The oceans take up more than 1 million tons of CO2 from the air per hour, about one-quarter of the anthropogenically released amount, leading to disrupted seawater chemistry due to increasing CO2 emissions. Based on the fossil fuel-intensive CO2 emission scenario (A1F1; Houghton et al., 2001), the H+ concentration or acidity of surface seawater will increase by about 150% (pH drop by 0.4) by the end of this century, the process known as ocean acidification (OA; Sabine et al., 2004; Doney et al., 2009; Gruber et al., 2012). Seawater pH is suggested to decrease faster in the coastal waters than in the pelagic oceans due to the interactions of hypoxia, respiration, and OA (Cai et al., 2011). Therefore, responses of coastal algae to OA are of general concern, considering the economic and social services provided by the coastal ecosystem that is adjacent to human living areas and that is dependent on coastal primary productivity. On the other hand, dynamic environmental changes in the coastal waters can interact with OA (Beardall et al., 2009).
Macroalgae have diversified strategies in terms of inorganic carbon (Ci) acquisition, with different carboxylation efficiencies associated with different photosynthetic affinities for Ci (Johnston and Raven, 1990; Giordano et al., 2005; Zou and Gao, 2010). Most macroalgae can actively use bicarbonate or directly take up CO2 to provide a CO2 source for Rubisco, a mechanism known as the carbon-concentrating mechanism (CCM), while a few red and green macroalgae, known as “non bicarbonate users,” acquire Ci solely by diffusion of dissolved CO2 (Raven et al., 1995; Kübler et al., 1999). Therefore, macroalgae may respond differentially to increasing pCO2 (for partial pressure of CO2 in seawater) and the changing chemistry of seawater. Increasing atmospheric CO2 concentrations have been demonstrated to enhance the photosynthesis of intertidal macroalgae at low tide during emersion (Gao et al., 1999; Zou and Gao, 2005) and enhance the growth of the red algae Porphyra yezoensis, Gracilaria spp., and Lomentaria articulata (Gao et al., 1991, 1993; Kübler et al., 1999) and the brown alga Hizikia fusiforme (Zou, 2005). However, decreased growth rates under elevated CO2 concentrations are observed in Gracilaria tenuistipitata (Garcŕa-Sánchez et al., 1994), Porphyra leucostica (Mercado et al., 1999), and Porphyra linearis (Israel et al., 1999). Neutral effects of elevated CO2 levels (750 μatm) on the growth of several macroalgae are also reported (Israel and Hophy, 2002). Recent research shows that meiospore germination in the brown macroalga Macrocystis pyrifera benefits from the increased availability of CO2 (820 μatm; Roleda et al., 2012). Despite these differential responses, OA is notorious for reducing calcification of the red coralline algae (Gao et al., 1993; Gao and Zheng, 2010), green Halimeda spp. (Sinutok et al., 2011), and brown Padina spp. (Johnson et al., 2012). Furthermore, elevated CO2 has the potential to influence competition between noncalcareous macroalgae and coralline species (Hepburn et al., 2011).
Marine green algae represent a large paraphyletic group of green plants from which the higher plants (the embryophytes) developed (Douglas et al., 2004). They share the same photosynthetic pigments with, but live in a quite different environment from, terrestrial higher plants. These plants, mostly distributed in coastal waters, where biological production is high, experience dynamic environmental changes associated with reciprocal tides and human activities. In most coastal waters, because of the photosynthetic carbon removal from and respiratory carbon release to the ambient environment, fluctuation of pH usually shows a day-night (high to low) reversion pattern. Therefore, marine green algae are usually tolerant to acid-base perturbations (Larsson et al., 1997), although the mechanisms involved are not understood yet. From a physiological point of view, marine green algae may show fairly different responses from terrestrial plants to increasing CO2 concentration, if acidity change acts to affect their physiology. However, to our knowledge, little has been documented on this aspect.
Increased availability of ambient CO2 to about 1,000 μatm based on the projected future atmospheric CO2 rise may not be large enough to affect the influx of CO2 into cells because of the operation of CCMs, which will result in concentrated CO2 within the cells (Raven et al., 2011). Although CCMs can be down-regulated under elevated CO2 levels, they are supposed not to have been switched off (Wu et al., 2012), and intracellular CO2 concentration and energetics for algal growth can be altered (Hopkinson et al., 2011; Raven et al., 2011). On the other hand, increased external acidity associated with the enrichment of CO2 would either exert a stress that influences intracellular acid-base stability (Gao et al., 2012) or ease the acid-base regulation, since most algae maintain an average pH across the cell of between 7 and 7.5 (Smith and Raven, 1979). Therefore, energetics can also be affected due to changes of pH in the ambient environment. At the same time, the oxygenation process might be enhanced, since the CO2-to-oxygen ratio surrounding Rubisco may be changed due to the down-regulation of CCMs. Based on the above theoretical assumptions, we hypothesize that the effects of future CO2-induced OA on marine green algae will depend on light levels due to affected energetics associated with the down-regulation of CCMs as well as acid-base regulation; therefore, dark- and light-dependent respiration would be enhanced under stressful light levels due to changes in the algae’s energetics to cope with the increased acidity. We chose the green macroalga Ulva prolifera (Zhang et al., 2011), which is commonly found in the coastal waters of the Pacific Ocean, to test this hypothesis. This species is also notorious for forming large-scale green tides (Lu and Qiao, 2008) in recent years.
