effects of quantity and duration of simulated pollutant nitrogen deposition on root-surface phosphatase activities in calcareous and acid grassland: a bioassay approach. New Phytol 141: 433–442 [Google Scholar]
- Johnson D, Leake JR, Lee JA, Campbell CD. (1998) Changes in soil microbia and microbial activities in response to 7 years simulated pollutant nitrogen deposition on a heathland and two grasslands. Environ Pollut 103: 239–250 [Google Scholar]
- Johnson DW, Cheng W, Joslin JD, Norby RJ, Edwards NT, Todd DE., Jr (2004) Effects of elevated CO2 on nutrient cycling in a sweetgum plantation. Biogeochemistry 69: 379–401 [Google Scholar]
- Johnson MG, Rygiewicz PT, Tingey DT, Phillips DL. (2006) Elevated CO2 and elevated temperature have no effect on Douglas-fir fine-root dynamics in nitrogen-poor soil. New Phytol 170: 345–356 [DOI] [PubMed] [Google Scholar]
- Jonasson S, Castro J, Michelsen A. (2004) Litter, warming and plants affect respiration and allocation of soil microbial and plant C, N and P in arctic mesocosms. Soil Biol Biochem 36: 1129–1139 [Google Scholar]
- Jones AG, Power SA. (2012) Field-scale evaluation of effects of nitrogen deposition on the functioning of heathland ecosystems. J Ecol 100: 331–342 [Google Scholar]
- Jones CA, Jacobsen JS, Wraith JM. (2005) Response of malt barley to phosphorus fertilization under drought conditions. J Plant Nutr 28: 1605–1617 [Google Scholar]
- Jones MLM, Wallace HL, Norris D, Brittain SA, Haria S, Jones RE, Rhind PM, Reynolds BR, Emmett BA. (2004) Changes in vegetation and soil characteristics in coastal sand dunes along a gradient of atmospheric nitrogen deposition. Plant Biol (Stuttg) 6: 598–605 [DOI] [PubMed] [Google Scholar]
- Joseph G, Henry HAL. (2008) Soil nitrogen leaching losses in response to freeze-thaw cycles and pulsed warming in a temperate old field. Soil Biol Biochem 40: 1947–1953 [Google Scholar]
- Jupp AP, Newman EI. (1987) Phosphorus uptake from soil by Lolium perenne during and after severe drought. J Appl Ecol 24: 979–990 [Google Scholar]
- Jyske T, Hölttä T, Mäkinen H, Nöjd P, Lumme I, Spiecker H. (2010) The effect of artificially induced drought on radial increment and wood properties of Norway spruce. Tree Physiol 30: 103–115 [DOI] [PubMed] [Google Scholar]
- Kang H, Zhuang H, Wu L, Liu Q, Shen G, Berg B, Man R, Liu C. (2011) Variation in leaf nitrogen and phosphorus stoichiometry in Picea abies across Europe: an analysis based on local observations. For Ecol Manag 201: 195–202
- Kohli A, Narciso JO, Miro B, Raorane M. (2012) Root proteases: reinforced links between nitrogen uptake and mobilization and drought tolerance. Physiol Plant 145: 165–179 [DOI] [PubMed] [Google Scholar]
- Kowalenko CG. (2006) The effect of nitrogen and boron fertilizer applications on Willamette red raspberry growth, and on applied and other nutrients in the plant and soil over two growing seasons. Can J Plant Sci 86: 213–225 [Google Scholar]
- Krapp A, Berthomé R, Orsel M, Mercey-Boutet S, Yu A, Castaings L, Elftieh S, Major H, Renou JP, Daniel-Vedele F. (2011) Arabidopsis roots and shoots show distinct temporal adaptation patterns toward nitrogen starvation. Plant Physiol 157: 1255–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreyling J, Beierkuhnlein C, Jentsch A. (2010) Effects of soil freeze-thaw cycles differ between experimental plant communities. Basic Appl Ecol 11: 65–75 [Google Scholar]
- Kristensen HL, Gundersen P, Gallesen I, Reinds GJ. (2004) Throughfall nitrogen deposition has different impacts on soil solution nitrate concentration in European coniferous and deciduous forest. Ecosystems (New York) 7: 180–192 [Google Scholar]
- Kronzucker HJ, Siddiqi MY, Glass ADM. (1997) Conifer root discrimination against soil nitrate and the ecology of forest succession. Nature 385: 59–61 [Google Scholar]
- Kueffer C. (2010) Reduced risk for positive soil-feedback on seedling regeneration by invasive trees on a very nutrient-poor soil in Seychelles. Biol Invasions 12: 97–102 [Google Scholar]
- Kutiel P, Lavee H, Ackermann O. (1998) Spatial distribution of soil surface coverage on north and south facing hillslopes along a Mediterranean to extreme arid climatic gradient. Geomosphology 23: 245–256 [Google Scholar]
- Lagomarsino A, Moscatelli MC, Hoosbeck MR, De Angelis P, Grego S. (2008) Assessment of soil nitrogen and phosphorus availability under elevated CO2 and N-fertilization in a short rotation poplar plantation. Plant Soil 308: 131–147 [Google Scholar]
- Laungani R, Knops JMH. (2009) Species-driven changes in nitrogen cycling can provide a mechanism for plant invasions. Proc Natl Acad Sci USA 106: 12400–12405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffler AJ, Monaco TA, James JJ. (2011) Nitrogen acquisition by annual and perennial grass seedlings: testing the roles of performance and plasticity to explain plant invasion. Plant Ecol 212: 1601–1611 [Google Scholar]
- Leger EA, Howe KM, Gurevitch J, Woo E, Hickman J, Ashton IW, Lerdau M. (2007) The interaction between soil nutrients and leaf loss during early establishment in plant invasion. For Sci 53: 701–709
- Leishman MR, Haslehurst T, Ares A, Baruch Z. (2007) Leaf trait relationships of native and invasive plants: community- and global-scale comparisons. New Phytol 176: 635–643 [DOI] [PubMed] [Google Scholar]
- Leith ID, Hicks WK, Fowler D, Woodin SJ. (1999) Differential responses of UK uplands plants to nitrogen deposition. New Phytol 141: 277–289 [DOI] [PubMed] [Google Scholar]
- Lellei-Kovacs E, Kovacs-Lang E, Kalapas T, Botta-Dukat Z, Barabas S, Beier C. (2008) Experimental warming does not enhance soil respiration in a semiarid temperate ecosystem. Conser Ecol 9: 29–37 [Google Scholar]
- Levine JM, Vilà M, D’Antonio C, Dukes JS, Grigulis K, Lavorel S. (2002) Mechanisms underlying the impacts of exotic plant invasions. Proc R Soc Lond 270: 775–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilleskov EA, Fahey TJ, Horton TR, Lovett GM. (2002) Belowground ectomycorrhizal fungal community change over a nitrogen deposition gradient in Alaska. Ecology 83: 104–115 [Google Scholar]