PHOTOSYNTHETIC PERFORMANCE
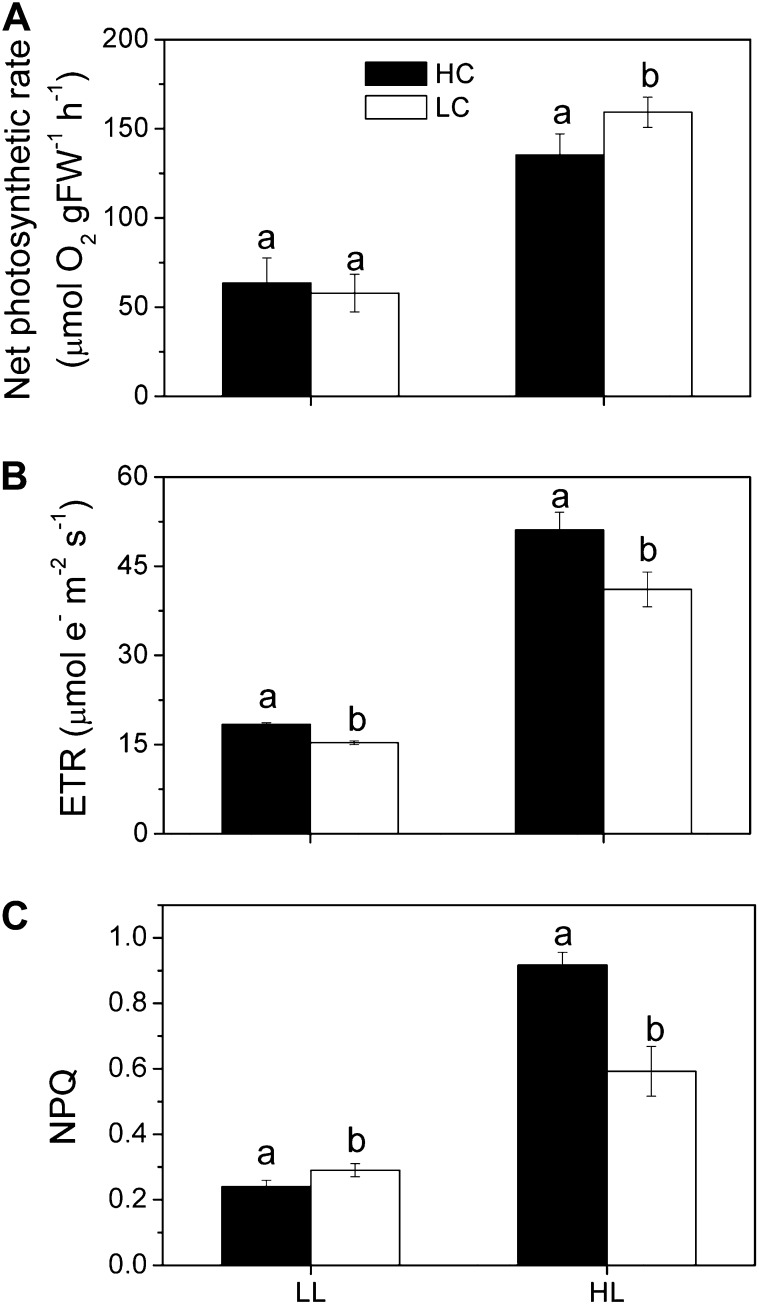
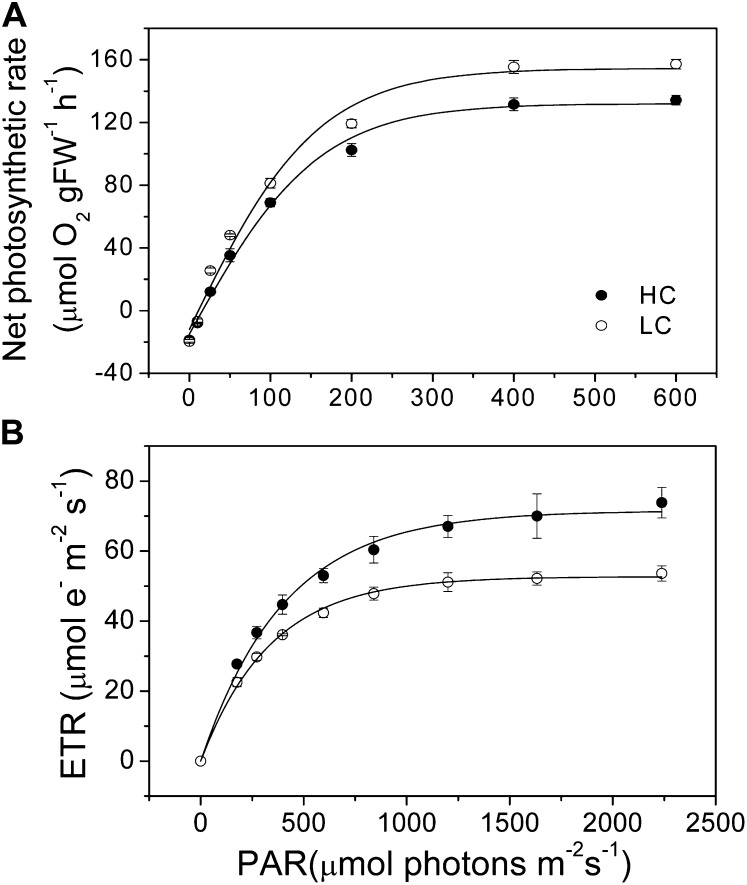
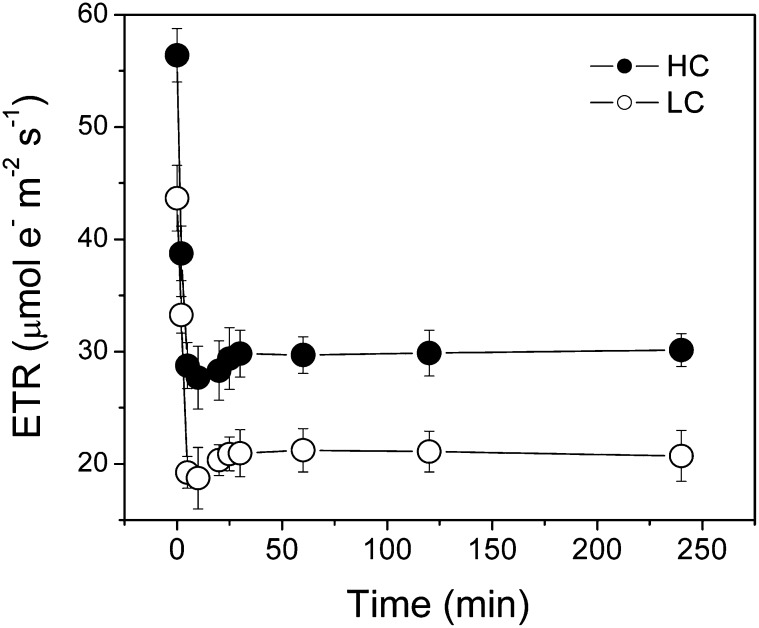
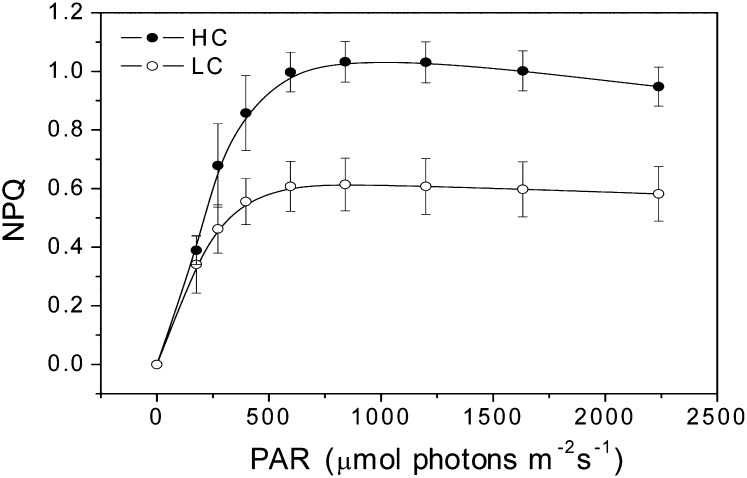
U. prolifera plants, developed from zoospores under different CO2 and pH levels, showed significant differences in growth and photosynthetic performance. The relative growth rate was significantly (P = 0.038) enhanced (Fig. 1) under elevated CO2 level. Under the growth light level (100 µmol photons m−2 s−1), electron transport rate (ETR) was higher (P = 0.001), while nonphotochemical quenching (NPQ) was significantly lower (P = 0.04), and no significant difference (P = 0.6) was found in the net photosynthetic (oxygen evolution) rate between the high-CO2 (HC)- and low-CO2 (LC)-grown plants. Under the high light levels (600 µmol photons m−2 s−1), both the ETR and NPQ were higher (P = 0.01), while the net photosynthetic oxygen evolution rate was significantly lower (P = 0.046) in the HC-grown plants (Fig. 2). When measured at the ambient CO2 level (pH 8.2), the light-saturated photosynthetic oxygen evolution rate (Pmax) and the apparent photosynthetic light use efficiency [α(P)] were significantly (P = 0.005 and 0.023, respectively) lower (Fig. 3A; Table I). However, the light-saturated ETR (ETRmax) and electron transport efficiency [α(ETR)] were higher (P = 0.01 and 0.038, respectively; Fig. 3B; Table I) in HC-grown than in LC-grown individuals. When examined in a CO2-free medium (pH 8.2), the ETR decreased immediately, with the depletion of the intracellular Ci pool within 5 min, and was then sustained for the following 4 h, with the rate of the HC-grown plants being significantly (P = 0.01) higher by 45% than that in the LC-grown ones (Fig. 4). The HC-grown alga increased their NPQ to a much higher extent (about twice) compared with the LC-grown ones when exposed to stressful high-light levels (Fig. 5), reflecting that the HC condition led to additional light stress.
Figure 1.
The relative growth rate (RGR) of U. prolifera plants grown at ambient (LC; 390 μatm) and elevated (HC; 1,000 μatm) CO2 levels. Different letters above the columns indicate significant (P = 0.038) differences.
Figure 2.
The net photosynthetic rate (A), ETR (B), and NPQ (C) of U. prolifera plants grown for 2 months at ambient (LC; 390 µatm) and elevated (HC; 1,000 µatm) CO2 levels measured at growth light (LL; 100 μmol m−2 s−1) and high light (HL; 600 μmol m−2 s−1). The reaction medium (sterilized seawater) was equilibrated with the ambient or elevated CO2 air before use. FW, Fresh weight.
Figure 3.
Photosynthetic oxygen evolution (A) and relative ETR (B) as a function of PAR in the U. prolifera plants grown for 2 months at ambient (LC; 390 µatm) and elevated (HC; 1,000 µatm) CO2 levels. The reaction medium (sterilized seawater) was equilibrated with ambient air before use. FW, Fresh weight.
Table I. Photosynthetic parameters of relationships of photosynthetic oxygen evolution (Fig. 3A) and ETR (Fig 3B) as a function of PAR in U. prolifera plants grown at ambient (LC; 390 µatm) and elevated (HC; 1,000 µatm) CO2 levels.
Data are means ± sd of three measurements; different letters represent significant differences between the CO2 levels (a/b, P < 0.05; a/a, P > 0.05). IK, Light saturation perimeter.
Figure 4.
Changes in ETR for U. prolifera plants grown for 2 months at ambient (LC; 390 µatm) and elevated (HC; 1,000 µatm) CO2 levels when measured in CO2- or DIC-free reaction medium (buffered with 20 mm Tris, pH 8.2) under a PAR of 600 μmol photons m−2 s−1 and 20°C.
Figure 5.
Changes in NPQ as a function of PAR in the U. prolifera plants grown for 2 months at ambient (LC; 390 µatm) and elevated (HC; 1,000 µatm) CO2. The reaction medium (sterilized seawater) was equilibrated with the ambient air before use.
RESPIRATORY RESPONSE, CCM ACTIVITY, AND PIGMENTS
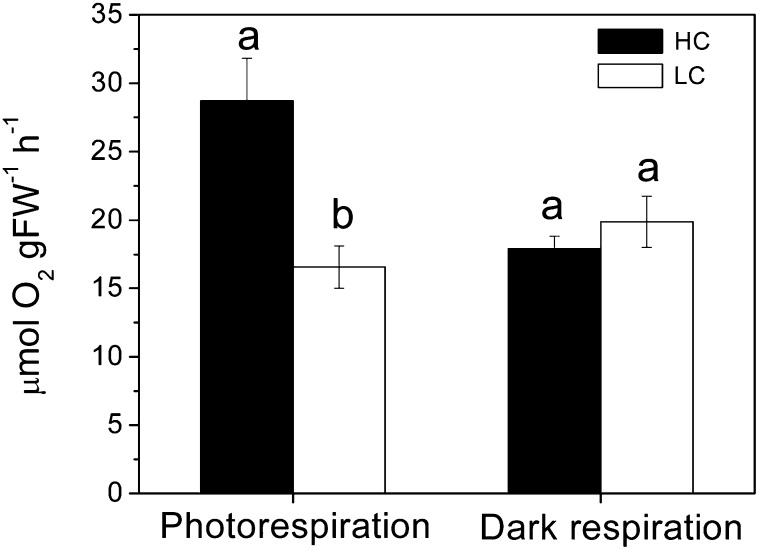
When light-dependent (photorespiration) and dark respirations were compared, the HC-grown thalli showed a higher (by 52%; P = 0.017) photorespiration rate than the LC-grown ones, but no significant (P = 0.41) difference was found in the dark respiration (Fig. 6).
Figure 6.
Changes in photorespiration (measured at 600 μmol photons m−2 s−1) and dark respiration for young U. prolifera grown for 2 months at ambient (LC; 390 µatm) and elevated (HC; 1,000 µatm) CO2 levels. Different letters above the bars represent significant (P < 0.05) differences between the CO2 levels. FW, Fresh weight.