- Limousin JM, Misson L, Lavoir AV, Martin NK, Rambal S. (2010) Do photosynthetic limitations of evergreen Quercus ilex leaves change with long-term increased drought severity? Plant Cell Environ 33: 863–875 [DOI] [PubMed] [Google Scholar]
- Link SO, Smith JL, Halvorson JJ, Bolton H., Jr (2003) A reciprocal transplant experiment within a climatic gradient in a semiarid shrub-steppe ecosystem: effects on bunchgrass growth and reproduction, soil carbon, and soil nitrogen. Glob Change Biol 9: 1097–1105 [Google Scholar]
- Lipson DA, Bowman WD, Monson RK. (1996) Luxury uptake and storage on nitrogen in the rhizomatous alpine herb, Bistorta bistortoides. Ecology 77: 1277–1285 [Google Scholar]
- Lipson DA, Wilson RF, Oechel WC. (2006) Effects of elevated atmospheric CO2 on soil microbial biomass activity, and diversity in a chaparral ecosystems. Appl Environ Microbiol 71: 8573–8580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloret F, Escudero A, Iriondo JM, Martínez-Vilalta J, Valladares F. (2012) Extreme climatic events and vegetation: the role of stabilizing processes. Glob Change Biol 18: 797–805 [Google Scholar]
- Londsdale WM, Miller IL, Forno IW. (1989) The biology of Australian weeds. 20. Mimosa pingra L. Plant Prot Q 4: 119–131 [Google Scholar]
- Lorenz K, Feger KH, Kandeler E. (2001) The response of soil microbial biomass and activity of a Norway spruce forest to liming and drought. J Plant Nutr Soil Sci 164: 9–19 [Google Scholar]
- Lorenzo P, González L, Reigosa MJ. (2010) The genus Acacia as invader: the characteristic case of Acacia dealbata link in Europe. Ann Sci 67: 101 [Google Scholar]
- Lund N, Christensen TR, Mastepanov M, Lindroth A, Ström L. (2009) Effects of N and P fertilization on greenhouse gas exchange in two northern peatlands with contrasting N deposition rates. Biogeosciences 6: 2135–2144 [Google Scholar]
- Luo Y. (2007) Terrestrial carbon-cycle feedback to climate warming. Annu Rev Ecol Evol Syst 38: 683–712 [Google Scholar]
- Luo Y, Su B, Currie WS, Dukes JS, Finzi A, Hartwig U, Hungate B, McMurtrie RE, Oren R, Parton WJ, et al. (2004) Progressive nitrogen limitation of ecosystem responses to rising atmospheric carbon dioxide. Bioscience 54: 731–739 [Google Scholar]
- MacDonald GK, Bennett EM, Potter PA, Ramankutty N. (2011) Agronomic phosphorus imbalances across the world’s croplands. Proc Natl Acad Sci USA 108: 3086–3091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie FT, Ver LM, Lerman A. (2002) Century-scale nitrogen and phosphorus controls of the carbon cycle. Chem Geol 190: 13–32 [Google Scholar]
- Maestre FT, Cortina J. (2003) Small-scale spatial variation in soil CO2 efflux in a Mediterranean semiarid steppe. Appl Soil Ecol 23: 199–209 [Google Scholar]
- Magill AH, Aber JD, Hendricks JJ, Bowden RD, Melillo JM, Steudler PA. (1997) Biogeochemical response of forest ecosystems to simulated chronic nitrogen deposition. Ecol Appl 7: 402–415 [Google Scholar]
- Maiquetía M, Cáceres A, Herrera A. (2009) Mycorrhization and phosphorus nutrition affect water relations and CAM induction by drought in seedlings of Clusia minor. Ann Bot (Lond) 103: 525–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdi H, Öhrvik J. (2004) Interactive effects of soil warming and fertilization on root production, mortality, and longevity in a Norway spruce stand in northern Sweden. Glob Change Biol 10: 182–186 [Google Scholar]
- Manning P, Newington JE, Robson HR, Saunders M, Eggers T, Bradford MA, Bardgett RD, Bonkowski M, Ellis RJ, Gange AC, et al. (2006) Decoupling the direct and indirect effects of nitrogen deposition on ecosystem function. Ecol Lett 9: 1015–1024 [DOI] [PubMed] [Google Scholar]
- Manning P, Saunders M, Bardgett RD, Bonkowski M, Bradford MA, Ellis RJ, Kandeler E, Marhan S, Tscherko D. (2008) Direct and indirect effects of nitrogen deposition on litter decomposition. Soil Biol Biochem 40: 688–698 [Google Scholar]
- Mansson KF, Falkengren-Grerup U. (2003) The effects of nitrogen deposition on nitrification, carbon and nitrogen mineralization and litter C:N ratios in oak (Quercus ilex L.) forest. For Ecol Manag 179: 455–467
- Manzoni S, Taylor P, Richter A, Porporato A, Agren GI. (2012) Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. New Phytol 196: 79–91 [DOI] [PubMed] [Google Scholar]
- Marklein AR, Houlton BZ. (2012) Nitrogen inputs accelerate phosphorus cycling rates across a wide variety of terrestrial ecosystems. New Phytol 193: 696–704 [DOI] [PubMed] [Google Scholar]
- Matías L, Castro J, Zamora R. (2011) Soil-nutrient availability under a global-change scenario in a Mediterranean mountain ecosystem. Glob Change Biol 17: 1646–1657 [Google Scholar]
- Matson PA, McDowell WH, Townsend AR, Vitousek PM. (1999) The globalization of N deposition: ecosystem consequences in tropical environments. Biogeochemistry 46: 67–83 [Google Scholar]
- Matzek V. (2011) Superior performance and nutrient-use efficiency of invasive plants over non-invasive congeners in a resource-limited environment. Biol Invasions 13: 3005–3014 [Google Scholar]
- Mayor AG, Bautista S. (2012) Multi-scale evaluation of soil functional indicators for the assessment of water and soil retention in Mediterranean semiarid landscape. Ecol Indic 20: 332–336 [Google Scholar]
- Mayor AG, Bautista S, Bellot J. (2009) Factors and interactions controlling infiltration, runoff, and soil loss at the microscale in a patchy Mediterranean semiarid landscape. Earth Surf Process Landf 34: 1702–1711 [Google Scholar]
- Meier IC, Leuschner C. (2008) Belowground drought response of European beech: fine root biomass and carbon partitioning in 14 mature stands across a precipitation gradient. Glob Change Biol 14: 2081–2095 [Google Scholar]
- Melillo JM, Butler S, Johnson J, Mohan J, Steudler P, Lux H, Burrows E, Bowles F, Smith R, Scott L, et al. (2011) Soil warming, carbon-nitrogen interactions, and forest carbon budgets. Proc Natl Acad Sci USA 108: 9508–9512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menge DNL, Field CB. (2007) Simulated global changes alter phosphorus demand in annual grassland. Glob Change Biol 13: 2582–2591 [Google Scholar]