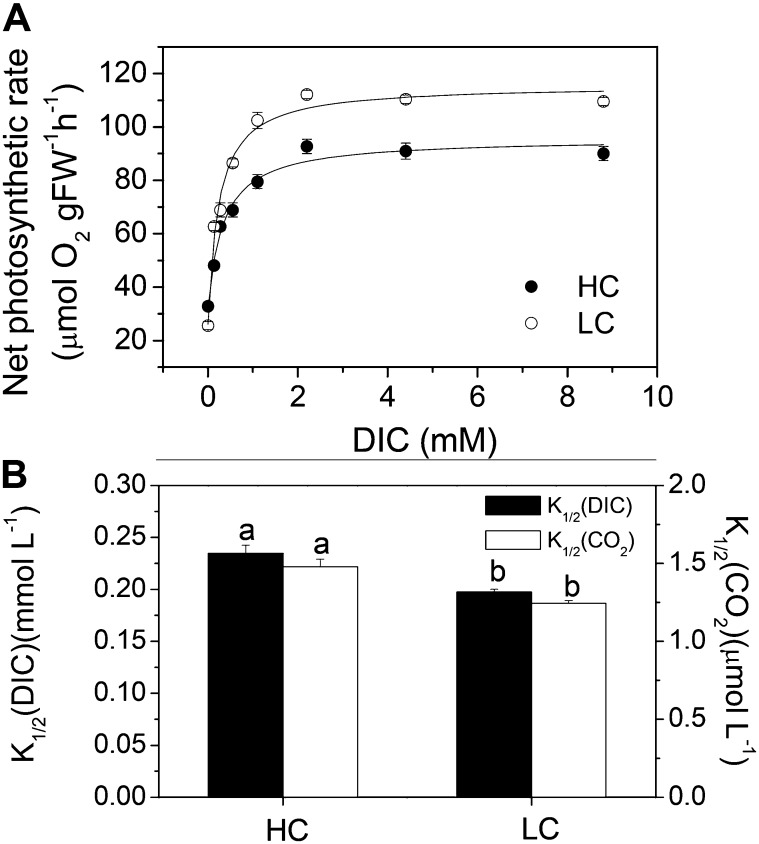
The relationship of photosynthetic oxygen evolution versus dissolved Ci concentration (Fig. 7A) showed higher K1/2DIC or K1/2CO2, reflecting reduced photosynthetic dissolved Ci (DIC) affinity (Fig. 7B), in the HC-grown plants. Such a reduced photosynthetic affinity for DIC and/or CO2 indicated a down-regulated CCM or a decrease in CCM activity. The efficiency of Ci acquisition, reflected by the ratio of Vmax to half-maximal photosynthetic rate (K1/2) for both DIC and CO2, decreased significantly (P = 0.0001) in the HC-grown plants, by up to 31%.
Figure 7.
A, Changes in net photosynthetic rate for U. prolifera plants grown for 2 months at ambient (LC; 390 µatm) and elevated (HC; 1,000 µatm) CO2 levels, measured at 600 μmol photons m−2 s−1 and 20°C, as a function of DIC concentration. B, DIC and CO2 concentrations for the K1/2, derived from A. Different letters above the bars represent significant (P < 0.05) differences between the CO2 levels. FW, Fresh weight.
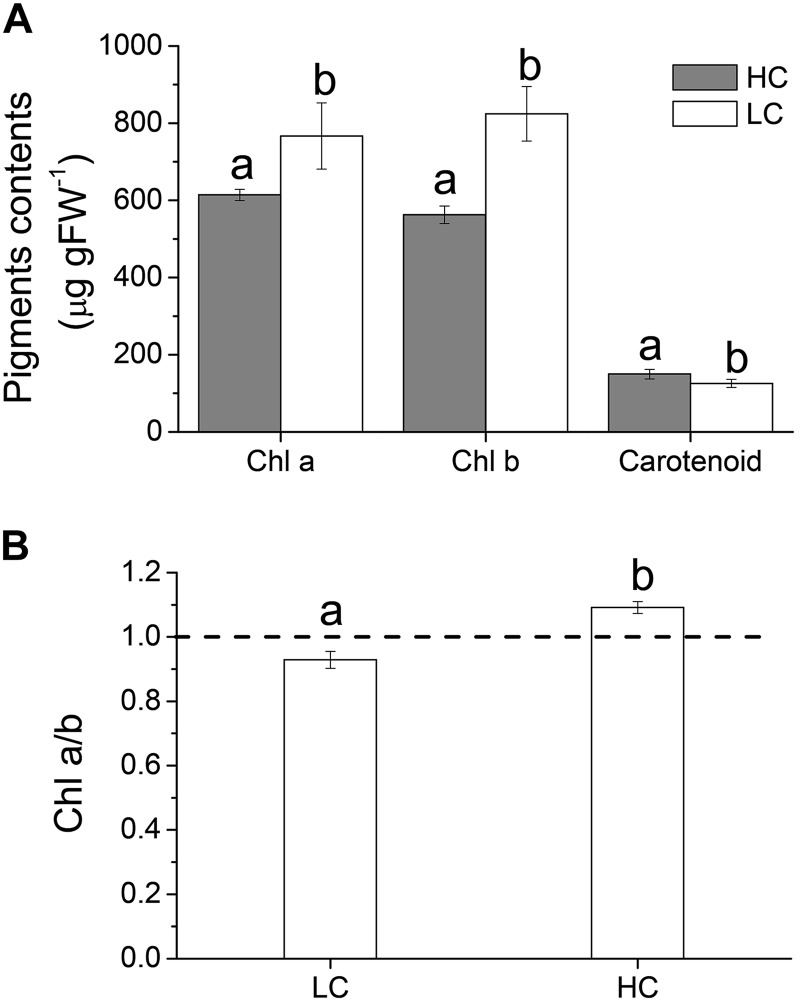
The contents of chlorophyll (Chl) a and Chl b decreased (P = 0.039 and 0.004, respectively), while that of carotenoids increased (P = 0.032) in the HC-grown thalli (Fig. 8A). The Chl a/b ratio in the thalli grown in the LC condition was significantly lower than that in the HC condition (P = 0.001; Fig. 8B).
Figure 8.
Pigment contents (Chl a, Chl b, and carotenoid; A) and Chl a/b ratio (B) of U. prolifera plants grown for 2 months at ambient (LC; 390 µatm) and elevated (HC; 1,000 µatm) CO2 levels. The different letters above the bars represent significant (P < 0.05) differences between the LC and HC conditions. FW, Fresh weight.
POSSIBLE MECHANISMS AND IMPLICATIONS
The green alga U. prolifera, when grown under the elevated CO2 concentration in a controlled seawater carbonate system, showed enhanced growth (Fig. 1) and reduced its maximal photosynthetic oxygen evolution rate and light use efficiency (Figs. 2A and 3A; Table I) as well as its efficiency to dissipate excessive light energy (Figs. 2C and 5), although acclimation to the elevated CO2 level led to a higher electron transfer rate under the growth light level (Figs. 2B and 3B), which is consistent with that previously reported by Liu et al. (2012) for the same species grown under the same high CO2 level but for a longer period (80 d). Along with the decreased photosynthetic affinity for HCO3− and/or CO2 (Fig. 7), photorespiration increased (Fig. 6), Chl a and b contents decreased, and carotenoid contents increased remarkably under the elevated CO2 (Fig. 8). The increased photorespiration and carotenoid contents, together with the down-regulated CCM, implied that the green alga increased its defensive strategy against CO2-driven seawater acidification at the cost of a reduced ratio of carboxylation to oxygenation.