- Merino-Martin L, Moreno-de las Heras M, Pérez-Domingo S, Espigarea T, Nicolau JM. (2012) Hydrological heterogeneity in Mediterranean reclaimed slopes: runoff and sediment yield at the patch and slope scales along a gradient of overland flow. Hydrol Earth Syst Sci 16: 1305–1320 [Google Scholar]
- Michopoulos P, Baloutsos G, Economou A, Nikolis N. (2004) Effects of nitrogen deposition on nitrogen cycling in an Aleppo pine stand in Athens, Greece. Sci Total Environ 323: 211–218 [DOI] [PubMed] [Google Scholar]
- Miki T. (2012) Microbe-mediated plant-soil feedback and its roles in a changing world. Ecol Res 27: 509–520 [Google Scholar]
- Milla R, Castro-Díez P, Maestro-Martínez M, Montserrat-Martí G. (2005) Relationships between phenology and the remobilization of nitrogen, phosphorus and potassium in branches of eight Mediterranean evergreens. New Phytol 168: 167–178 [DOI] [PubMed] [Google Scholar]
- Monaco TA, Johnson DA, Norton JM, Jones TA, Connors KJ, Norton JB, Redinbaugh MB. (2003) Contrasting responses of intermountain west grasses to soil nitrogen. J Range Manage 56: 282–290 [Google Scholar]
- Moreno-de las Heras M, Espigares T, Merino-Martín L, Nicolau JM. (2011) Water-related ecological impacts of rill erosion processes in Mediterranean-dry reclaimed slopes. Catena 84: 114–124 [Google Scholar]
- Morris TL, Esler KJ, Barger NN, Lacobs SM, Cramer MD. (2011) Ecophysiological traits associated with the competitive ability of invasive Australian acacias. Divers Distrib 17: 898–910 [Google Scholar]
- Moulton CA, Gough L. (2011) Effects of soil nutrient availability on the role of sexual reproduction in an Alaskan tundra. Arctic Antarctic Alpine Res 43: 612–620 [Google Scholar]
- Mozdzer TJ, Zieman JC. (2010) Ecophysiological differences between genetic lineages facilitate the invasion of non-native Phragmites australis in North American Atlantic coast wetlands. J Ecol 98: 451–458 [Google Scholar]
- Munné-Bosch S, Lalueza P. (2007) Age-related changes in oxidative stress markers and abscisic acid levels in a drought-tolerant shrub, Cistus clusii grown under Mediterranean field conditions. Planta 225: 1039–1049 [DOI] [PubMed] [Google Scholar]
- Murray MB, Smith RI, Friend A, Jarvis PG. (2000) Effect of elevated [CO2] and varying nutrient application rates on physiology and biomass accumulation of Sitka spruce (Picea sitchensis). Tree Physiol 20: 421–434 [DOI] [PubMed] [Google Scholar]
- Muth NZ, Pigliucci M. (2007) Implementation of a novel framework for assessing species plasticity in biological invasions: responses of Centaurea and Crepis to phosphorus and water availability. J Ecol 95: 1001–1013 [Google Scholar]
- Nadelhoffer KJ. (2000) The potential effects of nitrogen deposition on fine-root production in forest ecosystems. New Phytol 147: 131–139 [Google Scholar]
- Nagel JM, Griffin KL. (2001) Construction cost and invasive potential: comparing Lythrum salicaria (Lythraceae) with co-occurring native species along pond banks. Am J Bot 88: 2252–2258 [PubMed] [Google Scholar]
- Naya L, Ladrera R, Ramos J, González EM, Arrese-Igor C, Minchin FR, Becana M. (2007) The response of carbon metabolism and antioxidant defenses of alfalfa nodules to drought stress and to the subsequent recovery of plants. Plant Physiol 144: 1104–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves JP, Simöes MP, Ferreira LF, Madeira M, Gazarini LC. (2010) Comparison of biomass and nutrient dynamics between an invasive and a native species in a Mediterranean saltmarsh. Wetlands 30: 817–826 [Google Scholar]
- Newton PCD, Clark H, Bell CC, Glasgow EM, Tate KR, Ross DJ, Yates GW, Saggar S. (1995) Plant growth and soil processes in temperate grassland communities at elevated CO2. J Biogeogr 22: 235–240 [Google Scholar]
- Newton PCD, Lieffering M, Saman WM, Bowatte D, Brock SC, Hunt CL, Theobald PW, Ross DJ. (2010) The rate of progression and stability of progressive nitrogen limitation at elevated atmospheric CO2 in a grassland over 11 years of free air CO2 enrichment. Plant Soil 336: 433–441 [Google Scholar]
- Niklaus PA, Kandeler E, Leadley PW, Schmid B, Tscherko D, Körner C. (2001) A link between plant diversity, elevated CO2 and soil nitrate. Oecologia 127: 540–548 [DOI] [PubMed] [Google Scholar]
- Nilsen P, Borja I, Knutsen H, Brean R. (1998) Nitrogen and drought effects on ectomycorrhizae of Norway spruce, Picea abies L.(Karst.). Plant Soil 198: 179–184 [Google Scholar]
- Nilsson LO, Wallander H, Baath E, Falkengren-Grerup U. (2006) Soil N chemistry in oak forest along a nitrogen deposition gradient. Biogeochemistry 80: 43–55 [DOI] [PubMed] [Google Scholar]
- Nissinen A, Hari P. (1998) Effects of nitrogen deposition on tree growth and soil nutrients in boreal Scots pine stands. Environ Pollut 102: 61–68 [Google Scholar]
- Norby RJ, Iversen CM. (2006) Nitrogen uptake, distribution, turnover, and efficiency of use in a CO2-enriched sweetgum forest. Ecology 87: 5–14 [DOI] [PubMed] [Google Scholar]
- Norby RJ, Warren JM, Iversen CM, Medlyn BE, McMurtrie RE. (2010) CO2 enhancement of forest productivity constrained by limited nitrogen availability. Proc Natl Acad Sci USA 107: 19368–19373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norby RJ, Wullschleger SD, Gunderson CA, Johnson DW, Ceullemans R. (1999) Tree responses to rising CO2 in field experiments: implications for the future forest. Plant Cell Environ 22: 683–714 [Google Scholar]
- Ochoa-Hueso R, Hernandez RR, Pueyo JJ, Manrique E. (2011) Spatial distribution and physiology of biological soil crust from semi-arid central Spain are related to soil chemistry and shrub cover. Soil Biol Biochem 43: 1894–1901 [Google Scholar]
- Ogaya R, Peñuelas J. (2007) Tree growth, mortality, and above-ground biomass accumulation in a holm oak forest under a five-year experimental field drought. Plant Ecol 189: 291–299 [Google Scholar]
- Ogaya R, Peñuelas J. (2009) Changes in leaf δ13C and δ15N for three Mediterranean tree species in relation to soil water availability. Acta Oecol 34: 331–338 [Google Scholar]