Although light use efficiency increased due to saved energy from down-regulated CCM or easing acid-base regulation under the OA condition, decreased contents of photosynthetic pigments (Fig. 8) and/or enhanced photorespiration in the HC-grown thalli did lead to decreased oxygen evolution at the subsaturating and saturating light levels (Fig. 7), although their ETR increased either at deprived (Fig. 4) or elevated (Liu et al., 2012) DIC levels. Since the operation of CCMs demands energy and concentrated intracellular CO2 can acidify the thylakoid lumen, an essential component of the CCM (Raven, 1997), down-regulation of CCM in the HC-grown thalli might have decreased the intracellular CO2 level and have contributed to lower the NPQ at the same time. HC-grown red alga P. yezoensis showed decreased oxygen evolution rate (K. Gao, unpublished data), although its growth rate was stimulated (Gao et al., 1991). A cyanobacterium grown at an elevated DIC level also decreased its net photosynthetic reductant production (Mackenzie et al., 2004). The enhanced growth rate of U. prolifera might be attributed to the energy saved due to the down-regulated CCM operation as well as nitrogen metabolism. Elevated CO2 concentrations stimulated the uptake of NO3− in another Ulva species, Ulva rigida (Gordillo et al., 2001), and enhanced the activity of nitrate reductase in U. rigida (Gordillo et al., 2001, 2003) and Ulva linza (data not shown).
The persistence of photosynthetic electron transport without supply of ambient Ci from the ambient environment (Fig. 4) indicated the involvement of some pathways that continuously drained the electrons when the plants were exposed to oversaturating light levels. Consumption or reduction of O2 by both photorespiration and the Mehler reaction must have played a critical role in photoprotection, sustaining the electron flow (Ort and Baker, 2002). The fact that the sustained ETR was much higher in the HC-grown than in the LC-grown plants, even in the CO2-free medium, reflected that these pathways were enhanced under the HC condition. Photorespiration was indeed much higher in the HC-grown than in the LC-grown plants (Fig. 6). On the other hand, light-stimulated synthesis of fatty acids might also play a role in additional electron drainage (Willms et al., 1999).
The elevated CO2 concentrations, projected for the end of this century, are known to down-regulate algal CCMs (Rost et al., 2003; Wu et al., 2010), but they may not be adequate to supply enough CO2 by diffusion to sustain intracellular CO2 concentrations. While intracellular Ci pools might be reduced in HC-grown algae to be lower than that in the LC-grown ones (Spijkerman, 2011; Raven et al., 2012), intracellular CO2 availability around Rubisco in U. prolifera would have decreased under OA, so that the photosynthetic oxygen evolution rate was reduced (Figs. 2A and 3A) and photorespiration was enhanced (Fig. 6). On the other hand, the enhancement of ETR in the HC-grown plants (Figs. 2B, 3B, and 4) indirectly indicated that the enhancement of photorespiration or other metabolic pathways, such as the water-water cycle (Asada, 1999), led to additional electron drainage while playing the photoprotective role under excessive light conditions (Crawley et al., 2010). At the same time, NPQ increased faster in the HC-grown plants (Figs. 2C and 5). Stimulated NPQ and photorespiration under OA were recently noted in diatoms and phytoplankton assemblages when exposed to high light levels (Gao et al., 2012).
Under growth subsaturating light, down-regulated CCM due to increased external CO2 availability might have saved some energy for its operation, and this saved energy, such as ATP generated by transmembrane H+-ATPase, could be used for carboxylation, leading to decreased energy-dependent nonphotochemical quenching (the key component of NPQ; Kanazawa and Kramer, 2002) and increased ETR (Fig. 2B; Liu et al., 2012) and growth (Fig. 1). However, when the alga was exposed to excessive light levels, enhanced defensive pathways under the OA condition, such as photorespiration and NPQ, as well as the synthesis of carotenoids would have taken their toll on the energetics (Figs. 6 and 8A). At the same time, the alga decreased its light-capture pigments (Fig. 8A). Such a “pigment economy” phenomenon could avoid the overexcitation of electron transport as a sign of adaptation, and reduction of the antenna size and an increase in the Chl a/b ratio (Fig. 8B) provided further evidence to support this.
For terrestrial green plants, short-term exposures to increased CO2 levels stimulate the net photosynthesis of both C3 and even some C4 plants (Ziska and Bunce, 2006), while long-term exposures to elevated CO2 concentration often lead to a declined photosynthetic rate in many plant species (Thomas and Strain, 1991). Dark respiration and photorespiration are also reduced at the leaf level, while plant growth is stimulated (Bunce, 2004). In addition, intracellular acid-base regulation is affected by elevated CO2 levels in C3 higher plants (Yin et al., 1990). In contrast, the green alga U. prolifera has to cope with both decreasing pH and increasing partial pressure of CO2 in the marine environment because of the atmospheric CO2 rise. Such an acid-based perturbation would affect ion channels across the cell membrane and the associated energetic cost in order to maintain intracellular pH stability; therefore, OA can be a stressor for marine plants, although it may favor their energy budget when light energy is limited (Gao et al., 2012). When other environmental stressors, such as excessive light, coexisted with OA, the green alga’s defensive strategy was enhanced (Figs. 2B, 5, and 6). From an evolutionary point of view, of advantage to the green plants evolving to terrestrial environments might have been avoidance of the stress of OA, which is known to have occurred at the end of the Ordovician period (Veron, 2008). Green plants have been suggested to have evolved about 400 million years ago (Leliaert et al., 2011), when atmospheric CO2 was as high as 3,000 μL L−1 and the extinction of marine organisms occurred with an ancient OA event (Berner, 2006; Veron, 2008).
In the natural environment, although U. prolifera can tolerate some extent of pH change, increasing seawater acidity due to the ongoing atmospheric CO2 rise would lower the baseline of coastal pH and challenge most organisms’ ability to acclimate or adapt to coastal OA, which is suggested to proceed faster than in the pelagic waters (Cai et al., 2011). Our study obviously demonstrates that OA mediated the photochemical and photorespiratory pathways of the green alga grown under OA conditions, but how this affects its life cycle (interchange of sporophyte and gametophyte life stages) remains to be investigated.