- Olsrud M, Carlsson BA, Svensson BM, Michelsen A, Melillo JM. (2010) Responses of fungal root colonization, plant cover and leaf nutrients to long-term exposure to elevated atmospheric CO2 and warming in a subarctic birch forest understory. Glob Change Biol 16: 1820–1829 [Google Scholar]
- Onipchenko VG, Makarov MI, van Logtestijn RSP, Ivanov VB, Akhmetzhanova AA, Tekeev DK, Ermak AA, Salpagarova FS, Kozhevnikova AD, Cornelissen JHC. (2009) New nitrogen uptake strategy: specialized snow roots. Ecol Lett 12: 758–764 [DOI] [PubMed] [Google Scholar]
- Packett CR, Chambers RM. (2006) Distribution and nutrients status of haplotypes of the marsh grass Phragmites australis along the Rappahannock River in Virginia. Estua Coast 68: 1222–1225 [Google Scholar]
- Papanikolaou N, Britton AJ, Helliwell RC, Johnson D. (2010) Nitrogen deposition, vegetation burning and climate warming act independently on microbial community structure and enzyme activity associated with decomposing litter in low-alpine heath. Glob Change Biol 16: 3120–3132 [Google Scholar]
- Peng RH, Fang CM, Li B, Chen JK. (2011) Spartina alterniflora invasion increases soil inorganic nitrogen pools through interactions with tidal subsidies in the Yangtze Estuary, China. Oecologia 165: 797–807 [DOI] [PubMed] [Google Scholar]
- Peñuelas J, Estiarte M. (1997) Trends in plant carbon concentration and plant demand for N through this century. Oecologia 109: 69–73 [DOI] [PubMed] [Google Scholar]
- Peñuelas J, Estiarte M, Llusia J. (1997) Carbon-based secondary compounds at elevated CO2. Photosynthetica 33: 313–316 [Google Scholar]
- Peñuelas J, Filella I. (2001) Herbaria century record of increasing eutrophication in Spanish terrestrial ecosystems. Glob Change Biol 7: 427–433 [Google Scholar]
- Peñuelas J, Gordon C, Llorens L, Nielsen T, Tietema A, Beier C, Bruna P, Emmett B, Estiarte M, Gorissen A. (2004) Nonintrusive field experiments show different plant responses to warming and drought among sites, seasons, and species in a north-south European gradient. Ecosystems (New York) 7: 598–612 [Google Scholar]
- Peñuelas J, Matamala R. (1990) Changes in N and S leaf content, stomatal density and specific leaf area of 14 plant species during the last three centuries of CO2 increase. J Exp Bot 41: 1119–1124 [Google Scholar]
- Peñuelas J, Matamala R. (1993) Variations in the mineral composition of herbarium plant species collected during the last three centuries. J Exp Bot 44: 1523–1525 [Google Scholar]
- Peñuelas J, Sardans J, Llusia J, Owen SM, Carnicer J, Giambelluca TW, Rezende E, Waite M, Niinemetts Ü. (2010) Faster return on “leaf economics” and different biogeochemical niche in invasive compared with native plant species. Glob Change Biol 16: 2171–2185 [Google Scholar]
- Peñuelas J, Sardans J, Rivas-Ubach A, Janssens IA. (2012) The human imbalance between C, N and P in Earth’s life system. Glob Change Biol 18: 3–6 [Google Scholar]
- Perring MP, Hedin LO, Levin SA, McGroddy M, de Mazancourt C. (2008) Increased plant growth from nitrogen addition should conserve phosphorus in terrestrial ecosystems. Proc Natl Acad Sci USA 105: 1971–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters GP, Marland G, Le Quéré C, Boden T, Canadell JG, Rapauch MR. (2011) Rapid growth in CO2 emissions after the 2008–2009 global financial crisis. Nat Clim Change 2: 2–4 [Google Scholar]
- Phillips RP, Finzi AC, Bernhardt ES. (2011) Enhanced root exudation induces microbial feedbacks to N cycling in a pine forest under long-term CO2 fumigation. Ecol Lett 14: 187–194 [DOI] [PubMed] [Google Scholar]
- Phoenix GK, Booth RE, Leake JB, Read DJ, Grime JP, Lee JA. (2003) Simulated pollutant nitrogen deposition increases P demand and enhances root-surface phosphatase activities of three plant functional types in a calcareous grassland. New Phytol 161: 279–289 [Google Scholar]
- Phoenix GK, Emmett BA, Britton AJ, Caporn SJM, Dise NB, Helliwell R, Jones L, Leake JB, Leith ID, Sheppard LJ, et al. (2012) Impacts of atmospheric nitrogen deposition: responses of multiple plant and soil parameters across contrasting ecosystems in long-term field experiments. Glob Change Biol 18: 1197–1215 [Google Scholar]
- Planchet E, Rannou O, Ricoult C, Boutet-Mercey S, Maia-Grondard A, Limami AM. (2011) Nitrogen metabolism responses to water deficit act through both abscisic acid (ABA)-dependent and independent pathways in Medicago truncatula during post-germination. J Exp Bot 62: 605–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley HW, Johnson HB, Mayeux HS. (1997) Leaf physiology, production, water use, and nitrogen dynamics of the grassland invader Acacia smallii at elevated CO2 concentrations. Tree Physiol 17: 89–96 [DOI] [PubMed] [Google Scholar]
- Pritchard SG, Rogers HH. (2000) Spatial and temporal deployment of crop roots in CO2-enriched environments. New Phytol 147: 55–71 [Google Scholar]
- Prieto P, Peñuelas J, Llusia J, Asensio D, Estiarte M. (2009) Effects of experimental warming and drought on biomass accumulation in a Mediterranean shrubland. Plant Ecol 205: 179–191 [Google Scholar]
- Pysek P, Jarosik V, Hulme PE, Pergl J, Hejda M, Schaffner U, Vilà M. (2012) A global assessment of invasive plant impacts on resident species, communities and ecosystems: the interaction of impact measures, invading species’ traits and environment. Glob Change Biol 18: 1725–1737 [Google Scholar]
- Quaggiotti S, Ruperti B, Borsa P, Destro T, Malagoli M. (2003) Expression of a putative high-affinity NO3− transporter and of an H+-ATPase in relation to whole plant nitrate transport physiology in two maize genotypes differently responsive to low nitrogen availability. J Exp Bot 54: 1023–1031 [DOI] [PubMed] [Google Scholar]
- Radin JW, Ackerson RC. (1981) Water relations of cotton plants under nitrogen deficiency. Plant Physiol 67: 115–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid I, Reshi ZA. (2012) Allelopathic interaction of an alien invasive species Anthemis cotula on its neighbours Conyza canadiensis and Galinsoga parviflora. Allelopathy J 29: 77–91 [Google Scholar]
- Reed HE, Seastedt TR, Blair JM. (2005) Ecological consequences of C4 grass invasion of a C4 grassland: a dilemma for management. Ecol Appl 15: 1560–1569 [Google Scholar]
- Reich PB, Hungate BA, Luo Y. (2006) Carbon-nitrogen interactions in terrestrial ecosystems in response to rising atmospheric carbon dioxide. Annu Rev Ecol Evol Syst 37: 611–636 [Google Scholar]
- Reich PB, Tjoelker MG, Pregitzer KS, Wright IJ, Oleksyn J, Machado JL. (2008) Scaling of respiration to nitrogen in leaves, stems and roots of higher land plants. Ecol Lett 11: 793–801 [DOI] [PubMed] [Google Scholar]
- Remans T, Nacry P, Pervent M, Girin T, Tillard P, Lepetit M, Gojon A. (2006) A central role for the nitrate transporter NRT2.1 in the integrated morphological and physiological responses of the root system to nitrogen limitation in Arabidopsis. Plant Physiol 140: 909–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinnan R, Michelsen A, Baath E, Jonasson S. (2007) Mineralization and carbon turnover in subarctic heath soil as affected by warming and additional litter. Soil Biol Biochem 39: 3014–3023 [Google Scholar]
- Rivas-Ubach A, Sardans J, Pérez-Trujillo M, Estiarte M, Peñuelas J. (2012) Strong relationship between elemental stoichiometry and metabolome in plants. Proc Natl Acad Sci USA 109: 4181–4186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson CH, Saunders PW, Madan NJ, Pryce-Miller EJ, Pentecost A. (2004) Does nitrogen deposition affect soil microfungal diversity and soil N and P dynamics in a high arctic ecosystem? Glob Change Biol 10: 1065–1079 [Google Scholar]
- Roldán A, Díaz-Vivancos P, Hernández JA, Carrasco L, Caravaca F. (2008) Superoxide dismutase and total peroxidase activities in relation to drought recovery performance of mycorrhizal shrub seedlings grown in an amended semiarid soil. J Plant Physiol 165: 715–722 [DOI] [PubMed] [Google Scholar]
- Rout ME, Chrzanowski TH. (2009) The invasive Sorghum halepense harbors endophytic N2-fixing bacteria and alters soil biogeochemistry. Plant Soil 315: 163–172 [Google Scholar]
- Rudgers JA, Orr S. (2009) Non-native grass alters growth of native tree species via leaf and soil microbes. J Ecol 97: 247–255 [Google Scholar]
- Ruffel S, Freixes S, Balzergue S, Tillard P, Jeudy C, Martin-Magniette ML, van der Merwe MJ, Kakar K, Gouzy J, Fernie AR, et al. (2008) Systemic signaling of the plant nitrogen status triggers specific transcriptome responses depending on the nitrogen source in Medicago truncatula. Plant Physiol 146: 2020–2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Lozano JM, Azcon R, Gómez M. (1995) Effects of arbuscular-mycorrhizal Glomus species on drought tolerance: physiological and nutritional plant responses. Appl Environ Microbiol 61: 456–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Lozano JM, Ázcon R, Palma JM. (1996) Superoxide dismutase activity in arbuscular mycorrhizal Lactuca sativa plants subjected to drought stress. New Phytol 134: 327–333 [Google Scholar]
- Ruiz-Sinoga JD, Gabarón Galeote MA, Martínez Murillo JF, Garcia Marín R. (2011) Vegetation strategies for soil consumption along a pluviometric gradient in southern Spain. Catena 84: 12–20 [Google Scholar]
- Ruiz-Sinoga JD, Martínez-Murillo JF. (2009) Effects of soil surface components on soil hydrological behavior in a dry Mediterranean environment (Southern Spain). Geomorphology 108: 234–245 [Google Scholar]
- Ruiz-Sinoga JD, Pariente S, Romero Díaz A, Martínez Murillo JF. (2012) Variability of relationships between soil organic carbon and some soil properties in Mediterranean rangelands under different climatic conditions (south of Spain). Catena 94: 17–25 [Google Scholar]
- Ryan MG, O’Toole P, Farrell EP. (1998) The influence of drought and natural rewetting on nitrogen dynamics in a coniferous ecosystem in Ireland. Environ Pollut (Suppl I) 102: 445–451
- Saiya-Cork KR, Sinsabaugh RL, Zak DR. (2002) The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol Biochem 34: 1309–1315 [Google Scholar]
- Sala A, Verdaguer D, Vilà M. (2007) Sensitivity of the invasive geophyte Oxalis pes-caprae to nutrient availability and competition. Ann Bot (Lond) 99: 637–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarakoon AB, Gifford RM. (1995) Soil water content plants at high CO2 concentration and interactions with the direct CO2 effects: a species comparison. J Biogeogr 22: 193–202 [Google Scholar]
- Sanaullah M, Rumpel C, Charrier X, Chabbi A. (2012) How does drought stress influence the decomposition of plant litter with contrasting quality in a grassland ecosystem? Plant Soil 352: 277–288 [Google Scholar]
- Sanz-Pérez V, Castro-Díez P, Millard P. (2009) Effects of drought and shade on nitrogen cycling in the leaves and canopy of Mediterranean Quercus seedlings. Plant Soil 316: 45–56 [Google Scholar]
- Sardans J, Peñuelas J. (2004) Increasing drought decreases phosphorus availability in an evergreen Mediterranean forest. Plant Soil 267: 367–377 [Google Scholar]
- Sardans J, Peñuelas J. (2005) Drought decreases soil enzyme activity in a Mediterranean Quercus ilex L. forest. Soil Biol Biochem 37: 455–461 [Google Scholar]
- Sardans J, Peñuelas J. (2007) Drought changes phosphorus and potassium accumulation patterns in an evergreen Mediterranean forest. Funct Ecol 21: 191–201 [Google Scholar]
- Sardans J, Peñuelas J. (2008) Drought changes nutrient sources, content and stoichiometry in the bryophyte Hypnum cupressiforme Hedw. growing in a Mediterranean forest. J Bryol 30: 59–65 [Google Scholar]
- Sardans J, Peñuelas J. (2010) Soil enzyme activity in a Mediterranean forest after six years of drought. Soc Soil Sci Am J 74: 838–851 [Google Scholar]
- Sardans J, Peñuelas J. (2012a) Plant-soil relationships in Mediterranean forest and shrublands: perspectives in the framework of CO2-rich atmosphere and climatic change. Plant Soil (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardans J, Peñuelas J. (2012b) Tree growth changes with climate and forest type are associated to relative allocation of nutrients, especially P, to leaves and wood. Glob Ecol Biogeogr (in press) [Google Scholar]