MATERIALS AND METHODS
Thalli and Culture Conditions
Thalli of Ulva prolifera were collected in June 2009 from the coastal water of Lianyungang (119.3oE, 34.5oN), Jiangsu province of China, where the alga caused a green tide (Keesing et al., 2011). Selected thalli were cleaned of epiphytes and cultured in the laboratory at 100 µmol photons m−2 s−1 photosynthetically active radiation (PAR; 12/12 h of light/dark) and at 20°C before the released spores were collected and allowed to settle on glass slides in darkness. After adhesion, the spores were cultured under ambient (390 µatm; LC) and elevated (1,000 µatm; HC) CO2 levels and at the above light and temperature conditions. The HC level was obtained in a CO2 plant chamber, which automatically controlled the CO2 concentration in it with variation of less than 5%. The LC level was obtained by pumping the ambient air from outside (top of the building) of the laboratory. The pH levels were maintained at 8.18 and 7.82 in the LC and HC cultures, respectively, with variations of less than 0.04, by constantly removing increased algal biomass and renewing the medium every 24 h. The dissolved pCO2 and HCO3− levels were 32.4 and 1,936 μm, respectively, increased by 149% and 113%, and CO32− concentration was 75 μm, decreased by 50%, in the HC compared with the LC treatment Table II), representing typical chemical changes in the carbonate system associated with OA. Culture under the two CO2 levels lasted for about 2 months before the physiological performances were investigated during the last week.
Table II. Parameters of the seawater carbonate system under the ambient (LC; 390 µatm) and elevated (HC; 1,000 µatm) CO2 concentrations.
DIC, pH, salinity, nutrient concentration, and temperature were used to derive all other parameters using a CO2 system-analyzing software (CO2SYS). Data are means ± sd of six measurements; different letters represent significant differences between the CO2 levels (a/b, P < 0.05; a/a, P > 0.05). NBS, National Bureau of Standards.
Measurement of Growth
The relative growth rate (RGR) of the young sporophytes was estimated after the plants had acclimated to the CO2 levels for more than 50 d as follows: RGR = 100 × (lnNt − lnN0)/t, where N0 is the initial fresh weight and Nt is that after t days.
Measurements of Photosynthetic Oxygen Evolution
Photosynthetic oxygen evolution was measured by using a Clark-type oxygen electrode (YSI model 5300A). About 1-cm-long segments of U. prolifera were prepared with scissors (the cutting damage was minimized by incubating the segments in natural seawater for 1 h). About 0.05-g samples (about 0.03–0.04 mg of Chl a) were transferred to the cuvette containing 8 mL of filtered fresh seawater (30% salinity, pH 8.2, 1.9 mm DIC) for determining the net photosynthetic rate as a function of light (P-E curve), and the temperature was controlled at 20°C. The net photosynthetic rate was also measured in seawater medium equilibrated with either high (1,000 μatm) or low (390 μatm) CO2 levels under growth light (100 μmol photons m−2 s−1) and high light (600 μmol photons m−2 s−1). The photosynthesis-versus-DIC concentrations curve was measured by adding sodium bicarbonate solution into DIC-free seawater medium buffered with 20 mm Tris (pH 8.2) at final DIC concentrations within a range of 0 to 8.8 mmol L−1. Parameters for the P-E curves were analyzed as follows (Jassby and Platt 1976): P = Pmax × tanh(α × E/Pmax) + Rd, where P is the photosynthetic rate, tanh is the hyperbolic tangent, E is the irradiance, and Rd is the dark respiration rate. The K1/2 (reciprocal of photosynthetic affinity) values for DIC or CO2 were calculated using the Michaelis-Menten equation.
Determination of Photorespiration
Photorespiration was estimated as the difference in net photosynthetic oxygen evolution of the thalli at reduced (2%) and ambient (21%) O2 levels (Björk et al., 1993). The Tris-buffered seawater (pH 8.2) was flushed with either pure N2 or air to establish the low (2%) or air-saturated (21%) levels of dissolved O2. Oxygen concentration was measured as above.
Chl Fluorescence Measurements
After 10 min of dark adaptation, fluorescence induction curves were measured with a xenon-pulse amplitude-modulated fluorometer (Walz) for examining the photochemical performance of U. prolifera grown at different CO2 levels. The actinic light was set at 100 and 600 µmol photons m−2 s−1, respectively, and the saturating pulse was 5,000 µmol photons m−2 s−1 (0.8 s). The NPQ was attained as follows: NPQ = (Fm − Fm′)/Fm′, where Fm is the maximum fluorescence yield after dark adaptation and Fm′ is the maximum fluorescence yield under actinic light. The ETR was calculated as follows (Genty et al., 1989): ETR (µmol e− m−2 s−1) = Fv′/Fm′ × 0.5 × PFD × A, where Fv′/Fm′ represents the effective PSII quantum yield, PFD is the photosynthetically active photon flux density, and A is the fraction of incident photons absorbed by U. prolifera thallus (Franklin and Badger, 2001). The rapid light curves for ETR and NPQ were measured under eight different PAR levels (every measurement lasted for 10 s), and the reaction medium was the same as that used for the measurement of the P-E curve. The parameters of the ETR curves were analyzed according to Webb et al. (1974): ETR = ETRmax × (1 − e(−α*E/ETRmax)), where α is the efficiency of electron transport and E is the irradiance. To investigate the ETR in CO2-free seawater, DIC-free seawater medium was obtained by adding 1.0 m HCl to lower the pH to less than 3.0, then sparging for at least 1 h with high-purity N2 gas, and buffering with 20 mm Tris (pH 8.2).
Determination of Photosynthetic Pigments
About 100 mg fresh weight of thalli was extracted with 10 mL of absolute methanol at 4°C for 24 h in darkness, and Chl a, Chl b, and carotenoid concentrations were estimated according to Wellburn (1994).
Data Analysis
The data are shown as means ± sd of three measurements. Statistical analysis was performed with one-way ANOVA (Tukey’s) or Student’s t test. The 95% confidence level was used in all analyses.
Acknowledgments
Prof. John Hodgkiss of the University of Hong Kong is thanked for his assistance with English.