- Sardans J, Peñuelas J, Coll M, Vayreda J, Rivas-Ubach A. (2012a) Stoichiometry of potassium is largely determined by water availability and growth in Catalonian forests. Funct Ecol 26: 1077–1089 [Google Scholar]
- Sardans J, Peñuelas J, Estiarte M. (2006) Warming and drought alter soil phosphatase activity and soil P availability in a Mediterranean shrubland. Plant Soil 289: 227–238 [Google Scholar]
- Sardans J, Peñuelas J, Estiarte M. (2008a) Changes in soil enzymes related to C and N cycle and in soil C and N content under prolonged warming and drought in a Mediterranean shrubland. Appl Soil Ecol 39: 223–235 [Google Scholar]
- Sardans J, Peñuelas J, Estiarte M, Prieto P. (2008b) Warming and drought alter C and N concentration, allocation and accumulation in a Mediterranean shrubland. Glob Change Biol 14: 2304–2316 [Google Scholar]
- Sardans J, Peñuelas J, Ogaya R. (2008c) Drought-induced changes in C and N stoichiometry in a Quercus ilex Mediterranean forest. For Sci 54: 513–522
- Sardans J, Peñuelas J, Ogaya R. (2008d) Experimental drought reduced acid and alkaline phosphatase activity and increased organic extractable P in soil in a Quercus ilex Mediterranean forest. Eur J Soil Biol 44: 509–520 [Google Scholar]
- Sardans J, Peñuelas J, Prieto P, Estiarte M. (2008e) Drought and warming induced changes in P and K concentration and accumulation in plant biomass and soil in a Mediterranean shrubland. Plant Soil 306: 261–271 [Google Scholar]
- Sardans J, Rivas-Ubach A, Peñuelas J. (2011) Factors affecting nutrient concentration and stoichiometry of forest trees in Catalonia (NE Spain). For Ecol Manag 262: 2024–2034
- Sardans J, Rivas-Ubach A, Peñuelas J. (2012b) The C:N:P stoichiometry of organisms and ecosystems in a changing world: a review and perspectives. Perspect Plant Ecol Evol Syst 14: 33–47 [Google Scholar]
- Sardans J, Rivas-Ubach A, Peñuelas J. (2012c) The elemental stoichiometry of aquatic and terrestrial ecosystems and its relationships with organismic lifestyle and ecosystem structure and function: a review and perspectives. Biogeochemistry (in press) [Google Scholar]
- Sardans J, Rodà F, Peñuelas J. (2005) Effects of water and a nutrient pulse supply on Rosmarinus officinalis growth, nutrient content and flowering in the field. Environ Exp Bot 53: 1–11 [Google Scholar]
- Sas L, Marschner H, Römheld V, Mercik S. (2003) Effect of nitrogen forms on growth and chemical changes in the rhizosphere of strawberry plants. Acta Physiol Plant 25: 241–247 [Google Scholar]
- Scharfy D, Eggenschwiler H, Venterink OH, Edwards PJ, Güsewell S. (2009) The invasive alien plant species Solidago gigantea alters ecosystem properties across habitats with differing fertility. J Veg Sci 20: 1072–1085 [Google Scholar]
- Scharfy D, Funk A, Venterink HO, Güsewell S. (2011) Invasive forbs differ functionally from native graminoids, but are similar to native forbs. New Phytol 189: 818–828 [DOI] [PubMed] [Google Scholar]
- Schmidt I, Tietema A, Williams D, Gundersen P, Gordon C, Beier C, Emmett B, Estiarte M. (2004) Soil solution chemistry and element fluxes in three European heathlands and their responses to warming and drought. Ecosystems (New York) 7: 638–649 [Google Scholar]
- Schumacher E, Kueffer C, Edwards PJ, Dietz H. (2009) Influence of light and nutrient conditions on seedling growth of native and invasive trees in the Seychelles. Biol Invasions 11: 1941–1954 [Google Scholar]
- Schuster WS, Monson RK. (1990) An examination of the advantages of C3-C4 intermediate photosynthesis in warm environments. Plant Cell Environ 13: 903–912 [Google Scholar]
- Shah MA, Reshi ZA, Khasa DP. (2009) Arbuscular mycorrhizas: drivers or passengers of alien plant invasion. Bot Rev 75: 397–417 [Google Scholar]
- Shah MA, Reshi ZA, Rasool N. (2010) Plant invasion induce a shift in Glomalean spore diversity. Trop Ecol 51: 317–323 [Google Scholar]
- Sharma GP, Raghubanshi AS. (2009) Lantana invasion alters soil nitrogen pools and processes in the tropical dry deciduous forest of India. Appl Soil Ecol 42: 134–140 [Google Scholar]
- Shen H, Du H, Wang Z, Huang B. (2009) Differential responses of nutrients to heat stress in warm-season and cool-season turfgrasses. Hortic Sci 44: 2009–2014 [Google Scholar]
- Shen XY, Peng SL, Chen BM, Pang JX, Chen LY, Xu HM, Hou YP. (2011) Do higher resource capture ability and utilization efficiency facilitate the successful invasion of native plants? Biol Invasions 13: 869–881 [Google Scholar]
- Shi L, Guttenberger M, Kottke I, Hampp R. (2002) The effect of drought on mycorrhizas of beech (Fagus sylvatica L.): changes in community structure, and the content of carbohydrates and nitrogen storage bodies of the fungi. Mycorrhiza 12: 303–311 [DOI] [PubMed] [Google Scholar]
- Shimono H, Bunce A. (2009) Acclimation of nitrogen uptake capacity of rice to elevated atmospheric CO2 concentration. Ann Bot (Lond) 103: 87–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh DK, Sale PWG, Pallachy CK, McKenzie BM. (2000) Phosphorus concentrations in the leaves of defoliated white clover affect abscisic acid formation and transpiration in drying soil. New Phytol 146: 249–259 [DOI] [PubMed] [Google Scholar]
- Sinsabaugh RL, Gallo ME, Lauber C, Waldrop MP, Zak DR. (2005) Extracellular enzyme activities and soil organic matter dynamics for northern hardwood forest receiving simulated nitrogen deposition. Biogeochemistry 75: 201–215 [Google Scholar]
- Sorensen PL, Michelsen A. (2011) Long-term warming and litter addition affects nitrogen fixation in a subarctic heath. Glob Change Biol 17: 528–537 [Google Scholar]
- Sparrius LB, Sevink J, Kooijman AM. (2012) Effects of nitrogen deposition on soil and vegetation in primary succession stages in inland drift sands. Plant Soil 353: 261–272 [Google Scholar]
- Staddon PL, Gregersen R, Jakobsen I. (2004) The response of two Glomus mycorrhizal fungi and a fine endophyte to elevated atmospheric CO2, soil warming and drought. Glob Change Biol 10: 1909–1921 [Google Scholar]
- Sterner RW, Elser JJ.2002. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere. Princeton University Press, Princeton, NJ.
- Stevens CJ, Dise NB, Gowing DJG, Mountford JO. (2006) Loss of forb diversity in relation to nitrogen deposition in the UK: regional trends and potential controls. Glob Change Biol 12: 1823–1833 [Google Scholar]
- Stevens CJ, Duprè C, Dorland E, Gaudnik C, Gowing DJG, Bleeker A, Diekmann M, Alard D, Bobbink R, Fowler D, et al. (2011) The impact of nitrogen deposition on acid grasslands in the Atlantic region of Europe. Environ Pollut 159: 2243–2250 [DOI] [PubMed] [Google Scholar]
- Streeter JG. (2003) Effects of drought on nitrogen fixation in soybean root nodules. Plant Cell Environ 26: 1199–1204 [Google Scholar]
- Suralta RR. (2010) Plastic root system development responses to drought-enhanced nitrogen uptake during progressive soil drying conditions in rice. Philipp Agric Scientist 93: 458–462 [Google Scholar]
- Suriyagoda LDB, Ryan MH, Renton M, Lambers H. (2011) Above- and below-ground interactions of grass and pasture legume species when grown together under drought and low phosphorus availability. Plant Soil 348: 281–297 [Google Scholar]
- Suzuki S, Kudo G. (2000) Responses of alpine shrubs to simulated environmental change during three years in the mid-latitude mountain, northern Japan. Ecography 23: 553–564 [Google Scholar]
- Szente K, Nagy Z, Tuba Z, Fekete G. (1996) Photosynthesis of Festuca rupicola and Bothriochloa ischaemum under degradation and cutting pressure in a semiarid loess grassland. Photosynthetica 32: 399–407 [Google Scholar]
- Tao L, Hunter MD. (2012) Does anthropogenic nitrogen deposition induce phosphorus limitation in herbivorous insects? Glob Change Biol 18: 1843–1853 [Google Scholar]
- Taub DR, Wang X. (2008) Why are nitrogen concentrations in plant tissues lower under elevated CO2? A critical examination of the hypotheses. J Integr Plant Biol 50: 1365–1374 [DOI] [PubMed] [Google Scholar]
- Taylor SH, Ripley BS, Woodward FI, Osborne CP. (2011) Drought limitation of photosynthesis differs between C3 and C4 grass species in a comparative experiment. Plant Cell Environ 34: 65–75 [DOI] [PubMed] [Google Scholar]
- Tietema A. (1998) Microbial carbon and nitrogen dynamics in coniferous forest floor material collected along a European nitrogen deposition gradient. For Ecol Manag 101: 29–36
- Tilman D, Knops J, Wedin D, Reich P, Ritchie M, Siemann E. (1997) The influence of functional diversity and composition on ecosystem processes. Science 277: 1300–1302 [Google Scholar]
- Tobita H, Uemura A, Kitao M, Kitaoka S, Utsugi H. (2010) Interactive effects of elevated CO2 phosphorus deficiency, and soil drought on nodulation and nitrogenase activity in Alnus hirsuta and Alnus maximowiczii. Symbiosis 50: 59–69 [Google Scholar]
- Tognetti R, Peñuelas J. (2003) Nitrogen and carbon concentrations, and stable isotope ratios in Mediterranean shrubs growing in the proximity of a CO2 spring. Biol Plant 46: 411–418 [Google Scholar]
- Tomassen HBM, Smolders AJP, Lamers LPM, Roelofs JGM. (2003) Stimulated growth of Betula pubescens and Molinia caerulea on ombrotrophic bogs: role of high levels of atmospheric nitrogen deposition. J Ecol 91: 357–370 [Google Scholar]
- Treseder KK. (2004) A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies. New Phytol 164: 347–355 [DOI] [PubMed] [Google Scholar]
- Turner BL, Baxter R, Whitton BA. (2002) Seasonal phosphatase activity in three characteristic soils of the English uplands polluted by long-term atmospheric nitrogen deposition. Environ Pollut 120: 313–317 [DOI] [PubMed] [Google Scholar]
- Updegraff K, Bridgham SD, Pastor J, Weishampel P, Harth C. (2001) Response of CO2 and CH4 emissions from peatlands to warming and water table manipulation. Ecol Appl 11: 311–326 [Google Scholar]
- van Kleunen M, Weber E, Fischer M. (2010) A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol Lett 13: 235–245 [DOI] [PubMed] [Google Scholar]
- Van Meeteren MJM, Tietema A, van Loon EE, Verstraten JM. (2008) Microbial dynamics and litter decomposition under a changed climate in a Dutch heathland. Appl Soil Ecol 38: 119–127 [Google Scholar]
- Vicca S, Luyssaert S, Peñuelas J, Campioli M, Chapin FS, III, Ciais P, Heinemeyer A, Högberg P, Kutsch WL, Law BE, et al. (2012) Fertile forests produce biomass more efficiently. Ecol Lett 15: 520–526 [DOI] [PubMed] [Google Scholar]
- Vitousek PM. (1990) Biological invasion and ecosystem processes towards an integration of population biology and ecosystem studies. Oikos 57: 7–13 [Google Scholar]
- Vitousek PM, Walker LR, Whiteaker LD, Mueller-Dombois D, Matson PA. (1987) Biological invasion by Myrica faya alters ecosystem development in Hawaii. Science 238: 802–804 [DOI] [PubMed] [Google Scholar]
- Volder A, Gifford RM, Evans JR. (2007) Effects of elevated atmospheric CO2, cutting frequency, and differential day/night atmospheric warming on root growth and turnover of Phalaris swards. Glob Change Biol 13: 1040–1052 [Google Scholar]
- Von Lützow M, Kögel-Knabner I. (2009) Temperature sensitivity of soil organic matter decomposition: what do we know? Biol Fertil Soils 46: 1–15 [Google Scholar]
- Vourlitis GL, Zorba G. (2007) Nitrogen and carbon mineralization of semi-arid shrubland soil exposed to long-term atmospheric nitrogen deposition. Biol Fertil Soils 43: 611–615 [Google Scholar]
- Vourlitis GL, Zorba G, Pasquini SC, Mustard R. (2007) Chronic nitrogen deposition enhances nitrogen mineralization potential of semiarid shrublands soils. Soil Sci Soc Am J 71: 836–842 [Google Scholar]
- Waldrop MP, Zak DR. (2006) Response of oxidative enzyme activities to nitrogen deposition affects soil concentrations of dissolved organic carbon. Ecosystems (New York) 9: 921–933 [Google Scholar]