Glossary
- OA
ocean acidification
- Ci
inorganic carbon
- CCM
carbon-concentrating mechanism
- ETR
electron transport rate
- NPQ
nonphotochemical quenching
- HC
high-CO2
- LC
low-CO2
- Pmax
photosynthetic oxygen evolution rate
- ETRmax
light-saturated electron transport rate
- DIC
dissolved inorganic carbon
- Chl
chlorophyll
- PAR
photosynthetically active radiation
- P-E curve
net photosynthetic rate as a function of light
- K1/2
half-maximal photosynthetic rate
- Pmax
light-saturated photosynthetic oxygen evolution rate
References
- Asada K. (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50: 601–639 [DOI] [PubMed] [Google Scholar]
- Beardall J, Sobrino C, Stojkovic S. (2009) Interactions between the impacts of ultraviolet radiation, elevated CO2, and nutrient limitation on marine primary producers. Photochem Photobiol Sci 8: 1257–1265 [DOI] [PubMed] [Google Scholar]
- Berner RA. (2006) Inclusion of the weathering of volcanic rocks in the GEOCARBSULF model. Am J Sci 306: 295–302 [Google Scholar]
- Björk M, Haglund K, Ramazanov Z, Pedersen M. (1993) Inducible mechanisms for HCO3− utilization and repression of photorespiration in protoplasts and thalli of 3 species of Ulva (Chlorophyta). J Phycol 29: 166–173 [Google Scholar]
- Bunce JA. (2004) A comparison of the effects of carbon dioxide concentration and temperature on respiration, translocation and nitrate reduction in darkened soybean leaves. Ann Bot (London) 93: 665–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai WJ, Hu X, Huang WJ, Murrell MC, Lehrter JC, Lohrenz SE, Chou WC, Zhai W, Hollibaugh JT, Wang Y. (2011) Acidification of subsurface coastal waters enhanced by eutrophication. Nat Geosci 4: 766–770 [Google Scholar]
- Crawley A, Kline DI, Dunn S, Anthony K, Dove S. (2010) The effect of ocean acidification on symbiont photorespiration and productivity in Acropora formosa. Glob Change Biol 16: 851–863 [Google Scholar]
- Doney SC, Fabry VJ, Feely RA, Kleypas JA. (2009) Ocean acidification: the other CO2 problem. Annu Rev Mar Sci 1: 169–192 [DOI] [PubMed] [Google Scholar]
- Douglas SE, Raven JA, Larkum AWD. (2004) The algae and their general characteristics. In AWD Larkum, SE Douglas, JA Raven, eds, Photosynthesis in Algae. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 1–10 [Google Scholar]
- Franklin LA, Badger MR. (2001) A comparison of photosynthetic electron transport rates in macroalgae measured by pulse amplitude modulated chlorophyll fluorometry and mass spectrometry. J Phycol 37: 756–767 [Google Scholar]
- Gao K, Aruga Y, Asada K, Ishihara T, Akano T, Kiyohara M. (1991) Enhanced growth of the red alga Porphyra yezoensis ueda in high CO2 concentrations. J Appl Phycol 3: 355–362 [Google Scholar]
- Gao K, Aruga Y, Asada K, Kiyohara M. (1993) Influence of enhanced CO2 on growth and photosynthesis of the red algae Gracilaria sp and G. chilensis. J Appl Phycol 5: 563–571 [Google Scholar]
- Gao K, Ji Y, Aruga Y. (1999) Relationship of CO2 concentrations to photosynthesis of intertidal macroalgae during emersion. Hydrobiologia 399: 355–359 [Google Scholar]
- Gao K, Xu J, Gao G, Li Y, Hutchins DA, Huang B, Wang L, Zheng Y, Jin P, Cai X, et al. (2012) Rising CO2 and increased light exposure synergistically reduce marine primary productivity. Nat Climate Change 2: 519–523 [Google Scholar]
- Gao K, Zheng Y. (2010) Combined effects of ocean acidification and solar UV radiation on photosynthesis, growth, pigmentation and calcification of the coralline alga Corallina sessilis (Rhodophyta). Glob Change Biol 16: 2388–2398 [Google Scholar]
- Garcŕa-Sánchez MJ, Fernandez JA, Niell X. (1994) Effect of inorganic carbon supply on the photosynthetic physiology of Gracilaria tenuistipitata. Planta 194: 55–61 [Google Scholar]
- Genty B, Briantais JM, Baker NR. (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990: 87–92 [Google Scholar]
- Giordano M, Beardall J, Raven JA. (2005) CO2 concentrating mechanisms in algae: mechanisms, environmental modulation, and evolution. Annu Rev Plant Biol 56: 99–131 [DOI] [PubMed] [Google Scholar]
- Gordillo FJL, Figueroa FL, Niell FX. (2003) Photon- and carbon-use efficiency in Ulva rigida at different CO2 and N levels. Planta 218: 315–322 [DOI] [PubMed] [Google Scholar]
- Gordillo FJL, Niell FX, Figueroa FL. (2001) Non-photosynthetic enhancement of growth by high CO2 level in the nitrophilic seaweed Ulva rigida C. Agardh (Chlorophyta). Planta 213: 64–70 [DOI] [PubMed] [Google Scholar]
- Gruber N, Hauri C, Lachkar Z, Loher D, Frölicher TL, Plattner GK. (2012) Rapid progression of ocean acidification in the California current system. Science 337: 220–223 [DOI] [PubMed] [Google Scholar]
- Hepburn CD, Pritchard DW, Cornwall CE, McLeod RJ, Beardall J, Raven JA, Hurd CL. (2011) Diversity of carbon use strategies in a kelp forest community: implications for a high CO2 ocean. Glob Change Biol 17: 2488–2497 [Google Scholar]
- Hopkinson BM, Dupont CL, Allen AE, Morel FMM. (2011) Efficiency of the CO2-concentrating mechanism of diatoms. Proc Natl Acad Sci USA 108: 3830–3837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton JT, Ding Y, Griggs DJ, Noguer M, van der Winden PJ, Dai X. (2001) Climate Change 2001: The Scientific Basis. Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, UK [Google Scholar]
- Israel A, Hophy M. (2002) Growth, photosynthetic properties and Rubisco activities and amounts of marine macroalgae grown under current and elevated seawater CO2 concentrations. Glob Change Biol 8: 831–840 [Google Scholar]
- Israel A, Katz S, Dubinsky Z, Merrill JE, Friedlander M. (1999) Photosynthetic inorganic carbon utilization and growth of Porphyra linearis (Rhodophyta). J Appl Phycol 11: 447–453 [Google Scholar]
- Jassby AD, Platt T. (1976) Mathematical formulation of relationship between photosynthesis and light for phytoplankton. Limnol Oceanogr 21: 540–547 [Google Scholar]
- Johnson VR, Russell BD, Fabricius KE, Brownlee C, Hall-Spencer JM. (2012) Temperate and tropical brown macroalgae thrive, despite decalcification, along natural CO2 gradients. Glob Change Biol 18: 2792–2803 [DOI] [PubMed] [Google Scholar]
- Johnston AM, Raven JA. (1990) Effects of culture in high CO2 on the photosynthetic physiology of Fucus serratus. Br Phycol J 25: 75–82 [Google Scholar]