- Waldrop MP, Zak DR, Sinsabaugh RL. (2004a) Microbial community response to nitrogen deposition in northern forest ecosystems. Soil Biol Biochem 36: 1443–1451 [Google Scholar]
- Waldrop MP, Zak DR, Sinsabaugh RL, Gallo M, Lauber C. (2004b) Nitrogen deposition modifies soil carbon storage through changes in microbial enzymatic activity. Ecol Appl 14: 1171–1177 [Google Scholar]
- Wallenda T, Kottke I. (1998) Nitrogen deposition and ectomycorrhizas. New Phytol 139: 169–187 [Google Scholar]
- Waraich EA, Ahmad R, Saifullah, Ashraf MY, Ehsanullah (2011) Role of mineral nutrition in alleviation of drought stress in plants. Aust J Crop Sci 5: 764–777 [Google Scholar]
- Wardle DA, Nicholson KS, Ahmed M, Rahman A. (1994) Interference effects of the invasive plant Carduus nutans L. against the nitrogen fixation ability of Trifolium repens L. Plant Soil 163: 287–297 [Google Scholar]
- Weidenhamer JD, Callaway RM. (2010) Direct and indirect effects of invasive plants on soil chemistry and ecosystem function. J Chem Ecol 36: 59–69 [DOI] [PubMed] [Google Scholar]
- Weih M, Bonosi L, Ghelardini L, Rönnberg-Wästljung AC. (2011) Optimizing nitrogen economy under drought: increased leaf nitrogen is an acclimation to water stress in willow (Salix spp.). Ann Bot (Lond) 108: 1347–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CS, Moore DI, Craig JA. (2004) Regional-scale drought increases potential soil fertility in semiarid grassland. Biol Fertil Soils 40: 73–78 [Google Scholar]
- Wittenmayer L, Merbach W. (2005) Plant responses to drought and phosphorus deficiency: contribution of phytohormones in root-related processes. J Plant Nutr Soil Sci 168: 531–540 [Google Scholar]
- Wookey PA, Robinson CH, Parsons AN, Welker JM, Press MC, Callaghan TV, Lee JA. (1995) Environmental constraints on the growth, photosynthesis and reproductive development of Dryas octopetala at a high arctic polar semi-desert, Svalbard. Oecologia 192: 478–489 [DOI] [PubMed] [Google Scholar]
- Wu QS, Zou YN, He XH. (2011a) Differences of hyphal and soil phosphatase activities in drought-stressed mycorrhizal trifoliate orange (Poncirus trifoliate) seedlings. Sci Hortic (Amsterdam) 129: 294–298 [Google Scholar]
- Wu Z, Dijkstra P, Koch GW, Peñuelas J, Hungate BA. (2011b) Responses of terrestrial ecosystems to temperature and precipitation change: a meta-analysis of experimental manipulation. Glob Change Biol 17: 927–942 [Google Scholar]
- Xu CY, Griffin KL, Schuster WSF. (2007) Leaf phenology and seasonal variation of photosynthesis of invasive Berberis thunbergii (Japanese barberry) and two co-occurring native understory shrubs in a northeastern United States deciduous forest. Oecologia 154: 11–21 [DOI] [PubMed] [Google Scholar]
- Xu ZZ, Zhou GS. (2006) Nitrogen metabolism and photosynthesis in Leymus chinensis in response to long-term soil drought. J Plant Growth Regul 23: 252–266 [Google Scholar]
- Yarie J, van Cleve K. (1996) Effects of carbon, fertilizer, and drought on foliar chemistry of tree species in interior Alaska. Ecol Monogr 6: 815–827 [Google Scholar]
- Yavitt JB, Wright SJ, Weider RK. (2004) Seasonal drought and dry-season irrigation influence leaf-litter nutrients and soil enzymes in a moist, lowland forest in Panama. Austral Ecol 29: 177–188 [Google Scholar]
- Yelenik SG, Levine JM. (2011) The role of plant-soil feedbacks in driving native-species recovery. Ecology 92: 66–74 [DOI] [PubMed] [Google Scholar]
- Yelenik SG, Stock WD, Richardson DM. (2004) Ecosystem level impacts of invasive Acacia saligna in the South African fynbos. Restor Ecol 12: 44–51 [Google Scholar]
- Zabinsky CA, Quinn L, Callaway RM. (2002) Phosphorus uptake, not carbon transfer, explains arbuscular mycorrhizal enhancement of Centaurea maculosa in the presence of native grassland species. Funct Ecol 16: 758–765 [Google Scholar]
- Zaimes GN, Emmanouloudis D, Iakovoglou V. (2012) Estimating soil erosion in Natura 2000 areas located on three semi-arid Mediterranean islands. J Environ Biol 33: 277–282 [PubMed] [Google Scholar]
- Zak DR, Holmes WE, Finzi AC, Norby RJ, Schlesinger WH. (2003) Soil nitrogen cycling under elevated CO2: a synthesis of forest face experiments. Ecol Monogr 13: 1508–1514 [Google Scholar]
- Zak DR, Pregitzer KS, King JS, Holmes WE. (2000) Elevated atmospheric CO2, fine roots and the response of soil microorganisms: a review and hypothesis. New Phytol 147: 201–222 [Google Scholar]
- Zanetti S, Hartwig UA, Lüscher A, Hebeisen T, Frehner M, Fischer BU, Hendrey GR, Blum H, Nösberger J. (1996) Stimulation of symbiotic N2 fixation in Trifolium repens L. under elevated atmospheric pCO2 in a grassland ecosystem. Plant Physiol 112: 575–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeglin LH, Stursova M, Sinsabaugh RL, Collins SL. (2007) Microbial responses to nitrogen addition in three contrasting grassland ecosystems. Oecologia 154: 349–359 [DOI] [PubMed] [Google Scholar]
- Zhang Q, Yao LJ, Yang RY, Yang XY, Tang JJ, Chen X. (2007) Potential allelophatic effects of an invasive species Solidago canadensis on the mycorrhizae of native plant species. Allelophatic J 20: 71–77 [Google Scholar]
- Zhang YL, Hu YY, Luo HH, Chow WS, Zhang WF. (2011) Two distinct strategies of cotton and soybean differing in leaf movement to perform photosynthesis under drought in the field. Funct Plant Biol 38: 567–575 [DOI] [PubMed] [Google Scholar]
- Zhao D, Reddy KR, Kakani VG, Reddy VR. (2005) Nitrogen deficiency effects on plant growth, leaf photosynthesis, and hyperspectral reflectance properties of sorghum. Eur J Agron 22: 391–403 [Google Scholar]