- Kanazawa A, Kramer DM. (2002) In vivo modulation of nonphotochemical exciton quenching (NPQ) by regulation of the chloroplast ATP synthase. Proc Natl Acad Sci USA 99: 12789–12794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keesing JK, Liu DY, Fearns P, Garcia R. (2011) Inter- and intra-annual patterns of Ulva prolifera green tides in the Yellow Sea during 2007-2009, their origin and relationship to the expansion of coastal seaweed aquaculture in China. Mar Pollut Bull 62: 1169–1182 [DOI] [PubMed] [Google Scholar]
- Kübler JE, Johnston AM, Raven JA. (1999) The effects of reduced and elevated CO2 and O2 on the seaweed Lomentaria articulata. Plant Cell Environ 22: 1303–1310 [Google Scholar]
- Larsson C, Axelsson L, Ryberg H, Beer S. (1997) Photosynthetic carbon utilization by Enteromorpha intestinalis (Chlorophyta) from a Swedish rockpool. Eur J Phycol 32: 49–54 [Google Scholar]
- Leliaert F, Verbruggen H, Zechman FW. (2011) Into the deep: new discoveries at the base of the green plant phylogeny. Bioessays 33: 683–692 [DOI] [PubMed] [Google Scholar]
- Liu Y, Xu J, Gao K. (2012) CO2-driven seawater acidification increases photochemical stress in a green alga. Phycologia 51: 562–566 [Google Scholar]
- Lu X, Qiao F. (2008) Distribution of sunken macroalgae against the background of tidal circulation in the coastal waters of Qingdao, China, in summer 2008. Geophys Res Lett 35: L23614 [Google Scholar]
- Mackenzie TDB, Burns RA, Campbell DA. (2004) Carbon status constrains light acclimation in the cyanobacterium Synechococcus elongatus. Plant Physiol 136: 3301–3312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado JM, Javier F, Gordillo L, Niell FX, Figueroa FL. (1999) Effects of different levels of CO2 on photosynthesis and cell components of the red alga Porphyra leucosticta. J Appl Phycol 11: 455–461 [Google Scholar]
- Ort DR, Baker NR. (2002) A photoprotective role for O2 as an alternative electron sink in photosynthesis? Curr Opin Plant Biol 5: 193–198 [DOI] [PubMed] [Google Scholar]
- Raven JA. (1997) CO2-concentrating mechanisms: a direct role for thylakoid lumen acidification? Plant Cell Environ 20: 147–154 [Google Scholar]
- Raven JA, Giordano M, Beardall J, Maberly SC. (2011) Algal and aquatic plant carbon concentrating mechanisms in relation to environmental change. Photosynth Res 109: 281–296 [DOI] [PubMed] [Google Scholar]
- Raven JA, Giordano M, Beardall J, Maberly SC. (2012) Algal evolution in relation to atmospheric CO2: carboxylases, carbon-concentrating mechanisms and carbon oxidation cycles. Philos Trans R Soc Lond B Biol Sci 367: 493–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven JA, Walker DI, Johnston AM, Handley LL, Kübler JE. (1995) Implications of 13C natural abundance measurements for photosynthetic performance by marine macrophytes in their natural environment. Mar Ecol Prog Ser 123: 193–205 [Google Scholar]
- Roleda MY, Morris JN, McGraw CM, Hurd CL. (2012) Ocean acidification and seaweed reproduction: increased CO2 ameliorates the negative effect of lowered pH on meiospore germination in the giant kelp Macrocystis pyrifera (Laminariales, Phaeophyceae). Glob Change Biol 18: 854–864 [Google Scholar]
- Rost B, Riebesell U, Burkhardt S, Sültemeyer D. (2003) Carbon acquisition of bloom-forming marine phytoplankton. Limnol Oceanogr 48: 55–67 [Google Scholar]
- Sabine CL, Feely RA, Gruber N, Key RM, Lee K, Bullister JL, Wanninkhof R, Wong CS, Wallace DW, Tilbrook B, et al. (2004) The oceanic sink for anthropogenic CO2. Science 305: 367–371 [DOI] [PubMed] [Google Scholar]
- Sinutok S, Hill R, Doblin MA, Wuhrer R, Ralph PJ. (2011) Warmer more acidic conditions cause decreased productivity and calcification in subtropical coral reef sediment-dwelling calcifiers. Limnol Oceanogr 56: 1200–1212 [Google Scholar]
- Smith FA, Raven JA. (1979) Intracellular pH and its regulation. Annu Rev Plant Biol 30: 289–311 [Google Scholar]
- Spijkerman E. (2011) The expression of a carbon concentrating mechanism in Chlamydomonas acidophila under variable phosphorus, iron, and CO2 concentrations. Photosynth Res 109: 179–189 [DOI] [PubMed] [Google Scholar]
- Thomas RB, Strain BR. (1991) Root restriction as a factor in photosynthetic acclimation of cotton seedlings grown in elevated carbon dioxide. Plant Physiol 96: 627–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veron JEN. (2008) Mass extinctions and ocean acidification: biological constraints on geological dilemmas. Coral Reefs 27: 459–472 [Google Scholar]
- Webb WL, Newton M, Starr D. (1974) Carbon dioxide exchange of Alnus rubra: mathematical model. Oecologia 17: 281–291 [DOI] [PubMed] [Google Scholar]
- Wellburn R. (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144: 307–313 [Google Scholar]
- Willms JR, Salon C, Layzell DB. (1999) Evidence for light-stimulated fatty acid synthesis in soybean fruit. Plant Physiol 120: 1117–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Gao G, Giordano M, Gao K. (2012) Growth and photosynthesis of a diatom grown under elevated CO2 in the presence of solar UV radiation. Fund Appl Limnol 180: 279–290 [Google Scholar]
- Wu Y, Gao K, Riebesell U. (2010) CO2-induced seawater acidification affects physiological performance of the marine diatom Phaeodactylum tricornutum. Biogeosciences 7: 2915–2923 [Google Scholar]
- Yin Z, Neimanis S, Heber U. (1990) Light-dependent pH changes in leaves of C3 plants. II. Effect of CO2 and O2 on the cytosolic and the vacuolar pH. Planta 182: 253–261 [DOI] [PubMed] [Google Scholar]
- Zhang X, Xu D, Mao Y, Li Y, Xue S, Zou J, Lian W, Liang C, Zhuang Z, Wang Q, et al. (2011) Settlement of vegetative fragments of Ulva prolifera confirmed as an important seed source for succession of a large-scale green tide bloom. Limnol Oceanogr 56: 233–242 [Google Scholar]
- Ziska LH, Bunce JA. (2006) Plant responses to rising atmospheric carbon dioxide. In JIL Morison, MD Morecroft, eds, Plant Growth and Climate Change. Blackwell Publishing, Oxford, pp 17–47 [Google Scholar]
- Zou D. (2005) Effects of elevated atmospheric CO2 on growth, photosynthesis and nitrogen metabolism in the economic brown seaweed, Hizikia fusiforme (Sargassaceae, Phaeophyta). Aquaculture 250: 726–735 [Google Scholar]
- Zou D, Gao K. (2005) Ecophysiological characteristics of four intertidal marine macroalgae during emersion along Shantou coast of China, with a special reference to the relationship of photosynthesis and CO2. Acta Oceanol Sin 24: 105–113 [Google Scholar]
- Zou D, Gao K. (2010) Physiological responses of seaweeds to elevated atmospheric CO2 concentrations. In J Seckbach, R Einav, A Israel, eds, Seaweeds and Their Role in Globally Changing Environments. Springer, Dordrecht, The Netherlands, pp 115–126 [Google Scholar]